Organic manure input and straw cover improved the community structure of nitrogen cycle function microorganism driven by water erosion
2022-09-26YulongShiQingwenZhangXingrenLiuXuekaiJingChangShiLiZheng
Yulong Shi,Qingwen Zhang,Xingren Liu,Xuekai Jing,Chang Shi,Li Zheng
Agricultural Clean Watershed Resewarch Group,Institute of Environment and Sustainable Development in Agriculture,Chinese Academy of Agricultural Sciences,Beijing,100081,China
Keywords:Water erosion Soil erodibility Organic amendment Nitrifier Denitrifier N-fixing bacteria
A B S T R A C T Water erosion process induces differences to the nitrogen(N)functional microbial community structure,which is the driving force to key N processes at soil-water interface.However,how the soil N transformations associated with water erosion is affected by microorganisms,and how the microbial respond,are still unclear.The objective of this study is to investigate the changes of microbial diversity and community structure of the N-cycle function microorganisms as affected by water erosion under application of organic manure and straw cover.On the basis of iso-nitrogen substitution,four treatments were set up:1)only chemical fertilizer with N 150 kg ha-1,P2O5 60 kg ha-1 and K2O 90 kg ha-1(CK);the N was substituted 20%by 2)organic manure(OM);3)straw(SW);and 4)organic manure+straw(1:1)(OMSW).The results showed that applying organic manure and straw to sloping farmland can increase soil N contents,but reduce runoff depth,Kw,sediment yield and N loss,especially in the OMSW.Straw cover and straw+organic manure increased the diversity(Chao1)of nitrifier(AOB),and both diversity and uniformity(Shannon)of denitrifier(nirK/S)were increased in the OMSW.All erosion control measures reduced N-fixing bacteria diversity and increased their uniformity,and the combined application of organic manure and straw cover was a better erosion control measure than the single application of them.Improved soil chemistry and erodibility were the main drives for the changes of N-functional microbial community structure and the appearance of dominant bacteria with different organic materials.
1.Introduction
The essence of non-point source nitrogen pollution occurrence from the sloping cropland is the transformation and losses of nitrogen and phosphorus driven by the water erosion process.Currently,China's 23.9 million hectares sloping farmland,the production land that local people depend on for living and development,is also the main source of soil erosion.Sloping farmland accounts for about 8%of the country's total soil erosion area,but the amount of soil erosion accounts for nearly one-third of the country's total soil erosion(the 13th Five-Year Plan for comprehensive soil erosion control).The on-site effect of water erosion is that the nitrogen loss due to water erosion from sloping farmland result in a serious soil depletion,while the off-site effect of soil erosion and the losses of nitrogen in a dissolved or particle state,has exacerbated the water eutrophication problem to the receiving water bodies(Chen et al.,2017).
Straw mulching is an important measure to control soil erosion and N losses during the rainfall season(Ahmad,Mustafa,&Gideon,2020).Straw cover can avoid direct raindrop impact and promote infiltration,which in turn will reduce the water and sediment loaded N losses(Cerd`a et al.,2017).Keesstra et al.(2019)have reported that the straw mulch reduced the sediment concentration from 16.7 g L-1to 3.6 g L-1in the citrus orchard of Mediterranean area.In addition,the application of organic manure can enhance aggregation and improve soil buffering properties,and is an effective strategy to restore the eroded soil and reduce N losses(Cates & Ruark,2017;Miao et al.,2019;Sarker et al.,2018).Miao et al.(2019)found that application of cattle manure for 10 years significantly increased soil organic carbon accumulation and soil aggregation compared to the chemical fertilization only,and animal manure was recommended for restoring severely eroded soils.The sloping farmland of purple soil in Sichuan hills is mainly planted with wheat and maize with high cropping intensity and serious soil erosion.Therefore,applying organic manure and straw cover are the key practices for soil and water losses control and reduction of dissolved and sediment loaded N losses(Ahmad,Mustafa,& Gideon,2020).
The previous studies have focused on the patterns of nitrogen loss under water erosion(Bahadori et al.,2019;Liu,Li,Chang,et al.,2018;Shi & Schulin,2018;Wang et al.,2008;Zhang et al.,2000).Zhang et al.(2016)found that the soil erodibility significantly influenced the available N losses,and a positive logarithmic correlation best described soil erodibility and N losses relationship,andwas proven to be the main form of inorganic nitrogen loss.Bah et al.(2020)reported that the highest total nitrogen loss loadings were observed in the interflow that accounted for 92% of total hydrological loss,and thewas the major conduit of N loss.The mathematical models,Soil and Water Assessment Tool(SWAT)and Annualized Agricultural Non-Point Source(AnnAGNPS),were frequently used for evaluating the N losses and the effectiveness of best management practices for non-point source pollution control(Wang et al.,2018;Zhang et al.,2020),but they cannot show that the process and mechanism of the nitrogen transformation and loss under water erosion.The correlation between the transformation and the loss of soil nitrogen driven by water erosion remains unclear.Due to the driving forces of water erosion,mineral nitrogen of the slopeland soil is extracted and diffuses into the runoff.The ammonium nitrogen adsorbed on the surface of soil particles is transported by runoff into water bodies.Meanwhile,the particle binding nitrogen is lost along with the sediment transportation during water erosion processes.During water erosion processes,the nitrogen mineralization of the slopeland soil is strengthened,and at the same time,it is accompanied by the transient enhancement of nitrification and the temporary accumulation of nitrate nitrogen.Water erosion affects the nitrogen mineralization,nitrification and denitrification,and the available nitrogen can be release for hours,days,or longer.
The nitrogen transformation with microbial effect at water-soil interface plays an important role of engine to the nitrogen loss in sloping farmland.However,the N fixation,nitrification and denitrification of soil nitrogen by microorganisms under water erosion,and the microbial response,adaptation and feedback to nitrogen loss associated with water erosion are still unclear.This is a key scientific issue to be tackled urgently to increase soil N availability and reduce N loss by regulating microbial functional properties.Water erosion process changes soil properties and substrate availability,and induces structural difference to the nitrogen functional microbial community,and the microbial community influences the nitrogen transformation process through the feedback mechanism.Soil microorganisms are the driving force of key nitrogen processes in the ecosystem(Nelson et al.,2016).Soil microorganisms influence the nitrogen transformation through N fixation,nitrification,denitrification,mineralization and other processes,thus affecting the occurrence and development of non-point source nitrogen pollution.The diversity of microbial communities in nitrogen cycle was different in different water erosion habitats(Huang et al.,2013;Li et al.,2015;Xiao et al.,2017),and the abundance of the NFGs(NCycling functional Genes)is related to the process rate and available substrates in a water degradation environment.Because the NFGs replication value is expressed in different microbial groups(Ning et al.,2015),the NFGs approach is specific for the N-cycling processes.Water erosion can stimulate the activity of soil microorganisms,increase the mineralization rate of the soil organic matter and the inorganic nitrogen release,and affect the functional microbial properties involved in the N-cycling process,such as ammonia oxidation bacteria(AOB),ammonia oxidizing archaea(AOA),nitrifying bacteria and denitrifying bacteria,and thus change the N-cycling system of the sloping farmland.Severe water erosion can significantly reduce the bacterial abundance and fungal abundance(Huang et al.,2013).Qin et al.(2013)has reported that the potential nitrification rate was related to the diversity and abundance of AOA,but not to those of AOB,and the loss of NO3--N in runoff was significantly positively correlated with AOA copies in a long-term sloped land use experiment.
In the process of water erosion,sloping farmland has different hydrothermal environment and available substrate,but the process and mechanism of the microbial mediated N transformation and loss under water erosion are still unclear.Therefore,the objectives of this study are(1)to investigate the N losses in different forms driven by water erosion in different organic materials treatments;(2)to explore the diversity characteristics of the N cycle function microorganisms affected by organic manure input and straw cover under water erosion;(3)to analyze the structural changes of N cycle function microorganism community by different organic materials under water erosion using high-throughput sequencing;and(4)to better understand the drivers of the change of N cycle function microorganism community,and the relationship between the soil erodibility improvement and the variation of N cycle function microorganism after applying organic manure and straw cover.
2.Materials and methods
2.1.Site description
The field runoff monitoring site(30°36′N,105°1′E)is located in Xiangtanhe watershed of Zhongjiang County,Sichuan Province.This region has a subtropical monsoon climate with an average annual temperature of 16.7°C and an average annual precipitation of 958.2 mm in the past ten years.Rainfall from May to September accounts for more than 80% of the total annual rainfall.The cultivated land in this area is mainly sloping farmland covered by the purple soil,and the main crops are wheat and maize.The soil type is classified as an udorthent following the United States Department of Agriculture(USDA)soil taxonomy with organic matter of 12.00 g kg-1,total nitrogen(TN)of 0.98 g kg-1,total phosphorus(TP)of 0.75 g kg-1,total potassium(TK)of 20.44 g kg-1and soil bulk densities of 1.39 g cm-3in the tillage layer.The average contents of sand(2—0.05 mm),silt(0.05—0.001 mm),and clay(<0.001 mm)are about 21%,59%,and 20%,respectively.
2.2.Experimental design
The experiment began in April 2017 under winter wheat(Triticum aestivumL.)and summer maize(Zea mays L.)rotation.On the basis of organic manure and straw substituting chemical fertilizer with equal N,four soil amendment and erosion controlling treatments with three replications were set up:(1)no straw cover and organic manure,only applying chemical fertilizer with N 150 kg ha-1yr-1,P2O560 kg ha-1yr-1and K2O 90 kg hm-2(CK);(2)organic manure substituting 20%chemical fertilizer(OM);(3)straw substituting 20%chemical fertilizer(SW);(4)organic manure plus straw substituting 20% chemical fertilizer by ratio of organic manure:straw=1:1(OMSW).The amount of organic manure and wheat straw applied was 1035 kg ha-1(OM)and 3710 kg ha-1(SW),respectively.The 20% N and all P,K fertilizer and organic manure were applied in one time as base fertilizer on April 22,2017.After sowing,the wheat straw was artificially and evenly covered on the soil surface.The remaining 30%and 50%N were applied on May 22 and June 10,respectively.Urea(N 46.0%),calcium superphosphate(P2O512.0%),and potassium sulfate(K2O 52%)was used as the N,P and K fertilizers,respectively,and other management measures were consistent with local traditional practices.The organic manure contained 35.3% organic carbon,2.9% TN,5.5% TP and 2.2% TK,and the wheat straw contained 8.09 g/kg TN,0.42 g kg-1TP and 21.95 g/kg TK.The runoff plot and pools were built to collect surface runoff and sediment.Each plot had an area of 20 m×3 m=60 m2with a slope of 10°,and all plots were randomly arranged.
2.3.Sampling and measurements
Soil samples(0—20 cm)were collected using a 5 cm diameter sampling auger in the middle slope of each plot on June 22,2017.The soil samples were divided into three parts:the first one was stored at-80°C for functional genes sequencing,the second part was stored at 4°C for soil water content(SWC),ammonium nitrogen(NH4+-N)and nitrate nitrogen(NO3--N),and the third part was air-dried for soil cation-exchange capacity(CEC),organic matter,TN,available nitrogen(AN),available phosphorus(AP),available potassium(AK).A Rainfall amount of 50.2 mm before sampling was recorded using an automatic rain gauge.The water depth of each runoff pool was read immediately,and the surface runoff volume and runoff depth were calculated,after rainfall and runoff generation.Then,the pool water was manually stirred well and the 500 ml of mud-water was sampled,precipitated and filtered.The sediment was dried at 105°C and used to calculate the sediment concentration,and the filtrate was for the determination of TN,soluble total nitrogen(STN),TP and AP concentration of runoff water.
The TN and STN(the runoff was filtered by 0.45 μm microporous membrane)in runoff water were determined by a simultaneous TC/TN analyzer(multi C/N 2100 S,Analytik Jena,Germany).The NH4+-N and NO3--N of runoff were measured using an automated flow injection analyzer(Braun and Lübbe,Norderstedt,Germany).The TP of runoff was determined by the method of molybdenum—antimony anti-spectrophotometric after digestion with concentrated HClO4—H2SO4.The AP was also measured using molybdenum—antimony anti-spectrophotometric method.
Soil samples were sampled from the arable layer of each plot using a ring knife with a volume of 100 cm3,and the soil bulk density(ρb,g cm-3)was determined by the drying method(105°C,24 h).The Eijkelkamp Penetrologger was used to measure soil penetration resistance(PR,kPa).Shear strength was measured in situ by a portable three-head shear tester(Netherlands,10.4 Pocket Vane Tester).
The runoff depth was calculated using the following formula:

where Q is the runoff volume of each plot(m-3),A is the area of each plot(m2).
To calculate the sediment yield,the following formula was used:
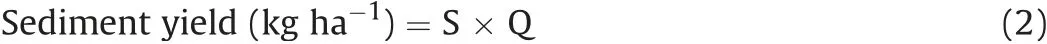
where S is the sediment concentration(kg m-3),Q is the runoff volume(m3ha-1).
Soil erodibility refers to the potential for soil damage by erosion and reflects the sensitivity of the soil to external forces of erosion,and is the basis for quantitative studies of soil erosion and a necessary parameter in many soil erosion forecasting models(Wang et al.,2013;Zhang et al.,2008),and it is the erosion amounts generated per unit area per unit runoff depth(Kw)that was used as an indicator of soil sensitivity to runoff erosion,which reflects the scouring effects of runoff and reveals the ability of soil itself to resist runoff erosion.As the runoff plot is used as the study unit and the single rainfall is taken as a study case,so the scouring power is calculated by per unit area per unit runoff.TheKwwas calculated using the following formula(Wu et al.,1993):

where the sediment yield is sediment yield of each plot(kg),A is the area of each plot(m2).
2.4.DNA extraction
The total DNA were extracted from each soil sample(0.5 g)using the Fast DNA SPIN extraction kits(MP Biomedicals,Santa Ana,CA,USA)according to the manufacturer's instructions,and the DNA samples were stored at-80°C for later use.The concentration and purity of DNA were determined using NanoDrop 2000 spectrophotometer(Thermo Scientific,USA)and agarose gel electrophoresis(1%),respectively.
2.5.Sequencing
The AOA-amoA,AOB-amoA,nirK,nirSandnifHgenes were sequenced at the Illumina MiSeq PE300 sequencing platform(Illumina,Inc.,CA,USA)by Beijing Allwegene Technology Inc.(Beijing,China).The following primers were used for PCR amplicons:Arch-amoA26F(GACTACATMTTCTAYACWGAYTGGGC)/ArchamoA417R(GGKGTCATRTATGGWGGYAAYGTTGG)for AOA-amoA(Park et al.,2008),amoA-1F(GGGGTTTCTACTGGTGGT)/amoA-2R(CCCCTCKGSAAAGCCTTCTTC)for AOB-amoA(Park et al.,2008),F1aCu(ATCATGGTSCTGCCGCG)/R3Cu(GCCTCGATCAGRTTGTGGTT)for nirK(Di et al.,2014),cd3aF(GTSAACGTSAAGGARACSGG)/R3cd(GASTTCGGRTGSGTCTTGA)for nirS(Di et al.,2014)and PolF1(TGCGAICCSAAIGCIGACTC)/AQER(GACGATGTAGATYTCCTG)for nifH(Yang,Bai,et al.,2019).The PCR reactions contained DNA sample 30 ng,forward primer(5 μM)1 μL,reverse primer(5 μM)1 μL,BSA(2 ng/μL)3 μL,2×Taq Plus Master Mix 12.5 μL and 7.5 μL of ddH2O.The PCR products were extracted by 2% agarose gels electrophoresis and purified by AxyPrep DNA Gel Extraction Kit(Axygen,USA).After quantification,the amplicons were pooled in equimolar and paired-end sequenced(2×300 bp)on the Illlumina MiSeq platform.
2.6.Sequence analysis
The sequencing data were de-multiplexed and quality-filtered using Quantitative Insights Into Microbial Ecology(QIIME,v1.8.0)(Wood et al.,2015).Low-quality sequences were removed that were shorter than 200 bp,including any ambiguous bases or mononucleotide repeats over 8,and the average Phred scores of sequences were over 20(Hou et al.,2018;Zhou et al.,2016).The operational taxonomic units(OTUs)were clustered by UCLUST(Edgar 2010)using high-quality sequences at 97% similarity.The representative sequences were selected from each OTU under default parameters,and each representative sequence was given its taxonomic information using the Ribosomal Database Project(RDP)Classifier(Ou et al.,2019).Taxonomic information was obtained and the community composition of each sample at each classification level was statistically calculated.
Sequence data were calculated using QIIME(v1.8.0)and R(v3.6.0).The α diversity indices including Chao1 and Shannon were calculated at OTU level.The principal coordinate analysis(PCoA)with weighted Unifrac distances was used to investigate the changes of microbial communities among samples in R(v3.6.0).The LEfSe analysis was used to detect the biomarkers with significant differences among groups with LDA Score=2 at genus level(http://huttenhower.sph.harvard.edu/galaxy/)(Segata et al.,2011).The redundancy analysis(RDA)and variance partitioning analysis were used to determine the key factors that changed the microbial community structure and the correlations between microorganisms and the soil physiochemical properties after removing collinearity among factors in Canoco 5.0.
2.7.Statistical analysis
The data of soil physical and chemical properties and soil erodibility were processed and calculated by Microsoft Office Excel 2019(Microsoft Corporation,USA),and the figures were produced by Origin Pro 8.5(Origin Lab,USA).All data were expressed as the mean±standard deviation.Analysis of variance(ANOVA)was used to determine the difference of soil and erosion indexes among treatments at a significance level of 0.05 by SPSS Statistics software(version 20)(IBM Software,Chicago,IL,USA).Pearson's correlation analysis was used to determine the correlation among the soil,erosion and diversity indexes,and the figures was produced in R(v3.6.0).
3.Results
3.1.Soil physical and chemical properties
Returning organic manure and straw to the field changed soil erodibility.The CK(17.67 mm)and OM(16.41 mm)treatments had a higher runoff depth than the amendments with straw(12.55 mm)and straw+organic manure(11.90 mm).Moreover,the sediment yields andKwwere significantly reduced in the OM(by 35.59%and 30.63%),SW(by 51.40% and 31.55%)and OMSW(by 56.60% and 36.14%)(Table 1).Application of organic manure,straw and organic manure plus straw had no significant change on soil bulk density,penetration resistance,shear strength,SWC(Table 1)and CEC(Table 2)compared with CK.The soil organic matter,available P and K increased in the OM,SM and OMSM(Table 2).Application of organic manure,straw and organic manure plus straw had a significant influence on soil N contents.The soil total N,NH4+-N,NO3--N and available N increased or had a growing trend under OM,SM and OMSM treatments.Soil nitrogen loss was dominated by soluble nitrogen(accounted for 60.0%—74.6%of TN in runoff).Input of organic manure and straw cover significantly reduced the losses of the total N,total soluble N,total P and available P contents from runoff while the reduction effect was not significant on the losses of NH4+-N and NO3--N(Table 3).

Table 1The changes of runoff depth,sediment yield,Kw,soil bulk density,penetration resistance,shear strength and soil water content after the rain.

Table 2The changes of soil CEC,organic matter,N,P and K contents after the rain.

Table 3The changes of N and P contents of runoff water.
3.2.The α-diversity of the nitrogen cycle function microorganism
Application of organic manure,straw and organic manure+straw changed the diversity of AOA,AOB,nitrogen-fixing bacteria,nirK-andnirS-type denitrifiers with different changing trends,as shown in Fig.1.For nitrifier,applying organic manure,straw and organic manure+straw reduced the Chao1 index of AOA,and Shannon of AOA was also reduced in OM.For AOB,straw amendment increased both Chao1 and Shannon,while organic manure+straw only increased the Chao1.Applying organic manure made no significant difference on the Chao1 and Shannon of AOB compared with CK.For N-fixing bacteria,the Chao1 decreased in OM,SW and OMSW,while manure,straw and organic manure+straw enhanced the Shannon index.Moreover,it showed a significant difference in one treatment for these two indexes.There were similar changes for Chao1 ofnirK-andnirS-type denitrifiers in each treatment.Only organic manure+straw increased the Chao1 and Shannon ofnirK/S-type denitrifiers.Organic manure showed a significant impact on Shannon of bothnirK-andnirS-type denitrifiers,increasing the Shannon ofnirK-type denitrifier but decreasing thenirS-type denitrifier.The Shannon ofnirK-type denitrifier was significantly enhanced by straw,but straw did not change the Shannon ofnirS-type denitrifier.The diversity of nitrifiers were lower than N-fixing bacteria,nirK-andnirS-type denitrifiers.
3.3.The compositions of the nitrogen cycle function microorganism
The PCoA showed bacterial community separation among the samples(Fig.2).The communities of AOA in OM,SW and OMSW were different from community in CK,and the communities were not clearly separated between SW and OMSW(Fig.2(a)).There was a significant difference of the community of AOB among different treatments(R=0.76,P=0.001,Fig.2(b)).The communities ofnirKtype denitrifier(R=0.82,P=0.001,Fig.2(c)),nirS-type denitrifier(R=0.92,P=0.002,Fig.2(d))and nitrogen-fixing bacteria(R=0.88,P=0.001,Fig.2(e))in the same treatment were clustered together,and were significantly different among the four treatments.
The dominant genera(relative abundance higher than 1%)of the soil nitrogen cycle function microorganism were presented in Fig.3.The most sequences of AOA were unidentified,and there was no significant difference forNitrososphaeraamong treatments(Fig.3(a)).A total of 3 major genera of AOB were identified in all treatments:Nitrosospira,NitrosovibrioandNitrosomonas(Fig.3(b)).ThenirK-type denitrifier communities were dominated by generaBradyrhizobium,Mesorhizobium,Sinorhizobium,Rhizobium,Lysobacter,Rhodopseudomonas,Chelatococcus,Chelativorans,EnsiferandBosea(Fig.3(c)).The dominant genera ofnirS-type denitrifiers wereRubrivivax,Azospirillum,Halomonas,Bradyrhizobium,Azospira,Cupriavidus,Rhodanobacter,Magnetospirillum,Pseudomonas,Azoarcus,Sulfuritalea,Alicycliphilus,Ideonella,Aromatoleum,Herbaspirillum,ZoogloeaandThermus(Fig.3(d)).The bacterial genetic sequences ofnifHrevealed thatSkermanella,Bradyrhizobium,Pseudarthrobacter,Pseudacidovorax,Burkholderia,Nostoc,Anabaena,Zoogloea,Azospirillum,Azotobacter,Klebsiella,Pelomonas,Candidatus_Methylomirabilis,Geobacte,Rubrivivax,Azoarcus,Anaeromyxobacter,Sinorhizobium,Streptomyces,Microcoleus,Desulfovibrio(>1%)were more notable in all of the soil samples(Fig.3(e)).

Fig.3.The community compositions of AOA,AOB,nirK-/nirS-type denitrifier and nitrogen-fixing bacteria.

Fig.1.Chao1 and Shannon of AOA,AOB,nirK/S-type denitrifier and nitrogen-fixing bacteria.

Fig.2.Principal coordinate analysis(PCoA)of the changes in AOA(a),AOB(b),nirK-(c)/nirS-type denitrifiers(d)and nitrogen-fixing bacteria(e)based on weighted UniFrac distances in different treatments.
The dominant genera of the nitrogen cycle function microorganism were similar in all treatments,but the application of organic manure and straw cover changed the relative abundance of the N cycle function microorganism.To further investigate the difference in the relative abundance of each genus of AOA,AOB,nitrogenfixing bacteria,nirK-andnirS-type denitrifier,the biomarkers in each treatment was discovered by the linear discriminant analysis(LDA)effect size(LEfSe)method at genus level.No biomarker of AOA was selected at the genus level in all treatments.For AOB,the predominant genera ofNitrosovibrio(4.3%)andNitrosomonas(1.9%)were overrepresented in the CK and OMSM(Fig.4(a)).Biomarkers ofnifHincludedBradyrhizobium(18.7%),Azospirillum(1.9%)andMicrocoleus(1.0%)emerging in the CK,Skermanella(24.6%),Nostoc(4.4%),Burkholderia(3.1%),Candidatus_Methylomirabilis(1.6%)andAzotobacter(1.6%)in the OM,Pseudarthrobacter(17.2%),Anabaena(3.7%),Zoogloea(3.0%),Geobacter(1.7%),Streptomyces(1.4%),Anaeromyxobacter(1.4%),Klebsiella(1.4%)andSinorhizobium(1.1%)in the SM andPelomonas(2.5%),Rubrivivax(1.5%),Desulfovibrio(1.0%)andAzoarcus(1.1%)in the OMSM(Fig.4(b)).FornirKtype denitrifier,Lysobacter(6.6%),Chelatococcus(4.3%)andChelativorans(2.6%)were biomarkers for CK.Sinorhizobium(5.7%)andEnsifer(1.9%)were biomarkers for SM.Mesorhizobium(11.2%),Rhodopseudomonas(4.6%),Rhizobium(5.0%)andBosea(1.1%)were biomarkers for OMSM(Fig.4(c)).FornirS-type denitrifier,Rubrivivax(15.0%),Magnetospirillum(5.3%),Azospira(5.7%),Zoogloea(1.1%)andRhodanobacter(4.9%)were more abundant in the CK compared with other treatments.The application of organic manure enriched the abundance ofAzospirillum(11.1%),Halomonas(9.9%),Thermus(1.0%)andAlicycliphilus(1.3%).The abundance ofPseudomonas(3.4%)andAzoarcus(3.3%)increased in the SM.Cupriavidus(6.7%),Bradyrhizobium(6.9%),Sulfuritalea(3.1%),Herbaspirillum(1.2%)andAromatoleum(1.4%)were biomarkers for OMSM(Fig.4(d)).
3.4.The correlations between soil environmental factors and nitrogen cycle function microorganism
The correlations among soil erodibility,physical and chemical properties and indexes of runoff were shown in Fig.5(a).The N and P contents in runoff were closely positively associated with soilKw,sediment yield and runoff depth.The soil N,P,K,organic matter,PR and τf were negatively correlated with runoff depth,sediment yield.The TN and STN in runoff were negatively associated with soil available nitrogen,NO3--N,NH4+-N,TN,organic matter,PR and τf.The AP of runoff water had a strong negative correlation with soil PR,τf and organic matter.The soil PR,τf,AK,AP,AN,NO3--N and TN were positively correlated with soil organic matter.
The relationships between the soil environmental factors and the diversities of N cycle function microorganism were showed in Fig.5(b).The Chao1 of AOA was significantly associated with soilKw,sediment yield,τf,SWC,AP,AK,NO3--N,NH4+-N and TN.For AOB,the Chao1 showed a negative correlation with soilKw,sediment yield and runoff depth,but was positively correlated with PR,τf,AK,AN and organic matter,and Shannon was negatively correlated with soil ρb.The Chao1 ofnifHshowed a positive correlation withKw,sediment yield and runoff depth and was negatively correlated with soil PR,τf,organic matter,AK and NH4+-N.Oppositely,the Shannon ofnifHwas negatively correlated withKwand sediment yield and positively correlated with PR,τf,organic matter,AP,AK,AN,NO3--N and TN.FornirK,the Chao1 was negatively correlated with soilKw,sediment yield and runoff depth and positively correlated with PR,τf,organic matter,AK,NH4+-N and TN,similarly the Shannon showed a negative correlation with soil runoff depth and a positive correlation with soil PR,organic matter and AN.TheKw,PR,τf,CEC,organic matter,AP,AK,AN,NO3--N and TN were significantly associated with the Chao1 and Shannon ofnirS-type denitrifier.
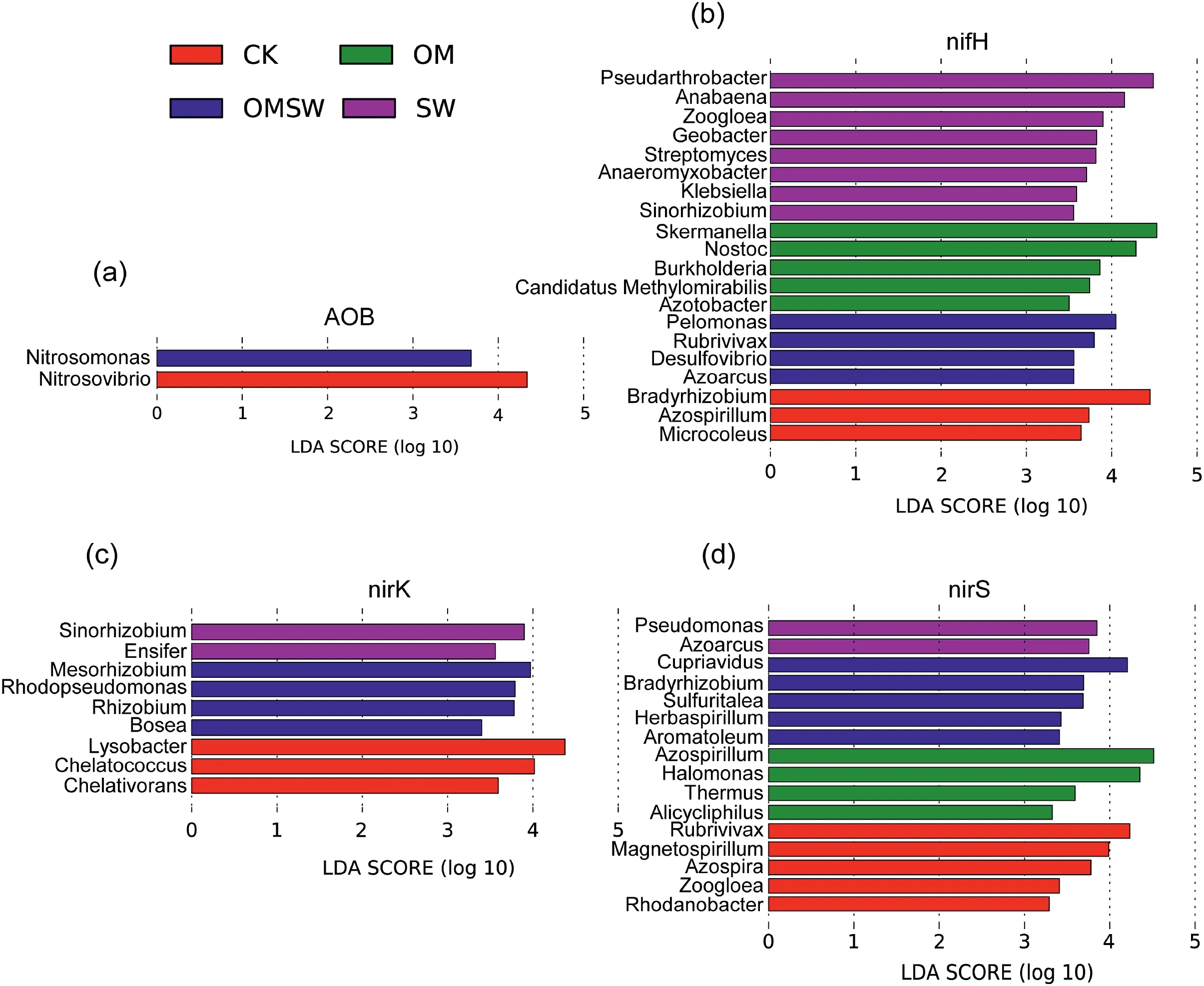
Fig.4.LEfSe analysis of AOB(a),nitrogen-fixing bacteria(b),nirK-(c)and nirS-type denitrifier(d)at genus level.Abbreviations:CK,control treatment with chemical fertilizer;OM,organic manure treatment;SW,straw treatment;OMSW,organic manure plus straw.
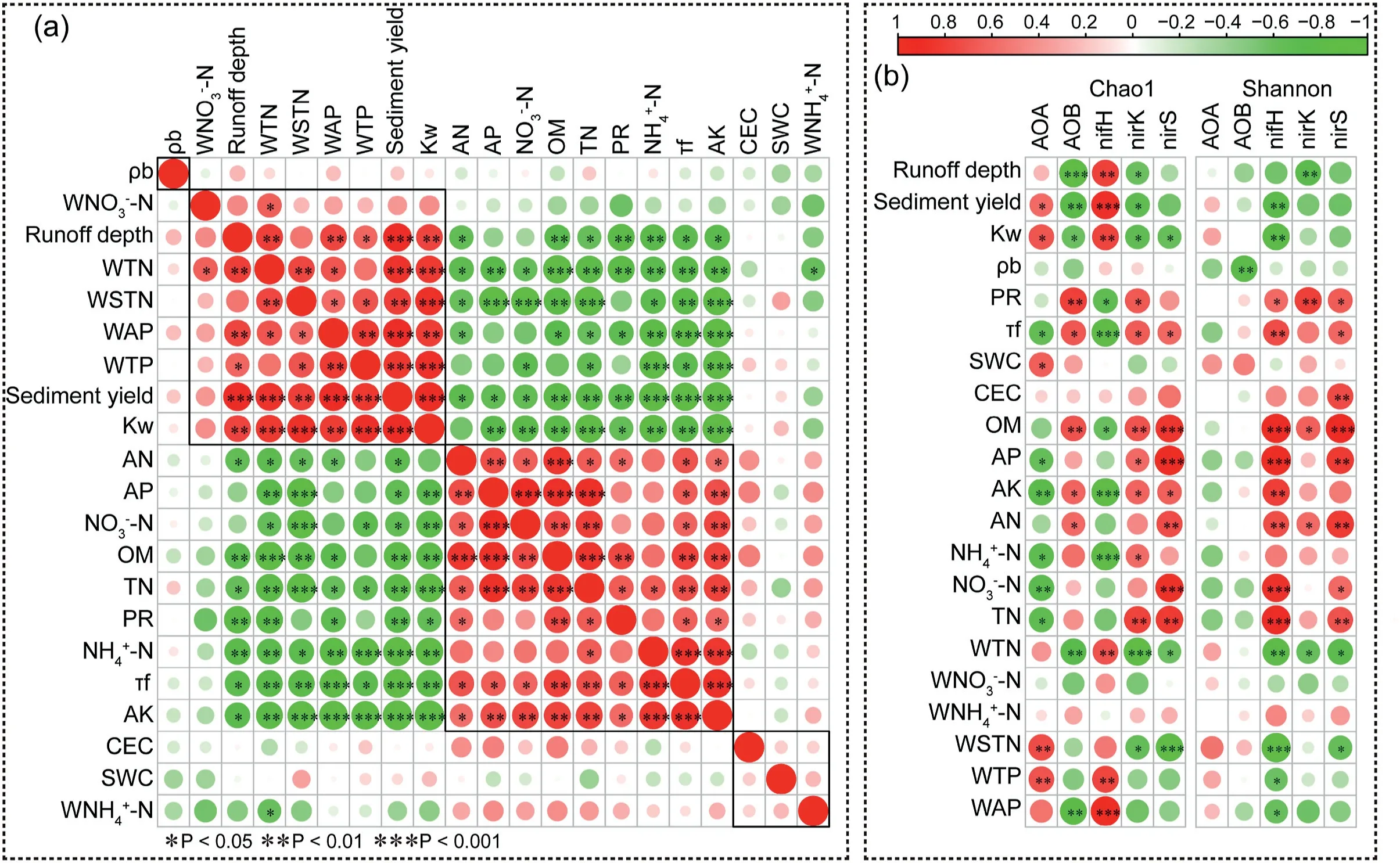
Fig.5.Relationship among soil properties,runoff indexes and diversities of cycle function microorganism.
The relationships between the soil environmental factors and soil N cycle function microorganism that from LEfSe analysis were showed in Fig.6.The soil NH4+-N(explain=79.6%),organic matter(8.3%)and AP(1.9%)showed a significant effect on soil communities of AOB(Fig.6(a)).The soil organic matter(53.5%),NO3--N(19.1%),NH4+-N(13.2%),SWC(5.2%),runoff depth(3.5%),sediment yield(2.6%)andKw(0.5%)were found to be main environmental factors affecting thenirK-type denitrifier community(Fig.6(b)).In addition,the change ofnirS-type denitrifier community was largely associated with soil runoff depth(28.7%),Kw(30.1%),organic matter(24.6%),AP(8.7%)and sediment yield(4.3%)(Fig.6(c)).The variations in nitrogen-fixing bacteria(nifH)communities were largely associated with soil sediment yield(34.7%),runoff depth(5.4%),AP(23.9%)and organic matter(32.3%),and these factors could explain 96.3% changes of N-fixing bacteria communities(Fig.6(d)).
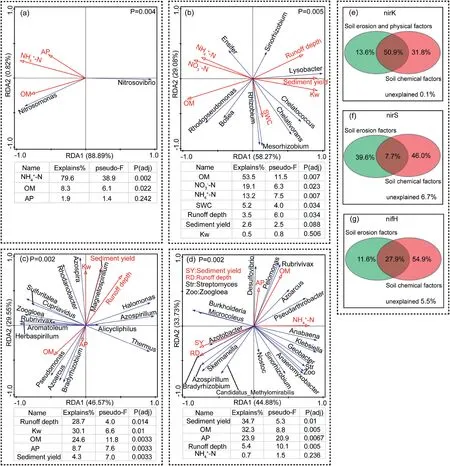
Fig.6.The redundancy analyses of the correlations between soil variables and AOB-aomA(a),nirK(b)and nirS(c)and nifH(d)at the genus level and the variation partitioning analysis(VPA)of nirK(e)and nirS(f)and nifH(g).
Soil chemical properties and soil erodibility are key factors affecting soil N cycling microorganisms.The variation partitioning analysis(VPA)showed that soil chemistry properties changes contributed to 31.8%,46.0% and 54.9% of thenirK-type denitrifier,nirS-type denitrifier and N-fixing bacteria variations(soil chemical properties changes explained 89.7% variations of AOB),respectively.Soil erodibility showed the greatest impact on soilnirS-type denitrifier and explained 39.6% variation.Together soil erodibility and chemical factors explained 27.9% of nitrogen-fixing bacteria variance,which was higher than soil erodibility(11.6%).The interaction between soil erodibility,physical factors and soil chemical factors explained 50.9% ofnirK-type denitrifier variations,higher than soil chemical factors.
4.Discussion
4.1.The effects of organic fertilizer and straw return on soil erodibility
The straw mulching and organic manure application have the potential benefits on reducing the runoff-associated soil N losses caused by water erosion.Accordingly,application of straw and organic fertilizer increased soil N contents and reduced runoff depth,sediment yields andKwto varying degrees,while it had a small impact on soil physical properties.Soil erodibility was closely related to the application of both organic manure and straw mulching.
TheKwvalue represented the soil susceptibility to water erosion(Zhang et al.,2011).Although PR and τf were significantly and negatively correlated withKw,the application of organic fertilizer and straw did not have a significant effect on them.Organic matter is considered to play an important role for the formation of a stable soil structure,and the changes in aggregates are closely related to soil N content(Kushwaha et al.,2001;Oades,1984).Kushwaha et al.(2001)suggested that the organic matter addition accelerated the formation of macroaggregates.Soil aggregates are the basic unit of soil structure and are an important component of soil(Rillig et al.,2015).Sarker et al.(2018)found that the organic matter input induced a rapid initial increase of aggregates,exceeding that of the controls by one to two orders of magnitude.The stability of soil aggregates has a great influence on soil erodibility and soil fertility(Lu et al.,2016).Liu,Mørkved,et al.(2010)reported that the amount of water-stable aggregates showed a positive correlation with their contribution to soil organic C,N and P.Organic matter was significantly negatively correlated with runoff depth,sediment yields andKwin this study.The application of organic fertilizer and straw mulching significantly promoted the soil organic matter contents(Table 2).On the one hand,organic matter can be used as a binder for the formation of aggregates,and humic acid will bind to the Ca2+and Mg2+in the soil(Wall & Choppin,2003),thereby forming a large number of water-stable aggregates that stabilize the soil water,enhance permeability,improve the soil structure(Ge et al.,2019)and increase the effect of runoff permeability,which may be the main reason for the mitigation of soil erodibility.Barth`es and Roose et al.(2002)found that runoff depth and soil loss were negatively correlated with water-stable aggregates of topsoil by simulated rainfall.On the other hand,straw covering the soil surface reduced the direct collision between rainwater and the soil surface,reduced the damage to the soil surface structure and the velocity of surface runoff(Prosdocimi et al.,2016),which may have led to a significantly smaller runoff depth in the SW treatments than in the OM treatment.Moreover,the combination of organic manure and straw was significantly better than the single application of organic fertilizer or straw.
The content of nitrogen,phosphorus and potassium were significantly increased while the erodibility decreased in the soil treated with organic manure and straw,and the loss of nutrients caused by runoff were also reduced,which were inseparable from the soil erodibility decrease and the soil organic matter increase.The soil nitrogen,phosphorus and potassium showed a significant negative correlation with runoff depth,sediment yields andKw(Fig.5(a)).On the one hand,soil organic matter improves the ability of soil aggregates to store nutrients(Cates & Ruark,2017;Sarker et al.,2018),and the release of nutrients from soil organic manure and straw is a slow process;on the other hand,the lower soil erodibility reduced damage to soil structure and transport of sediment particles,thereby reducing N and P losses through runoff(Zhang et al.,2016).This may be the main reason for the increase in soil nutrients and decrease in losses through runoff.Wang et al.(2014)reported that the soil erodibility was significantly positively correlated with the sediment-associated available N and available P.And the soil erodibility showed a significant positive correlation with sediment yield(Fig.5(a)).Moreover,N and P content in runoff were positively correlated with soil erodibility and negatively correlated with soil organic matter(Fig.5(a)).Nitrogen lost through runoff was mainly water-soluble.The loss of NO3--N that is fairly easy to cause nitrate pollution(Shrestha &Ladah,2002)is greater than that of the NH4+-N.This is mainly because NO3--N is not easy to be adsorbed by soil colloids(Dresler et al.,2011),and is easy to migrate with runoff,while NH4+-N with positive charge easily and steadily combine with soil clay and is not easy to be leached(Hossain & Sarker,2020).
4.2.The effects of organic fertilizer and straw on the nitrogen cycle function microorganism
Microorganisms are an important part of soil ecology system and are sensitive to changes in soil environment.The changes in Ncycling microorganisms in the sloping field were also different among treatments in this study.AOB and AOA were the main players in ammonia oxidation that was the rate-limiting step of nitrification,but there were significant differences in their diversity and structure.The soil N-cycling microorganisms'variation showed significant relationship with soil chemical factors in different treatments.The Chao1 of AOA reduced in the OM,SW and OMSW treatments,while Chao1 of AOB increased under the amendments with straw and organic manure+straw,and the changes in AOA and AOB were similar for Shannon.The response of AOB diversity to the change of environmental factors was almost opposite to that of AOA(Fig.5(b)).Applying organic manure and straw reduced soil erodibility during rainfall and increased nutrient content in soil.Chao1 of AOA demonstrated a negative correlation with soil N,P and K contents,and the dominant AOA was not found,which probably happened for that AOA appeared to be more active in acidic and low nutrient environment(Liu,Li,Chang,et al.,2018),while AOB show an opposite response of AOA to environmental factors(Shen et al.,2012).The more complex the organic carbon source in the presence of both heterotrophs and AOB simultaneously,the more diverse the heterotrophic microorganisms will be,and the diversity of the AOB community will increase(Deng et al.,2018),which may be the main reason why the Chao1 of AOB was the highest in OMSW treatment.Moreover,soil organic matter,NH4+-N and AP were the key factors leading to the change of AOB community structure.AOB are autotrophs that requires inorganic carbon to grow,but the increase of organic matter promoted the growth of heterotrophic microorganisms,which also increased the release of inorganic carbon,thus promoting the growth of AOB(Deng et al.,2018).Increased organic matter and NH4+-N may be the main reasons whyNitrosomonasbecame the dominant bacteria in the OMSW treatment,providing sufficient carbon and nitrogen sources for its activity(Fang et al.,2014;Gao et al.,2017).Tang et al.(2016)found that the AOB-amoAcopies decreased in the treatment with P addition.The decrease in relative abundance ofNitrosovibriomight be because of the increase of AP in the OM,SW and OMSW treatments(Zhang et al.,2017).Although the relative abundances ofNitrosovibrio(0.08%—4.3%)andNitrosomonas(0.8%—2.0%)were significantly changed in the OM,SW and OMSW treatments,their abundances were smaller asNitrosospira(72.8%—90.8%)still played a decisive role in the ammonia oxidation process.Therefore,the application of organic manure and straw cover can retain the nutrients in the soil,but it showed a minor impact on the soil ammonia oxidation.Compared with the control treatment,the combined application of organic manure and straw increased the Chao1 and Shannon of soilnirKandnirS-type denitrifier,better than applying organic fertilizer or straw alone.Soil organic carbon is not only a very important electron donor in denitrification,but also an important energy source for heterotrophic microorganisms(Si et al.,2018).Diverse carbon sources may have different effects on soil denitrifiers,and complex carbon sources may be utilized by a more diverse range of denitrifiers than a single carbon source(Yang,Wu,et al.,2013),which may contribute to the change ofnirKandnirS-type denitrifier diversities in the OMSW treatment.However,application of single organic manure or straw had little effect on the diversity of soil denitrifier,and even the Shannon of soilnirK-type denitrifier was reduced in the organic manure treatment,probably because organic manure stimulated the growth of heterotrophic denitrifiers,while autotrophic denitrifiers may gradually be at a disadvantage(Sheng et al.,2018).In addition,the diversity ofnirSwas higher than that ofnirK(Chao1),which was consistent with the results of Mosier and Francis(2010).Moreover,the communities structure ofnirKandnirS-type denitrifier were significantly altered by applying organic manure and straw.The organic matter,NO3--N and NH4+-N were the main drivers changing thenirK-type denitrifier community structure.In this study,organic matter explaining 53.5% of the totalnirK-type denitrifiers variance,and it may due to organic matter enhanced the stability of soil structure,and the decomposition of organic matter increased soil O2consumption,and the soil water content was higher after rainfall,which created better conditions for soil denitrifiers,and the increased mineral N provided a large amount of substrate for soil denitrification.The denitrifiers dominated in the CK,SW and OMSW treatments belonged to phylumProteobacteria,except forLysobacterwhich belongs to classGamma-Proteobacteriaand orderXanthomonadales,the others belong to classAlpha-Proteobacteriaand orderRhizobiales.Hou et al.(2018)found that mostnirK-type denitrifiers were in the classAlpha-Proteobacteriaand more than 50%nirK-type denitrifiers belonged to orderRhizobiales.Compared withnirK,nirS-type denitrifier was less affected by soil mineral N.It has been shown thatnirK-type denitrifier was more sensitive to changes in soil nutrients thannirS-type denitrifier(Barta et al.,2010).In addition to organic matter(only explained 24.6% of the variance),the AP was also one of the important factors affectingnirS-type denitrifier community structure and explained 8.7%of the variance ofnirS-type denitrifiers.The growth rate of microbial cells is inseparable from the phosphorus concentration,which requires P-rich ribosomes to produce new proteins(Tang et al.,2016).We found that soil AP were negatively correlated withMagnetospirillum,AzospiraandRhodanobacterand showed a positive relationship withPseudomonas,AzoarcusandBradyrhizobiumin this study.Moreover,a significant positive correlation betweennirStranscription numbers rather thannirKand soil AP was found by Che et al.(2018).Therefore,soil AP was considered as critical factor affectingnirS-type denitrifiers in this study.In addition,thenirS-type denitrifiers in this study were distributed inAlpha-Proteobacteria,Beta-andGamma-Proteobacteria(Hou et al.,2018;Long et al.,2018).
Biological nitrogen fixation is an important source of soil N,and microorganisms with the ability to fix N are widely distributed in the soil(Cleveland et al.,1999).Fertilization is a key factor in the diversity and community structure of soil diazotrophic bacteria.The application of organic manure and straw cover significantly reduced the Chao1 of N-fixing bacteria,but increased the Shannon.The increased Shannon index also showed that the uniformity of the N-fixing bacteria community was improved,and organic fertilizer+straw had the greatest impact on Shannon ofnifH.Soil chemistry factors were still the main factors changing N-fixing bacteria in this study(Fig.6(g)).Soil organic matter increased the organic carbon substrate,which provided monosaccharides or polysaccharides for soil heterotrophic or mixotrophic bacteria and facilitated the growth and reproduction of soil N-fixing bacteria(Chen et al.,2019;Rahav et al.,2016).Tang et al.(2012)found that applying organic manure greatly increased the types of N-fixing bacteria,which was conducive to the development and evolution of the diversity of N-fixing genes.Furthermore,the addition of organic matter also fundamentally altered the community structure of soil N-fixing bacteria(Berthrong et al.,2014;Yang,Bai,et al.,2019),and all heterotrophic diazotrophs use organic matter as an energy source(Liao et al.,2018;Rosch & Bothe,2009).Except for organic matter,the content of AP in soil was also a key driver affecting the community structure of N-fixing bacteria.Romero et al.(2012)reported that N fixation rate of soil treated with phosphorus was significantly higher than that of control treatment.Nitrogen-fixing bacteria can also convert insoluble mineral phosphate into soluble form by acidifying the surrounding environment(Dobbelaere et al.,2003).However,higher levels of N in the soil may counteract the effect of organic matter and inhibit the activity of N-fixing bacteria(Orr et al.,2011)and was no longer a limiting factor for changes in N-fixing bacterial communities,which will also make some Nfixing bacteria more adapted to this environment.The biomarkers ofnifHemerging includedSkermanella,Nostoc,Burkholderia,CandidatusMethylomirabilisandAzotobacterin the OM.Pseudarthrobacter,Anabaena,Zoogloea,Geobacter,Streptomyces,Anaeromyxobacter,KlebsiellaandSinorhizobiumwere dominant in the SM,andPelomonas,Rubrivivax,DesulfovibrioandAzoarcuswere dominant in the OMSM(Fig.4(b)).These dominant genera are mainly fromProteobacteria(Shu et al.,2012).
The increase in the diversity of nitrifying and denitrifying bacteria and the presence of biomarkers in the different treatments indicated that different erosion control measures increased the availability of soil N,accelerated the transformation of soil N and improved crop utilization of soil N.As a result,the need for N-fixing bacteria to fix nitrogen from the atmosphere may be reduced,which may also be an important reason for the decrease in the diversity of N-fixing bacteria.While increasing the diversity of nitrifier and denitrifier also increased the risk of potential gaseous nitrogen loss.
4.3.Coupling effects of practical treatments and water erosion on N cycle function microorganism
Soil erodibility singly or directly affected N cycle function microorganism which lessened the damage to soil structure caused by precipitation and affected the environment for soil microbial survival.Soil erodibility decreased in all organic treatments,which reduced the possibility of soil erosion,increased the stability of the soil structure,and weakened the damage to the living environment of soil N cycle functional microorganisms.There was a significant correlation between soil erodibility and the diversity of soil N cycling functional microorganisms,andKw,runoff depth and sediment yield significantly changed the community structure of soilnirK,nirS-type denitrifier and N-fixing bacteria(accounted for 3.5%—34.7% of the variances),which may indicate the direct influence of soil erodibility on soil N cycle functional microorganisms.Soil erosion leads to poor soil structure and has an important impact on soil nitrogen transfer and transformation in sloping land(Park et al.,2021).Soil aggregates,as the basic structural unit of soil,influence the distribution and activity of soil microorganisms(Xiao et al.,2017).Over 90%of soil bacteria associate with aggregates,and aggregates of different sizes and stability in soil create a complex of ecological niches that are different from physico-chemical and structural characteristics,which promotes the colonisation and maintenance of different microbial communities(Trivedi et al.,2015;Wilpiszeski et al.,2019).We found that soil erosion factors had the greatest direct effect on soilnirS-type denitrifier community structure,which was likely due to the wide distribution ofnirS-type denitrifier in soil(Braker et al.,2000;Liu,Mørkved,et al.,2010;Mosier & Francis,2010),and the diversity ofnirS-type denitrifier was the highest in this study,and they may be more sensitive to changes in soil structure.Although the direct effect of soil erodibility on soil microbial community structure is less than that of soil chemistry factors(Fig.e—g),the soil erosion factors are still main factor that cannot be ignored for soil nitrogen cycle functional microorganisms.
All erosion control measures retained the nutrients in the soil,which indirectly changed the diversity and community structure of soilnirK,nirS-type denitrifier and N-fixing bacteria.The decrease of soil erodibility reduced the losses of soil nutrients,which was a virtuous circle and also had a coupling effect on soil N-cycling microorganisms in the erosion control treatments.The changes of the diversity and community structure of N-cycling microorganisms were significantly associated with soil organic matter,N and P contents(Figs.5(b)and 6).The decomposing activities of organic matter enhanced the soil aggregate structure,increased soil porosity,reduced slope flow,or straw cover reduced the damage of precipitation to the soil surface(Girmay et al.,2009;Zhang et al.,2016),and increased the difficulty of soil erosion(Cates & Ruark,2017;Sarker et al.,2018;Park et al.,2021).Changes innirK-type denitrifiers depend on the joint role of soil erosion factors and chemical factors(explained 50.9% of the variance)in this study(Fig.6(e)).It has been shown thatnirK-type denitrifier was more sensitive to changes in soil nutrients thannirS-type denitrifier(Barta et al.,2010).We found that there was no dominantnirK-type denitrifier in the OM treatment.On the one hand,the straw cover avoided direct damage to the topsoil by rain(Peng et al.,2016),on the other hand,due to the reduction of soil erodibility,the community(orderRhizobiales)that uses straw as a carbon source and energy source(Li et al.,2019;Maruthamuthu et al.,2016)increased in the amendment with straw,which may be the main reasons for this result.Yang,Ma,et al.(2019)also found that straw return enriched the relative abundance of the genusRhizobium.While less coupling effects of soil erosion factors and chemical factors were found on the community structure ofnirS-type denitrifier(7.7%)and N-fixing bacteria(27.9%).Although soil chemical factors were the main factors affecting soil N cycle functional microorganisms,the increase in soil organic matter,N and P were not only from the input of organic manure and straw,but also from the reduction of soil erodibility to reduce soil nutrients losses(Mao et al.,2020).
5.Conclusions
The combined application of organic manure and straw was the most effective erosion control measure to reduce soil erosion and increase the diversity of AOB,nirK/S-type denitrifier and uniformity of N-fixing bacteria,which facilitated the transformation of N.All erosion control measures significantly altered the community structure of soil AOB,denitrifier and nitrogen-fixing bacteria,but had less effect on AOA.Soil chemical factor especially soil organic matter,N and P was found the largest contributors to the changes of N cycle function microorganism community structure.Soil erosion factors had the largest effect onnirS-type denitrifier,and the greatest joint effect of soil chemical and erosion factors was found onnirK-type denitrifier.Furthermore,the indirect effect of soil erosion factors on N cycle function microorganism may exist as well.The results indicated that organic materials substituting inorganic N fertilizer is an effective water erosion control measure,and helped us to better understand the mechanisms of erosion control measures on soil N cycle under water erosion conditions.
Acknowledgments
Financial support for this study was provided by the National Nature Science Foundation of China(No.41977072),the Special Fund for Agro-scientific Research in the Public Interest(201503119),and the Agricultural Science and Technology Innovation Program(ASTIP).
杂志排行
International Soil and Water Conservation Research的其它文章
- An updated isoerodent map of the conterminous United States
- Monitoring gully erosion in the European Union:A novel approach based on the Land Use/Cover Area frame survey(LUCAS)
- Unpaved road erosion after heavy storms in mountain areas of northern China
- Determination of rill erodibility and critical shear stress of saturated purple soil slopes
- Erosion risk assessment:A contribution for conservation priority area identification in the sub-basin of Lake Tana,north-western Ethiopia
- Mapping soil erodibility in southeast China at 250 m resolution:Using environmental variables and random forest regression with limited samples
