Synthesis and Characterization of Hyperbranched Epoxy with Terminal Ally Group and Its Application of Toughen Bismaleimide
2021-09-15ZHANGZeWANGXiangCHENDanxiaCHENYaoMAWeiCHENWeiping
ZHANG Ze, WANG Xiang, CHEN Danxia, CHEN Yao, MA Wei, CHEN Weiping
(School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China)
Abstract: A hyperbranched epoxy (AHEP) resin with terminal allyl groups was synthesized. The number average molecular weight of the synthesized AHEP is 1 260, the multi-dispersion coefficient is 1.88,and the branching degree is 0.9. The hyperbranched epoxy resin and bismaleimide(BMI) monomer were heated to extend the chain and then blended and cured with methyl nadic anhydride. Studies have found that when the molar ratio of AHEP: BDM is 1:2, the mechanical properties and heat resistance of the resin casting body reach the best. Among them, the impact toughness was 15.05 kg/m2, the bending strength was 101 MPa, and the heat resistance temperature index was 229.23 ℃. We used AHEP to toughen bismaleimide resin in three ways simultaneously: hyperbranched structure, allyl copolymerized brackets chain and flexible side chain (epoxy group) addition. Experiments have proved that AHEP can improve the flexibility of the BMI chain and maintain its heat resistance, thereby forming a matrix with excellent mechanical properties and processing properties.
Key words: hyperbranched; toughen; resins
1 Introduction
Bismaleimide resin[1-5](Bismaleimide, BMI) is a typical representative of heat-resistant thermosetting resin, which also has excellent electrical insulation properties, radiation resistance, fatigue resistance, and moisture resistance. While possessing these advantages,the BMI resin has a high rigidity of the molecular chain structure due to excessive cross-linking density, and the structure of the resin after curing is relatively brittle,with poor impact resistance, low elongation at break and low fracture toughness. At the same time, the high melting point of the monomer itself, poor solubility,high molding temperature and other shortcomings have severely restricted the application and development of BMI resins in various fields.
Among them, poor toughness is the most prominent shortcoming of BMI resin. At present, the toughening modification methods for BMI resin mainly include copolymerization with allyl compounds[6-10],diamine chain extension modification[11]2,3-bis(4-aminophenyl, inorganic filler modification[12-15], epoxy resin modification[16-23], synthesis of new BMI monomers[24-27]etc. Among these above methods, the most widely used and the most effective method is the copolymerization modification of BMI resin with allyl compound. Liuet al[28]synthesized 3-allyl-5, 5-dimethylhydantoin(ADMH), using ADMH to N, N’-(4, 4’-diphenylmethane) bismaleimide (BDM )/2,2’-Diallylbisphenol. The results demonstrated that 1-BDA had the best thermal and mechanical properties exhibiting excellent modification effect of ADMH.
Hyperbranched polymer[29-38]has a highly branched three-dimensional structure without entanglement between molecules, so the solubility is greatly improved. Compared with linear molecules of the same molecular weight, the solution viscosity and melt viscosity are lower, and the processing performance is better. By studying the structure of molecular monomers,a kind of hyperbranched polymer with allylic bond is chemically synthesized, using the good chain flexibility of hyperbranched polymer and a large number of terminal groups at the periphery of the molecule, such as epoxy groups, and BMI resin undergoes copolymerization toughening modification. While retaining the original excellent performance, it can also effectively enhance the heat resistance of the cured resin, improve the process performance, and facilitate the application and development of actual industrial production. Liuet al[39]synthesized low-viscosity hyperbranched epoxy resin by polycondensation reaction using pentaerythritol andin-situprepared bisphenol a diglycidyl ether as raw materials. The study shows that the hyperbranched epoxy is superior in terms of physical properties as well as performance (especially, the toughness value is 800% higher) compared to the linear polymer.
Compared with the allyl toughening modification of the bismaleimide resin before, simply designing an allyl compound cannot solve the problem well. Epoxy resin is a kind of thermosetting resin which has been widely used in many fields[40]. For hyperbranched epoxy resins, poor heat resistance is still its disadvantage.Combining the above viewpoints, it is necessary to design a hyperbranched epoxy resin with allyl groups.
In this paper, trimethylpropane triglycidyl ether and diallyl bisphenol A are used as monomers, and tetrabutylammonium bromide is used as a catalyst to synthesize a hyperbranched epoxy resin (AHEP) with terminal allyl groups. Then use the C=C in the bismaleimide resin monomer (BDM) and the terminal allyl group in the AHEP to carry out the addition chain extension reaction. And use methylnadic anhydride as the epoxy group curing agent of AHEP. The curing agent makes the epoxy group and the bismaleimide resin monomer in the system crosslink and cure at the same time to form a crosslinked interpenetrating network.By introducing rigid molecules in BDM to improve the heat resistance of the resin, and by adding a low-viscosity hyperbranched epoxy resin to improve the toughness and processability of the resin, a matrix resin with excellent mechanical properties and good heat resistance is obtained. The structure principle is shown in Fig.1.
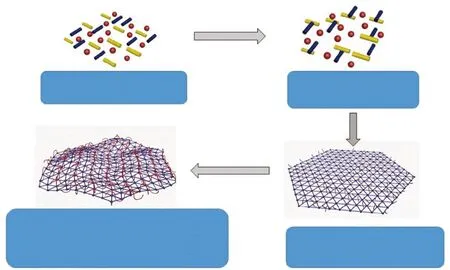
Fig.1 Schematic diagram of AHEP/BMI resin structure network skeleton
2 Experimental
2.1 Materials
Trimethylpropane triglycidyl ether (TMPGE,industrial grade) was purchased from Wuhan Penglei Biotechnology Co., Ltd. Diallyl bisphenol A (DABPA,industrial grade) purchased from Laizhou Laiyu Chemical Co., Ltd. Tetrabutylammonium bromide (TBAB,AR), tetrahydrofuran (THF, AR), acetone (AR), and hydrochloric acid (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. Diphenylmethane bismaleimide (BDM, industrial grade) was purchased from Honghu Shuangma New Materials Co., Ltd. Methylnadic anhydride (industrial grade) was purchased from Jiangsu Minsheng Trading Co., Ltd. Ethanol (AR) was purchased from Tianjin Fuyu Fine Chemical Co., Ltd.Sodium hydroxide (AR) and phenolphthalein (AR)were purchased from Tianjin Beichen Fangzheng Reagent Factory.
2.2 Synthesis of hyperbranched epoxy resin(AHEP)
Trimethylpropane triglycidyl ether, diallyl bisphenol A and tetrabutylammonium bromide were added to a three-necked flask with a nitrogen atmosphere. The reaction monomer was then heated in a constant temperature water bath at 80 ℃ and stirred continuously for 24 hours. After the reaction was over, the reactants were poured into a beaker and cooled to room temperature. The mixture was added an appropriate amount of distilled water several times and stirred and let stand after adding tetrahydrofuran. The solvent and residual water were removed and the remaining test solution in the rotary steaming flask was vacuum-heated in a vacuum oven for 10 h to obtain a hyperbranched epoxy resin. Fig.2 is a schematic diagram of the synthesis principle of AHEP.
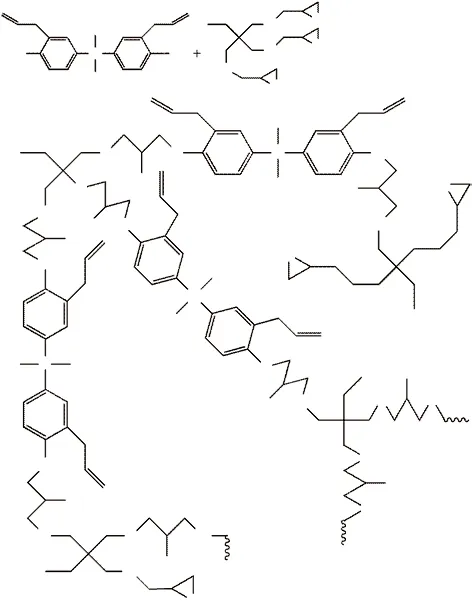
Fig.2 Schematic diagram of AHEP synthesis principle
2.3 Curing of the AHEP/BMI resins
The AHEP and bismaleimide monomer (molar ratio 1:2) were continuously stirred at 130 ℃ until the color became reddish brown. Added methylnadic anhydride and then poured into a mold heated curing.Finally it obtained a modified AHEP/BMI resin casting material. In addition, AHEP and methylnadic anhydride(molar ratio of 1:5) were separately mixed and casted as a blank control group for comparison, and the number was recorded as AHEP-0.
2.4 Characterization
The FTIR spectrum was recorded using a Nicolet FTIR spec-trophotometer (Nexus, Therno Nicolet,USA). The sample was prepared by pressing KBr pellets. The1H NMR and13C NMR spectra of the resins were recorded using THF as the solvent and TMS as the internal standard on a FTNMR spectrometer(Bruker avance 3). The molecular weights of the resins were determined using gel permeation chromatography (PLGPC 220, USA), using THF as the solvent. The epoxy value of AHEP was determined by the hydrochloric acid-acetone method. The resin curing system was calculated by using a differential scanning calorimeter (PerkinElmer, USA) DSC400 to characterize the AHEP/BMI resin prepolymer. The dynamic mechanical properties of AHEP/BMI resin were characterized by a synchronous thermal analyzer (STA449F3, Germany)in N2atmosphere, the temperature range was 30-800 ℃,and the heating rate was 10 ℃/min. The dynamic thermomechanical properties of the resin were characterized by the DMA8000( PE company, USA), the heating rate was 5 ℃/min, the test temperature range was -120℃-400 ℃, the test frequency was 1Hz, the method was three-point bending, and the sample size was 50 mm-6 mm-2 mm. The flexural strength and flexural modulus of the AHEP/BMI resin cast body were tested with a universal material tester (Instron 1341, UK), the sample size was 80 mm-15 mm-4 mm, and the beam displacement speed was 2 mm/min. The impact toughness of the AHEP/BMI resin cast body was tested by an impact testing machine(XJJ-5 type), the sample size was 120 mm-15 mm-10 mm, and the impact rate was 2.9 m/s. The cross-sectional morphology of the impact sample was observed by scanning electron microscope(-JSM-IT300, Japan).
3 Results and discussion
3.1 Synthesis and characterization of the AHEP
The structure of the synthesized AHEP is characterized by FTIR,1H-NMR and13C-NMR. Fig.3 is the FTIR diagram of AHEP. It can be seen from the figure that 3 448 and 1 101 cm-1are the strong absorption peak and weak absorption peak of the secondary alcohol, respectively, which indicates that the phenolic hydroxyl and epoxy groups in allyl bisphenol A have undergone ring opening reaction. The characteristic absorption peak at 1 230 cm-1is the phenolic hydroxyl group. There is no corresponding characteristic peak in the figure, indicating that the phenolic hydroxyl group in the reaction monomer has been completely reacted.The multi-segment characteristic absorption peak at 900-1 350 cm-1is C-O-C, which can also indicate that the epoxy group undergoes a ring-opening reaction.910 cm-1is the characteristic absorption peak of C-O on the epoxy group, indicating that there are residual epoxy groups in the reaction product. 1 638 cm-1is the characteristic absorption peak of stretching vibration of the allyl C=C double bond. 994 cm-1is the characteristic absorption peak of in-plane flexural vibration of terminal allyl C-H, which can both prove the existence of allyl in the reaction product.
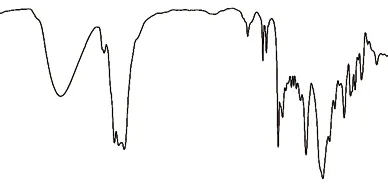
Fig.3 FTIR spectrum of AHEP
Fig.4 and Fig.5 are the1H-NMR and13C-NMR diagrams of AHEP. Fig.6 is the NMR chart of the central carbon atom of AHEP. From Fig.4, it can be seen that the proton characteristic peak on the benzene ring is at 6.93 ppm, and the proton characteristic peak at the allyl double bond is at 4.91 ppm, indicating that there is no reaction with epoxy groups and phenolic hydroxyl groups. Fig.5 shows that the characteristic absorption peak of carbon atoms of epoxy group appears at 50.87 ppm, and the characteristic absorption peak of carbon atoms in the reaction center appears at 43.96 ppm. The branching degree of AHEP is calculated according to the formula. The structure of a hyperbranched polymer should contain three different types of units: dendrites(D), linear (L) and terminal (T). The degree of branching DB is the ratio of the sum of D and T unit integrals to the sum of D, L and T unit integrals (Eq.(1)):
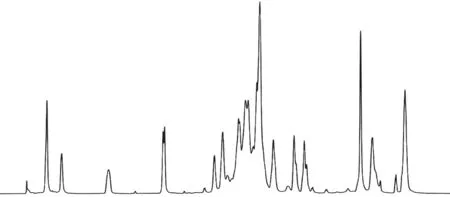
Fig.4 1H-NMR spectrum of AHEP
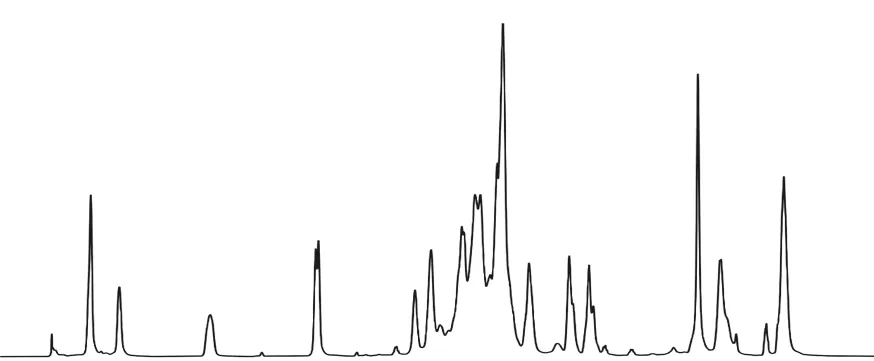
Fig.5 13C-NMR spectrum of AHEP
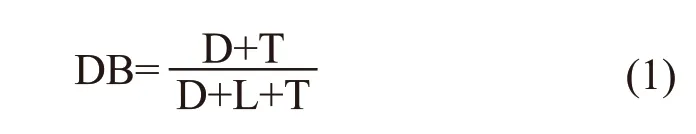
Fig.6 is a schematic diagram of the integration of the central carbon atom. It can be seen from Fig.6 that the integrated area of D of AHEP is 1.0, L is 0.3, and T is 0.12. According to the formula, the degree of branching DB = 0.9, which is very close to the dendritic polymer. In addition, the number average molecular weight of the synthesized AHEP is 1 260, the weight average molecular weight is 2 365, the polydispersity coefficient α is 1.88, and the average epoxy value is 0.50.
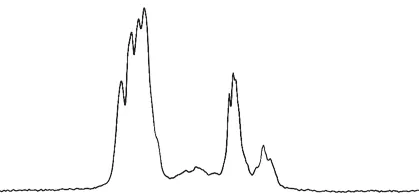
Fig.6 NMR spectrum of the carbon atom in the center of AHEP
3.2 The curing system of AHEP/BMI resin
Fig.7 shows the DSC scanning curves of AHEP/BMI resin prepolymer(AHEP-0.5) tested at different heating rates. Taking the DSC scanning curve with a heating rate of 10 ℃/min as an example, it is obvious that there is only one peak with a large area, which indicates that the resin has good compatibility during the reaction process. As shown in the figure that the peak temperature is 210 ℃. This is the main reaction exothermic peak of the curing and crosslinking of the reaction system. The curing reaction exothermic peak not only becomes getting sharper and sharper, but also moving towards the high temperature with the heating rate increase. It is a result of the activation energy of crosslinking reactions.
The characteristic temperature (T) of the resin curing stage is directly proportional to the heating rate(β). The characteristic temperature (T) of the resin curing stage is directly proportional to the heating rate (β).(Eq.(2)):

After measuring the characteristic temperature at different heating rates, the linear law equation of each characteristic temperature can be obtained by linear fitting. Extrapolating the equation to obtain the characteristic temperature valueT0atβ=0,T0is often used as the reference reaction temperature for the resin curing reaction. Table 1 lists the characteristic temperature values corresponding to differentβ.Tiis the gel temperature,Tpis the peak temperature, andTfis the post-treatment temperature.

Table 1 DSC characteristic temperature of AHEP/BMI resin prepolymer
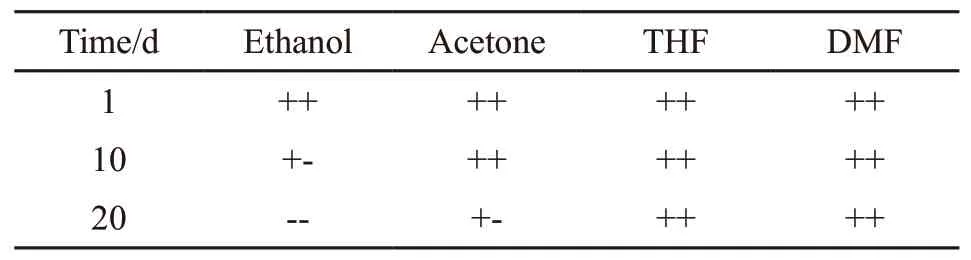
Table 2 Solubility of AHEP/BMI resin prepolymer
Plot and fit the characteristic temperature toβ,as shown in Fig.8. Whenβ=0, the gel temperatureTiis 127 ℃, the peak temperatureTpis 181 ℃, and the post-curing temperatureTfis 225 ℃.
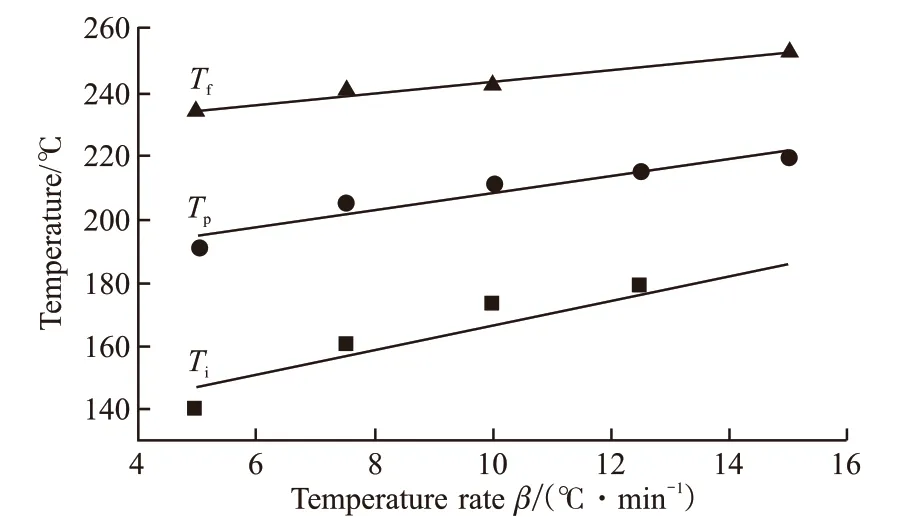
Fig.8 Fitting curve of characteristic temperature of AHEP/BMI prepolymer
Considering that there are many possible side reactions during the curing process, for example, excess carboxylic acid groups can undergo esterification reactions with hydroxyl groups releasing water, we choose to blend AHEP and BDM at 130 ℃ until the color turns red, then add methyl nadic acid anhydride, and quickly increase the temperature to 180 °C, which can effectively prevent the cross-linking reaction of epoxy group and acid anhydride from affecting the self-polymerization cross-linking reaction of BDM. Considering that the temperature range between 180 and 220 ℃ is very different, after many practical attempts, it was decided to insert an intermediate transition temperature in the middle.
Combined with the experience gained from many experiments, the curing system is determined to be 180℃/2 h+200 ℃/2 h+220 ℃/2 h.
3.3 Characterization of AHEP/BMI resin
Fig.9 is the infrared spectrum of AHEP/BMI resin before and after curing. It can be seen from Fig.9 that the vibrational peak of the carbonyl group on the five-membered BDM heterocycle at 1 716 cm-1and the skeletal vibrational absorption peak of the benzene ring at 1 512 cm-1did not change with the progress of the reaction. It proves that the five-membered ring structure of BDM and the benzene ring structure in the system did not change during the curing process.

Fig.9 Infrared spectrum of AHEP/BMI modified resin before and after curing
The wavelength of 910 cm-1is the characteristic absorption peak of the C-O bond of the epoxy group,and the characteristic absorption peak of the epoxy group gradually decreases with the progress of the reaction. The wavelength of 1 857 cm-1is the characteristic absorption peak of acid anhydride, which also decreases and disappears with the progress of the reaction. It proves that the curing and crosslinking reaction between the epoxy group and the acid anhydride has occurred. The wavelength of 1 638 cm-1is the weak absorption peak of the stretching vibration of the allyl C=C double bond in DABPA. As the reaction proceeds,the peak intensity gradually decreases until it disappears, indicating that almost all the allyl C=C double bond of DABPA are involved in the reaction.
In the modified resin system before curing, it is the characteristic peak of terminal allyl group at 1 638 cm-1. After curing, the peak disappears and does not appear. At the same time, it is the characteristic absorption of cis-carbon-carbon double bond in bismaleimide at 690 cm-1. The peak also disappears with the progress of the curing reaction, which indicates that the terminal allyl group of the AHEP in the reaction system and the C=C in the bismaleimide resin monomer have participated in the reaction.
The modified resin has obvious C-N-C characteristic peaks at 1 227 cm-1before curing, but the characteristic peaks obviously disappear after curing,and 1 180 cm-1is the characteristic peak formed by the self-polymerization and crosslinking of the bismaleimide resin monomer, and before the curing reaction it has never been found, which shows that the bismaleimide resin monomer has undergone self-polymerization and cross-linking reaction.
According to the infrared spectrum, the electron-deficient C=C double bond on the functional group of bismaleimide in the modified resin system reacted with the allyl group on AHEP by addition chain extension reaction, the methyl nadacic anhydride reacted with the epoxy active group on AHEP, and the self-polymerization cross-linking reaction is carried out by the monomer of bismaleimide resin. The three reactions continuously improve the curing reaction degree, and finally obtain a fully cured toughened modified resin with three-dimensional interpenetrating cross-linking network.
3.4 Solubility of AHEP/BMI resin prepolymer
The AHEP/BMI resin prepolymer are placed for different time and different solvents for solubility tests,in which the mass ratio of solute to solvent was 1:1.The test results are shown in Table 2. It can be seen from Table 2 that the prepolymer has good solubility in acetone, tetrahydrofuran (THF), and N, N’-dimethylformamide (DMF), and the solution is stable without layering. However, it can be completely dissolved into a suspension in an ethanol solution, but it cannot form a transparent and stable solution. This is because the BMI monomer has undergone a chain extension reaction with AHEP, which has severely damaged the structural regularity of the BMI resin and reduced the crystallization performance, and the large number of hydroxyl groups and ether bond groups in the AHEP have improved the resin prepolymer. With the extension of time, due to the principle of time-temperature equivalence, the prepolymer still undergoes different degrees of cross-linking.
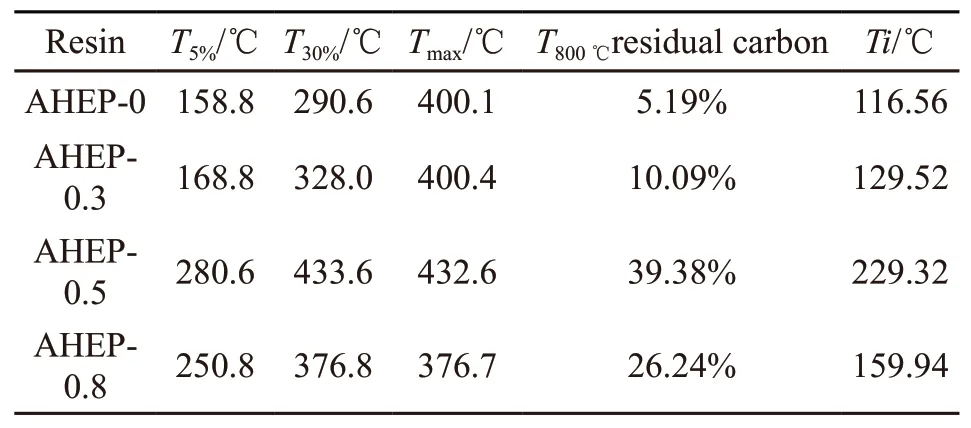
Table 3 Thermal performance parameters of AHEP/BMI resin
3.5 Thermal performance analysis of AHEP/BMI resin
Thermal analysis (TGA) was used to simultaneously measure the thermal weight loss.
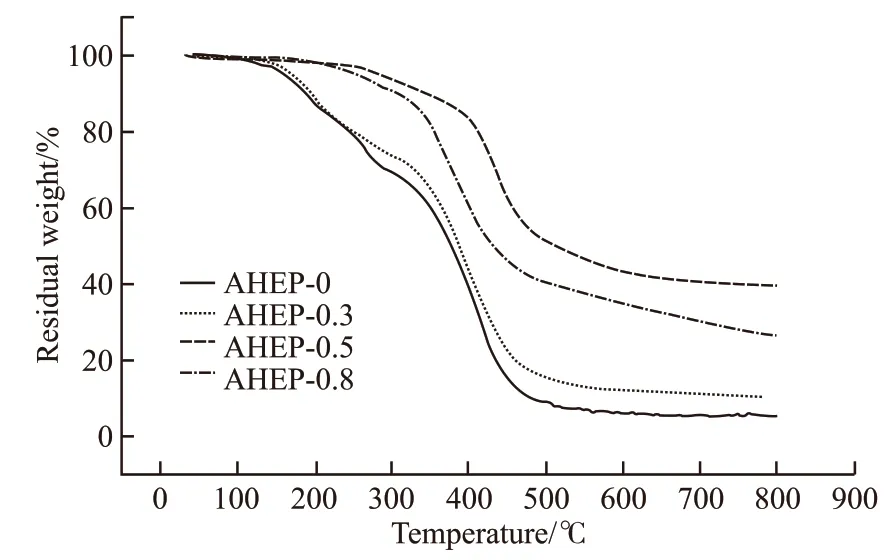
Fig.10 Thermal weight loss curve of AHEP/BMI resin
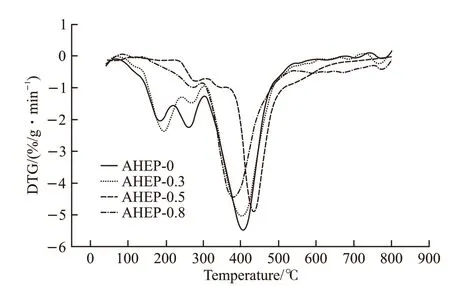
Fig.11 DTG curve of AHEP/BMI resin
Figs.10 and 11 show the thermogravimetric and DTG curves of cured AHEP/BMI resins with different formulations. As can be seen from the figure, with the increase of BDM content,T5%of the system and carbon residue rate at 800 ℃ showed a very obvious upward trend. This is because BDM molecules have more rigid groups and thus have higher thermal stability. As can be seen from the DTG curve, with the increase of BDM content, the maximum decomposition temperature first increased and then decreased. This is because the heat resistance of the network skeleton formed by cross-linking the autopolymers of bismaleimide is better than that of the epoxy cross-linking skeleton formed by AHEP, and the chain extension reaction of BDM and AHEP allyl in the AHEP-0.5 system has just been completed. When a double reticulation skeleton is formed, it is homogeneous and the cross-linking density of the resin is higher than single component system.However, the reason for the decrease is that with the continuous increase of BDM content, excessive BDM will be irregularly dispersed in the resin system, thus reducing the cross linking density of the resin, leading to a slight decrease in the heat resistance. However, the maximum decomposition temperature is still higher than that of AHEP-0.3 system. This is because the allyl chain reaction in the AHEP-0.8 system is more complete, the cross-linked network skeleton is more stable,and the heat resistance is relatively better, which can be proved by the residual carbon rate at 800 ℃.
Table 3 shows the thermal performance parameters of AHEP/BMI resin cured by different formulations. Combining itsT5%andT30%to calculate the heat-resistant temperature indexTiof each system resin, it is generally believed that at the heat-resistant temperature index, the mechanical properties of the resin are stable for a long time and can be kept at this temperature for a long time. The calculation formula is shown in Eq.(3):
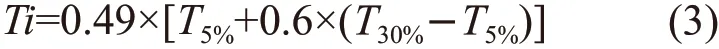
where,T5%,T30%are respectively 5% of thermal weight loss, and the corresponding temperature at 30%
Therefore, we can calculate the ability of modified resins with different formulation systems to be used for a long time at different temperatures. Compared with AHEP-0 blank control group, the temperature ofTiincreases with the increase of BDM content. When AHEP and BDM react completely,Tireaches the maximum value of 229.32 ℃.
3.6 Dynamic mechanical thermal analysis of AHEP/BMI resin
Figs.12 and 13 show the storage modulusE’-Tand Tanδ-Tcurves of AHEP/BMI resin. Tables 3-4 shows theTg(Tanδpeak value),βtransition temperature andE’(25 ℃) data of each resin system.
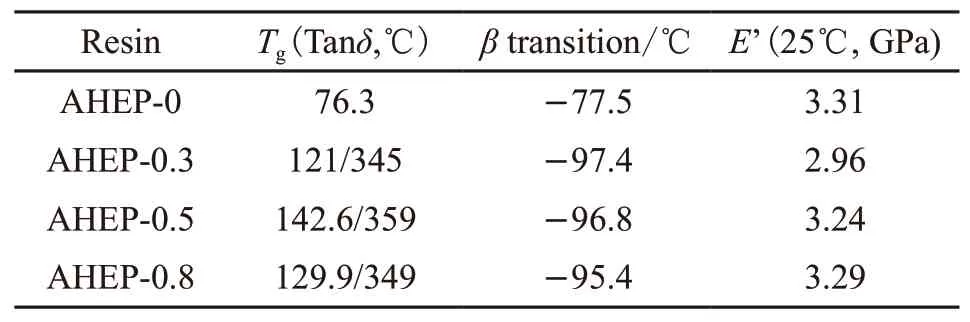
Table 4 Thermomechanical performance parameters of AHEP/BMI resin
It can be seen from Fig.12 that the energy storage modulus of the modified resin decreases gradually with the increase of temperature.However, when the BMI component was added, the temperature at which the modulus of the modified resin decreased was greatly increased, and the AHEP-0.5 component was the highest. This shows that the BMI component improves the heat resistance of the resin system. As can be seen from Fig.13, with the addition of BMI component, the peak corresponding temperature of the AHEP/BMI resin Tanδcurve increased significantly, while the loss factor Tanδvalue decreased accordingly. Compared with AHEP-0, the Tanδcurve of the resin system with BMI component has two peaks, which indicates not only the existence of two resin crosslinked skeleton,but also the heat resistance of epoxy matrix skeleton is significantly lower than that of bismaleimide resin crosslinked skeleton. It also proved the existence of the cross-linked interpenetrating network at the beginning of the experimental design. At the same time, the peak temperature of Tanδcurve of the AHEP-0.5 system is the highest, which are 142.6 ℃ and 359 ℃ respectively. This is because the BDM and AHEP allyl chain extension reaction in the AHEP-0.5 system is just complete. When the double network skeleton is formed, it is a homogeneous system. The cross linking density and structure regularity of the resin are higher than the single component system. BMI self-curing temperature is higher than the epoxy group, which will hinder the curing of the BMI resin to a certain extent during the curing process of the epoxy group, which will reduce the crosslinking density of the resin system and affect the overall heat mechanical behavior.
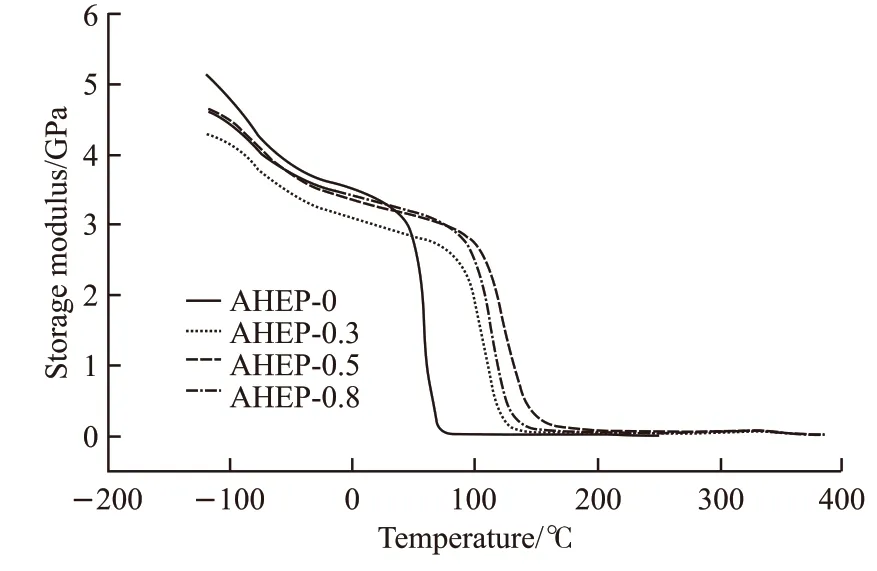
Fig.12 Storage modulus of AHEP/BMI resin
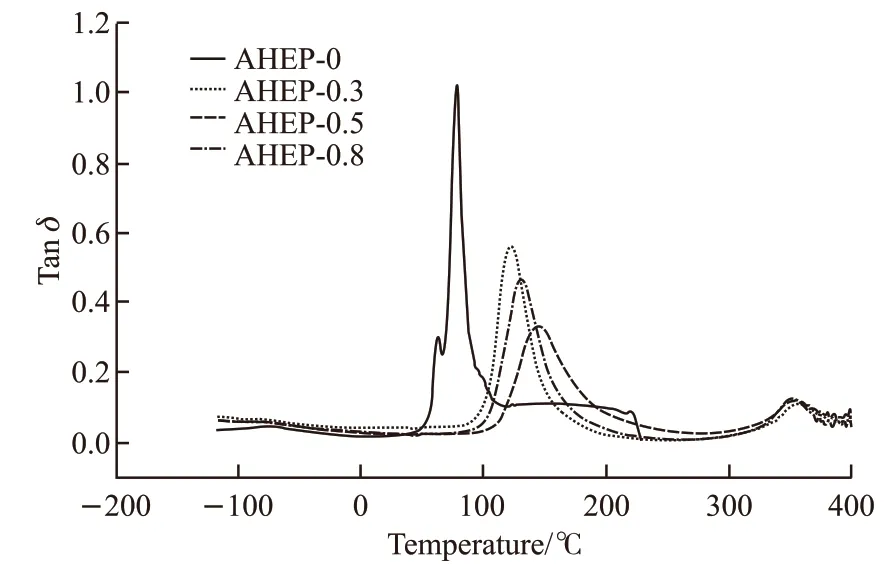
Fig.13 Tanδ curve of AHEP/BMI resin
Table 4 shows the thermal and mechanical properties of AHEP/BMI resin. It can be seen from Table 4 that theβtransition temperature of the AHEP/BMI resin decreases significantly with the addition of BMI components, but theβtransition temperature of the three resin systems of AHEP-0.3, AHEP-0.5 and AHEP-0.8 are all no significant changes. It shows that the increase of BMI content has no effect on the position of secondary transformation bee at low temperature. And with the increase of BMI components,the storage modulus value of AHEP/BMI resin at 25 ℃shows a downward trend, and they are all slightly lower than the AHEP-0 system.
3.7 Mechanical properties of AHEP/BMI resin
Table 5 shows the mechanical properties of the AHEP/BMI resin system after curing. According to the data in the table, the AHEP-0 system resin has the best toughness, impact toughness and bending properties.With the increase of BDM content, the flexural strength and flexural modulus decreased significantly compared with the blank control group AHEP-0. This is due to the fact that the addition of BDM destroys the structural system of epoxy group one-component curing and introduces. The rigid molecular chain increases the brittleness of the resin system. But from the perspective of the three groups of resin systems added with BDM,it can be concluded that the mechanical properties of the AHEP-0.5 system are the best. This is because the degree of allyl chain extension reaction of AHEP and BDM in different proportions is different. In AHEP-0.5, BDM and AHEP allyl chain extension have just reacted completely, and a cross-linked interpenetrating network of epoxy skeleton and bi-horse skeleton isformed, which increases the cross-link density to a certain extent. It has a higher modulus and more regular structure than other formulations. Therefore, the toughness of the resin is very excellent.
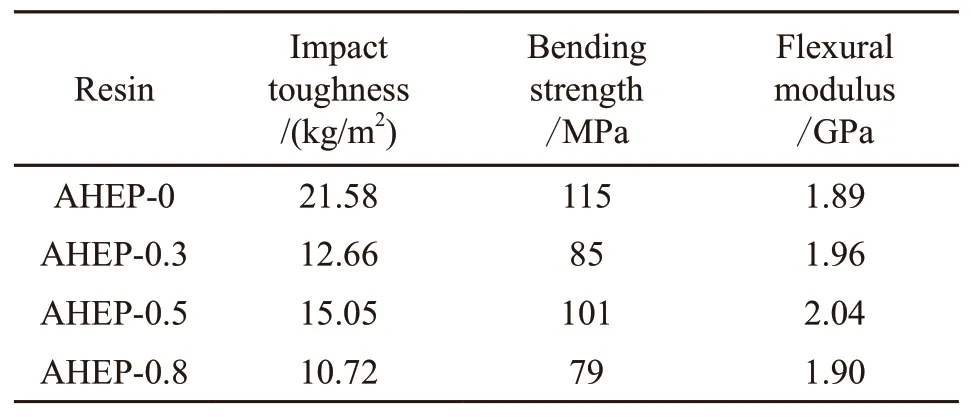
Table 5 Mechanical properties of AHEP/BMI resin system
4 Conclusions
In this paper, an allyl-terminated hyperbranched epoxy resin was synthesized and heated by mixing it with bismaleimide resin and methylnatican anhydride.The structures of hyperbranched oxygen resin and modified resin were characterized by Fourier transform infrared spectroscopy. The thermal properties of the modified resin were characterized by synchronous thermal analyzer and dynamic mechanical thermal analysis.Static mechanical properties of modified resin castable were characterized by universal mechanical tester and scanning electron microscope. The test results found that when the molar ratio of AHEP:BDM is 1:2, the mechanical properties and heat resistance of the resin cast body reach the best. Among them, the impact toughness is 15.05 kg/m2, the bending strength is 101 MPa, the flexural modulus is 20.4 GPa, the heat resistance temperature indexTiis 229.23 ℃, and the glass transition temperatureTgis 359 ℃. The experimental results show that the molecular structure and flexible side chain of hyperbranched epoxy resin can improve the toughness of bismaleimide resin and the structural rigidity of the bismaleimide resin can enhance the heat resistance of the hyperbranched epoxy resin.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Poly(dopamine)-assisted Bioactive Coating on the Surface of Porous Poly (Ether Ether Ketone) to Promote Osteogenic Differentiation of rBMSC
- Influence of Heat Treatment on Microstructure and Mechanical Properties of Plasma Sprayed FeCrMoCBY Amorphous Coatings
- Lamella Multiple Grained Structure Making 2205 Duplex Stainless Steel with Superior Strength and Ductility
- Progress in Light-weight High Entropy Alloys
- Synthesis and Characterization of Polyaniline/MgTiO3 Composite with Excellent Thermal and Electrochemical Performance
- Loose Sand Cemented by Microbial Cementitious Material:Composition, Microstructure and Mechanical Properties
