Poly(dopamine)-assisted Bioactive Coating on the Surface of Porous Poly (Ether Ether Ketone) to Promote Osteogenic Differentiation of rBMSC
2021-09-15WANGJinWANGYoufaWUQingzhi
WANG Jin, WANG Youfa, WU Qingzhi
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Biomedical Material and Engineering Center of Hubei Province, Wuhan University of Technology, Wuhan 430070, China)
Abstract: A facile modification strategy is developed to promote the proliferation and osteogenic differentiation of rat bone marrow stromal cells (rBMSCs) through deposition of a bioactive calcium silicate(CS) coating on the porous surface of poly (ether-ether-ketone) (PEEK) with the assistance of poly(dopamine)(PDA). The porous structures are etched on the surface of PEEK after sulfonation treatment. A poly(dopamine)layer is coated on the porous surface of the sulfonated PEEK (SPEEK), which provides anchoring groups for the subsequent deposition of the CS layer. Results show that the CS coating on the porous surface of SPEEK significantly improve the hydrophilicity and biomineralization formation of hydroxyapatite. Compared with PEEK, SPEEK-PDA-CS displays higher bioactivity to promote the proliferation and osteogenic differentiation of rBMSCs, including the increase of ALP activity and formation of calcium nodules, the expression of osteogenic differentiation-related genes. These results are beneficial to extending clinical applications of PEEKbased implants for bone tissue repair and orthopedic surgery.
Key words: poly (ether-ether-ketone); calcium silicate; poly(dopamine); bioactivity; osteogenic differentiation
1 Introduction
The large-scale and irregular bone defects can cause great physical and mental pain to patients due to severe trauma or pathological fracture (osteoporosis or primary malignant tumor), which often require bone tissue repair[1]. At present, titanium-based implants are widely used in the field of bone repair due to its good mechanical properties and biocompatibility[2,3]. However, the mismatched modulus of elasticity between the titanium-based implants and human bones can bring some clinical problems, such as inferior mechanical stability of the bone-implant interface[4-6].
Poly(ether ether ketone) (PEEK) as a semi-crystalline thermoplastic polymer possesses many advantages, such as high temperature resistance, abrasion resistance, and chemical resistance, as well as good biocompatibility and adaptable mechanical strength,which makes PEEK as an ideal candidate for bone tissue repair[7-10]. In particular, PEEK with an adaptable elastic modulus matched with that of natural bone can effectively reduce defects caused by stress shielding effects which are usually induced by conventional metal-based implants[4,11]. In addition, PEEK is currently used as an orthopedic material in orthopedic surgery[7,12]. However, PEEK does not integrate well with adjacent bone tissue owing to its bio-inertness,resulting in the failure when implanted into a living body[13,14]. Considerable efforts have been contributed to improve the osseointegration ability of PEEK[15-19].For example, Menget alreported the preparation of KR-12-coated PEEK with excellent bioactivity, which promoted osteogenesis and osseointegration[12]. Chanet alreported the preparation of PEEK/hydroxyapatite/carbon fibers composite with excellent thermal stability and bioactivity for load-bearing implant applications[20].
Calcium silicate (CS) has been well demonstrated with excellent osteoinductive activity and used in bone tissue engineering scaffolds[21-23]. Studies suggested that the PEEK/CS composites improved osteogenic differentiation of rBMSCs[24]. Dopamine with abundant catechol and aminol groups is widely used as a secondary modification platform for adhesive coating[25-27], which can undergo self-polymerization into poly(dopamine)(PDA) under weak alkaline conditions and deposit an adherent coating on all kinds of material surfaces[28,29].Ahmadet aldesigned a stable coating through polydopamine to control osteogenesis and osteoclastogenesis[30]. Liet alprepared a mussel-inspired novel nano silver/calcium phosphate (CaP) composite coating on the anodized Ti by polydopamine to maintain biological performance and long-term antibacterial ability[31].
In this work, a PDA-assisted strategy has been developed through the formation of bioactive CS coating on the porous surface of sulfonation-treated PEEK(SPEEK) in order to promote the proliferation and osteogenic differentiation of rBMSCs. As shown in Scheme 1, the porous structures were generated on the surface of PEEK after sulfonation etching, which were hydrothermally treated in order to remove the residual sulfuric acid. PDA was subsequently fixed on the porous surface of SPEEK under alkalic condition. The CS coating was formed after immersing the PDA-coated SPEEK into the CS precursor solution. The formation of CS-PDA-coated SPEEK (SPEEK-PDA-CS) was characterized through XRD, SEM/EDS and XPS. The wettability and the biomineralization formation of hydroxyapatite (HA) on the surface of SPEEK-PDACS were measured. Furthermore, the proliferation and osteogenic differentiation of rBMSCs on the surface of SPEEK-PDA-CS were evaluated.
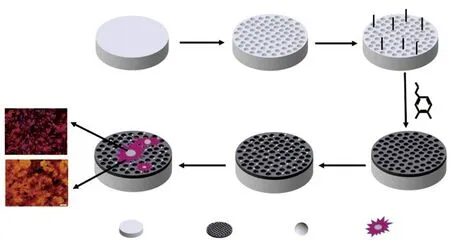
Scheme 1 Schematic of the preparation of bioactive CS-coated SPEEK with the assistance of PDA
2 Experimental
2.1 Materials
4,4-Difluorobenzophenone and dopamine hydrochloride were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., China. Diphenylsulfone was sourced from Macklin Biochemical Technology Co., Ltd., China. Phosphate buffered saline(PBS), α minimum essential medium (αMEM), fetal bovine serum (FBS), and penicillin and streptomycin were come from Hyclone. Other chemicals used were of analytical-reagent grade.
2.2 Preparation of PEEK and PEEK board
A certain quality of 4,4’- Difluorobenzophenone(10.9 g), hydroquinone (5.5 g), and diphenylsulfone(32.8 g) were added to a four-necked flask equipped with a reflux condenser and an electric stirring device under N2. The reaction temperature was controlled at 140 ℃ for 1 h, 180 ℃ for 1 h, 250 ℃ for 2 h, and 320℃ for 3 h, and Na2CO3(5.3 g) and K2CO3(0.69 g)were added to the flask when the reactive temperature was 140 ℃. A solid was appeared after the reaction[32].The collected solid was ground into powder and then washed with acetone, alcohol, and deionized water, respectively, to remove diphenylsulfone, inorganic salts,and residual monomers and oligomers. The collected PEEK powder was dried at 120 ℃ for 12 h to remove residue acetone and alcohol.
PEEK board was fabricated by injection molding method with the nozzle temperature 390 ℃. For various experiments and tests, the injected PEEK was machined to dimensions φ4 × 2 mm or φ21 × 2 mm for surface characterization, immersion tests, andinvitrostudies on 96-well /12-well culture plate.
2.3 Preparation of SPEEK-PDA-CS
In a typical process, PEEK was firstly treated with sulfuric acid (98%) in an ultrasonic bath at room temperature for 10 min to obtain a uniform porous structure on the surface, and then immediately washed with deionized water and hydrothermally treated at 120 ℃ for 5 h to remove residue sulfuric acid. The specimens were dried in an oven at 60 ℃ for 12 h,referred as sulfonated PEEK (SPEEK). The SPEEK was treated with sodium hydroxide solution (2.5 mol/L) for 3 h and washed with deionized water before drying. Then the hydroxylated SPEEK was put in a solution that 2 mg/mL dopamine was dissolved in 10 mM Tris-HCl buffered solution (pH=8.5) and the above mixture was placed in the shaker for 12 h. After the process, PDA was coated on the surface of SPEEK,which was labeled as SPEEK-PDA. To prepare the CS precursor solution, sodium silicate nonahydrate (Na-2SiO3·9H2O, 14.4 g) and calcium nitrate tetrahydrate[Ca(NO3)2·4H2O, 11.8 g] were separately dissolved in deionized water (50 mL), and polyvinylpyrrolidone K30 (PVP-K30, 2.0 g) was added into calcium nitrate solution. SPEEK-PDA was immersed in calcium nitrate solution under stirring and then the sodium silicate solution was added dropwise into the calcium nitrate solution through the peristaltic pump driver at a rate of 5 mL/min, and the reaction temperature was controlled at 55 ℃ for 5 h. The sample was gently rinsed with deionized water several times and marked as SPEEKPDA-CS.
2.4 Characterization of as-prepared SPEEK-PDA-CS
The surface structure and chemical composition of as-prepared SPEEK-PDA-CS were characterized using Fourier transform infrared spectroscopy (FTIR; Nexus,Therno Nicolet, USA), scanning electron microscope(SEM; JEOL, JSMIT200, Japan), X-ray photo electron spectroscopy (XPS; ESCALAB 250Xi, Thermo Fisher,U.S.A.), X-ray diffractometer (XRD; Empyrean, Netherlands) respectively. The hydrophilicity of the sample was assessed by measuring the water contact angles on a drop-shape analysis system (JC2000C, Shanghai Metallographic Environmental Technology Co., Shanghai,China) at ambient condition.
2.5 Biomineralization formation of HA on the surface of SPEEK-PDA-CS
SPEEK-PDA-CS, SPEEK-PDA, SPEEK and PEEK with a diameter of 21 mm were respectively immersed in 30 mL simulated body fluid (SBF) and placed in the shaker at 37 ℃ for 7 days, and SBF was replaced every 2 days. At the end of each time point,the specimens were rinsed with deionized water three times and dried overnight, then sputter-coated with gold and analyzed by scanning electron microscope (SEM;JSM-7610F, Japan) and energy dispersive spectrometry(EDS; JSM-7610FPlus, Japan) after mineralization.
2.6 Ions release
The release of Ca2+and SiO32-ions from SPEEKPDA-CS was monitored in PBS solution at 37 ℃ within 21 days. The concentrations of Ca2+and SiO32-ions in the PBS solution were determined at different intervals by inductively coupled plasma-optical emission spectrometry (ICP-OES, Leeman, USA) to obtain the cumulative ion release profiles.
2.7 Cell culture and proliferation
Rat bone marrow stromal cells (rBMSCs) were used to investigate the interaction between cells and as-prepared samples (with PEEK as a control). PEEK,SPEEK, SPEEK-PDA, and SPEEK-PDA-CS were disinfected with 75% alcohol for 4 h and rinsed with sterile PBS three times, then irradiated by UV for 30 min prior to cell culture. Cells with a density of 5 ×103cells per well were seeded on the specimens with a diameter of 4 mm in 96-well culture plates at 37 ℃in a humidified atmosphere with 5% CO2and 95% air.Culture medium was consisted of α-minimum essential medium (αMEM; Hyclone, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, USA), 1%penicillin (Hyclone, USA), and streptomycin sulfate(Hyclone, USA), which was changed every 2 days. A cell counting kit-8 (CCK-8) assay was used to detect the proliferation of rBMSCs at the time interval of 1, 4,and 7 days. 20 μL of CCK-8 solution (Beyotime Institute of Biotechnology, China) was added to each well and incubated for 2 h. After the incubation, 100 μL of the supernatant was transferred into a new 96-well plate and read at 450 nm using a microplate reader (Multiskan Go, Thermo Scientific, USA).
2.8 ALP activity and staining assay
0.1 μM dexamethasone, 50 μM ascorbic acid, and 10 mMβ-glycerol sodium phosphate (Sigma-Aldrich Co.) were added to the culture medium as the osteogenic inductive medium. Cells were seeded in a 12-well plate containing the samples at a density of 3 × 104cells per well, and the culture condition was the same as described above. After 24 h, the original medium was replaced by the osteogenic inductive medium, and the medium was replaced every 2 days. After 7 and 14 days of culture with the osteogenic inductive culture medium, ALP staining was carried out according to the procedure on the BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime Institute of Biotechnology,Shanghai, China). The OD values were measured with a microplate reader (Multiskan Go, Thermo Scientific,USA) at 405 nm and the total protein content was de-termined with the BCA protein kit (Beyotime Institute of Biotechnology, Shanghai, China), and then the ALP activity was normalized to the corresponding content of total protein, which was considered as an essential enzyme to promote early bone regeneration.
2.9 Alizarin red S (ARS) staining and quantitative assay
The osteogenesis assay using AR staining was employed to analyze calcium nodule formation (mineralization) on the surface of materials. Cells were cultured at a density of 3 × 104cells per well in a 12-well plate with the samples for 14 and 21 days in osteogenic inductive culture medium, as described previously. At the indicated time interval, the cells after co-incubation were rinsed with PBS three times and fixed in 4%paraformaldehyde for 15 min and then stained with 2%alizarin red solution (pH=8.3, Sigma-Aldrich Co.) for 20 min at room temperature. Before observing under an optical microscopy (Olympus IX71, Japan), the samples were washed with deionized water several times until orange color disappeared. For quantitative analysis, ARS was extracted using 10% cetylpyridinum chloride (pH=7.0; Sigma-Aldrich Co.) in sodium phosphate solution and then the OD values were measured with a microplate reader (Multiskan Go, Thermo Scientific,USA) at 570 nm.
2.10 Quantitative real-time PCR detection for osteogenic gene expression
The expression of osteogenesis-related genes was quantitatively determined via real-time polymerase chain reaction (RT-PCR). Each sample was seeded with 1 mL of cell suspensions at a density of 3 × 104cells per well and cocultured for 7 and 14 days in osteogenic inductive culture medium. At the indicated time interval, total RNA was extracted from cells grown on the samples with the TRIZOL reagent (Invitrogen, USA)according to the manufacturer’s protocol and then the reverse transcription was carried out. The sequences of the forward and reverse primers were listed in Table 1.The expression of osteogenesis-related genes, including collagen type I (COL1), run-related transcription factor 2 (RUNX2), osteoprotein (OPN), and osteocalcin (OCN) were normalized to the house-keeping gene[glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] and then quantified with the assistance of RTPCR (CFX Connect, Bio-rad).
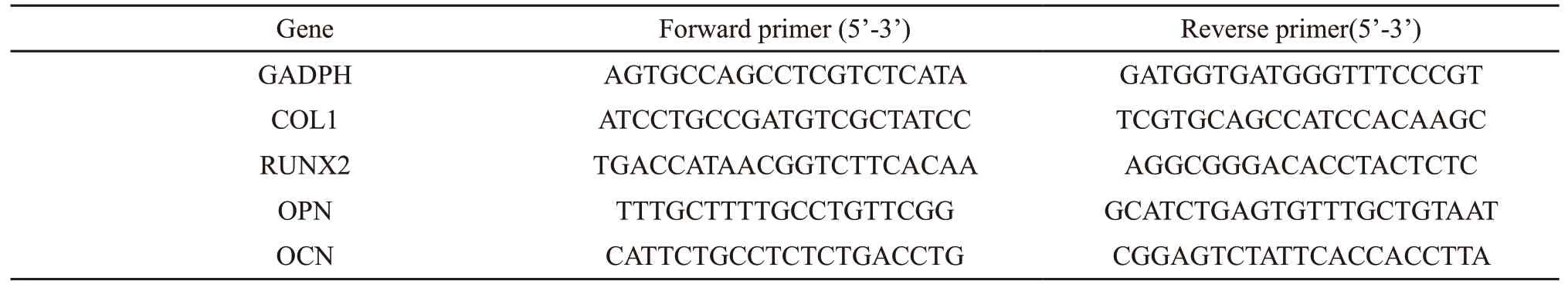
Table 1 Sequences of primers for RT-PCR assay
2.11 Statistical analysis
All the experiments were operated independently in triplicates. The data were presented as mean ±standard deviation and were analyzed using SPSS 22.0 software. The statistic significant differences between different groups were analyzed using a one-way analysis of variance (ANOVA). Ap-value < 0.05 was considered as significant, and ap-value < 0.01 was considered as highly significant.
3 Results and discussion
3.1 Surface characterization
PEEK has been considered as an ideal biomaterial for bone tissue repair and orthopedic surgery due to its good biocompatibility and adaptable mechanical properties[27,33]. However, the poor bio-interfacial interaction between PEEK and cells easily resulted in failure after implanted into human body[34].To improve the bio-interfacial activity of PEEK-based implants, a facile modification strategy has been developed through coating the bioactive CS layer on the porous surface of SPEEK with the assistance of PDA. In this study, porous structures were etched on the surface of PEEK after sulfonation treatment. The formation of numerous porous structures on the surface of PEEK provides anchor sites for integration of newly formed bone with implants.
Fig.1(a) shows the surface morphology of PEEK and the modified PEEK. The smooth surface of PEEK was changed into a laminated microporous structure with pore sizes ranging from 1 to 3 μm after sulfonation treatment. The porous structure of PEEK is mainly produced by the etching of concentrated sulfuric acid.A uniform PDA film was coated on the SPEEK surface which did not alter the surface microstructure. After the SPEEK-PDA was immersed in the CS precursor solution, it is obvious that abundant CS particles (white dots) were uniformly deposited on the porous surface of SPEEK-PDA. The formation of CS on the porous surface of PDA-coated was further characterized through XRD (Fig.1(b)). The diffraction peaks in the XRD pattern at approximate 29.7°, 32.8°, 47.6°, and 53.4°could be indexed to the characteristic (110), (200), (020), and(310) plane of CS (JCPDS card No. 09-0210), respectively. The results indicate that the CS particles were successfully coated on the PEEK surface.
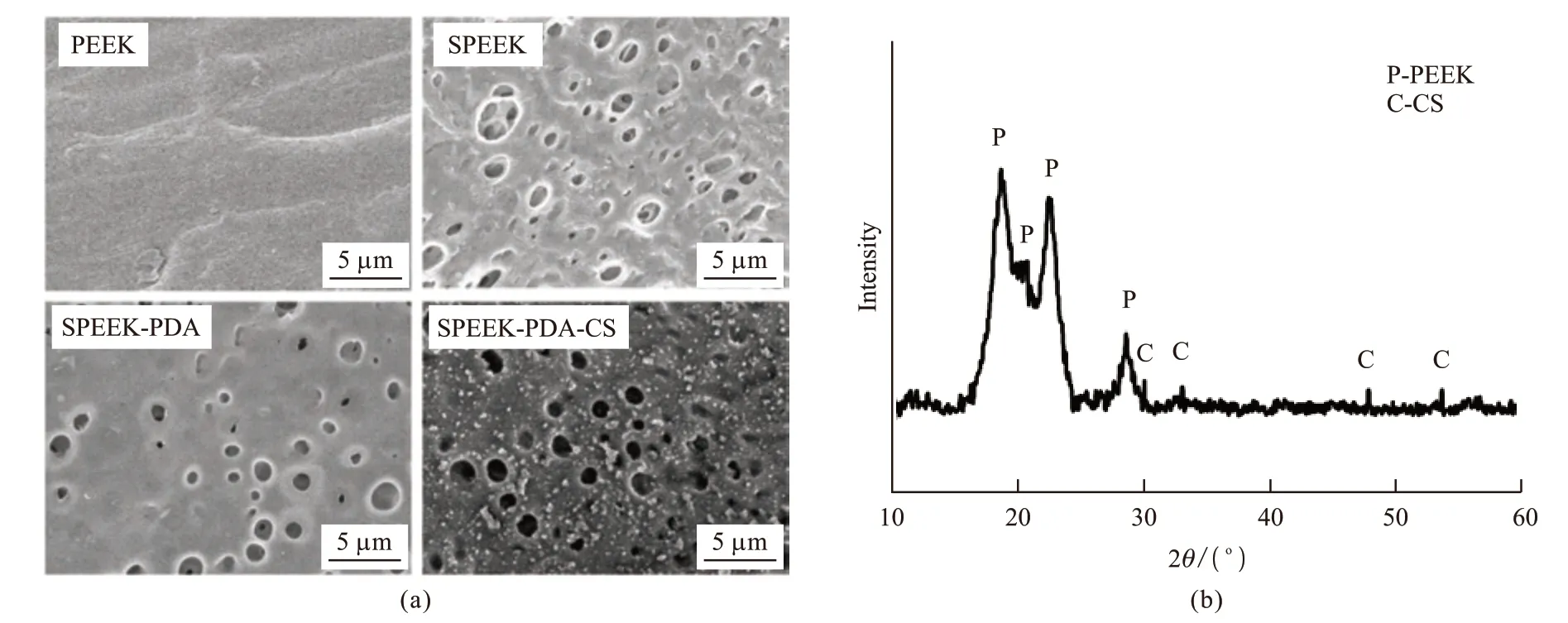
Fig.1 (a) SEM images of the surface of PEEK with different coatings; (b) The XRD pattern of SPEEK-PDA-CS
The chemical composition and elemental state on the surface of the samples were analyzed through XPS spectra (Fig.2). In the case of pure PEEK, a major peak at 283 eV was assigned to C1sand can be divided into three peaks with binding energy of 282.8, 283.2, and 284.3 eV, confirming the presence of C-C/C-H, C-O,and C=O, respectively. The peak for O 1sat 530.4 eV in the XPS spectrum can be deconvoluted into two peaks at 530.1 and 530.9 eV, respectively, corresponding to O=C and O-C. It is noteworthy that the peak for C-S at 283.9 eV was observed in the XPS spectrum of C 1sfor SPEEK. Two peaks at 166.9 and 168 eV were assigned to S 2p3/2and S 2p1/2, respectively. These results confirm the presence of high-oxidation-state SO3H group after sulfonation treatment. With PDA deposited on the surface of SPEEK, two characteristic peaks for N1s were observed at 398.5 and 400.1 eV, corresponding to N-H and -N=. After the coating of CS on the SPEEK-PDA surface, two characteristic peaks at 345.7 and 349.6 eV were observed in XPS spectrum for Ca 2p, and a characteristic peak at 100.8 eV was observed in XPS spectrum for Si 2p. These results confirm the successful preparation of PEEK-PDA-CS.
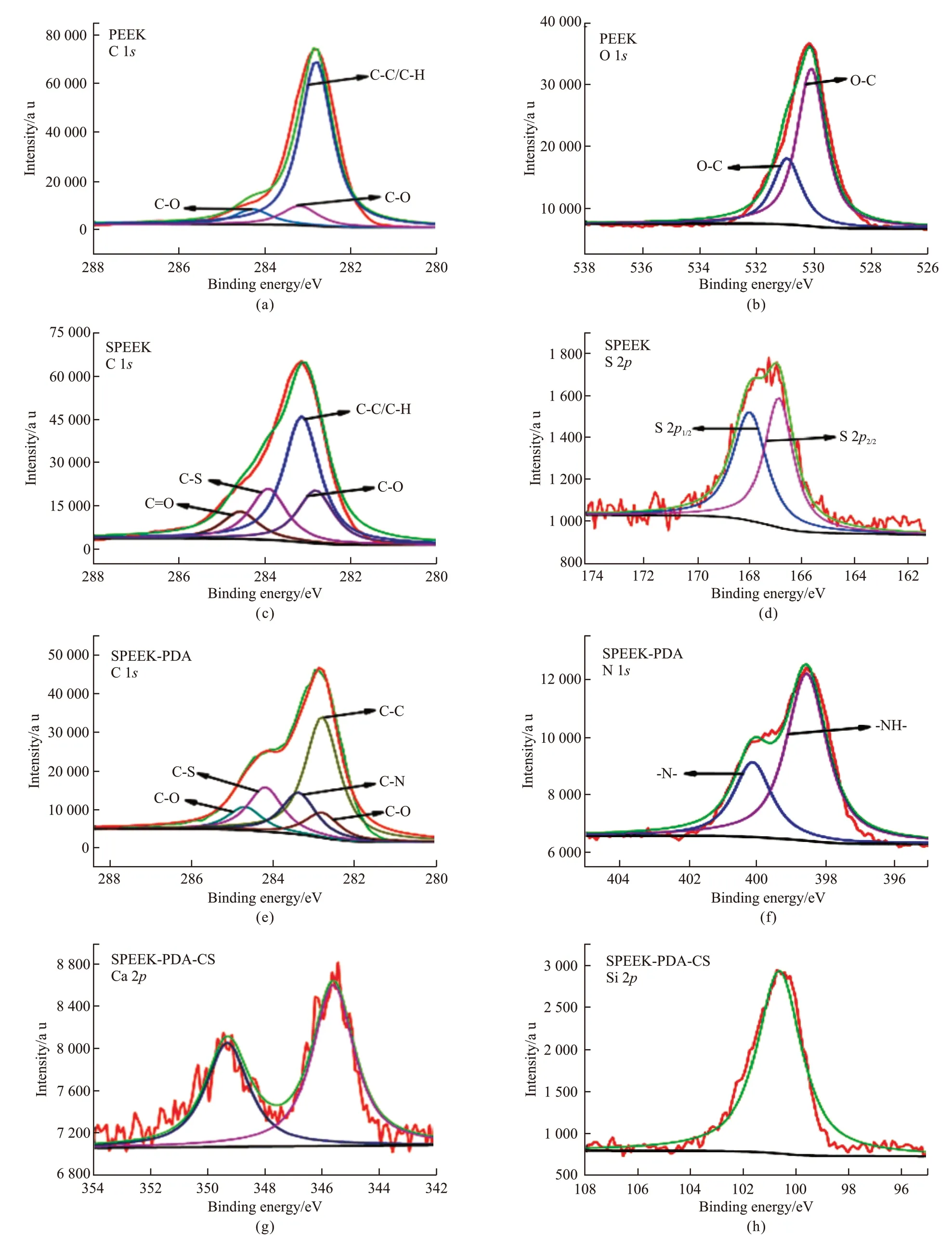
Fig.2 High resolution XPS spectra of C 1s, O 1s, S 2p, N 1s, Ca 2p, and Si 2p for PEEK with different coatings: (a, b) PEEK; (c, d) SPEEK; (e,f) SPEEK-PDA; (g, h) SPEEK-PDA-CS
Fig.3(a) and 3(b) show the contact angle of PEEK, SPEEK, SPEEK-PDA, and SPEEK-PDA-CS.The contact angle of PEEK was 88.6 ± 1.4°, while the contact angle was 96.3 ± 4.3° for SPEEK after sulfonation treatment. Notably, the PDA coating greatly enhanced the hydrophilicity with the contact angle of 56.8 ± 1.2°, which was lower than the pristine PEEK and SPEEK. The contact angle of SPEEK-PDA-CS was slightly decreased with a value of 46.4 ± 0.8°.The hydrophilicity of SPEEK-PDA has been significantly improved, which is mainly due to the excellent hydrophilicity of dopamine itself. In addition, the Ca2+released by the calcium silicate coated on the surface of SPEEK-PDA-CS also play a certain role in improving the hydrophilicity. A PDA layer was coated onto the porous surface of SPEEK to provide phenolic hydroxyl groups as anchor sites for deposition of the bioactive CS layer due to the excellent adhesive performance of PDA[35-37]. These results indicate that the hydrophilicity of SPEEK-PDA-CS was significantly improved due to the PDA-assisted CS coating.
3.2 Biomineralization formation of HA on the surface of SPEEK-PDA-CS
Fig.3(c) shows SEM images of the surface morphology of the samples after immersion in SBF for 7 days. Numerous spherical particles were observed on the surface of SPEEK, SPEEK-PDA, and SPEEKPDA-CS, while few particles were observed on the surface of PEEK. It is noteworthy that the surface of SPEEK-PDA-CS was fully covered by the newly formed particles. EDS spectra of spherical particles newly formed on the surface of the three samples show the Ca/P ratio of 1.44-1.58, confirming the biomineralization formation of HA. The CS coating significantly improved the hydrophilicity and biomineralization formation of HA of the surface of SPEEK-PDA-CS[38].
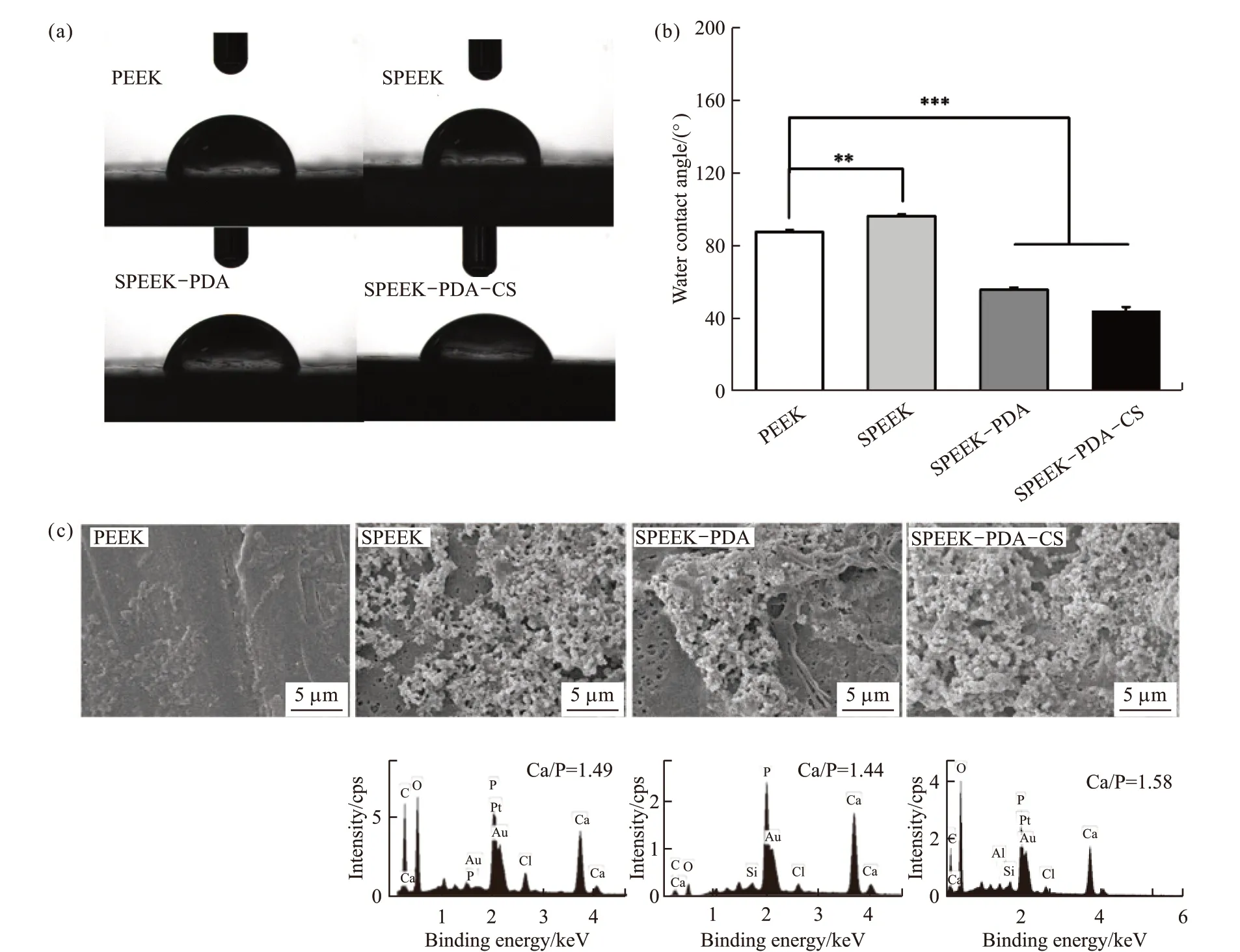
Fig.3 (a, b) Water contact angles of the PEEK with different coatings. *** donates significant differences compared with the other three groups (p<0.001); (c) SEM images and EDS spectra of HA formed on the surface of the PEEK with different coatings after immersion in the SBF solution for 7 days
3.3 Ion release analysis
Fig.4(a) shows the releasing profiles of Ca2+and SiO32-from SPEEK-PDA-CS in PBS within 21 days.The concentrations of both ions increased obviously from day 1 to day 14, implying the continuous release of both ions from SPEEK-PDA-CS.
3.4 Cell proliferation
Fig.4(b) shows cell viability of rBMSCs on the surface of the samples after co-incubation for 7 days.An obvious increase of cell viability after 7 days suggests good biocompatibility of these samples. Results also show that cell viability of rBMSCs in the SPEEKPDA-CS group was significantly higher than that in the PEEK group (p<0.001). These results indicate that the SPEEK-PDA-CS displayed good biocompatibility and significantly improved the proliferation of rBMSCs.
3.5 Osteogenic differentiation of rBMSCs on the surface of SPEEK-PDA-CS
To evaluate osteogenic differentiation of rBMSCs on the surface of SPEEK-PDA-CS, both ALP staining and Alizarin Red staining were carried out. As shown in Fig.4(c) and (d), ALP staining was remarkably more intense in the SPEEK-PDA-CS group than that in the PEEK group at the end of 7 and 14 days. The ALP activity in the SPEEK-PDA-CS group increased approximate 82% after 7 days and 86% after 14 days compared with that in the PEEK group.
Fig.4(e) and (f) show Alizarin red staining and analysis of rBMSCs after co-cultured with the samples for 14 and 21 days. Numerous calcium nodules were observed in the SPEEK-PDA-CS group, which was approximate 110% and 91% higher than that in the PEEK group at the end of 14 and 21 days, respectively. These results indicate that SPEEK-PDA-CS significantly improved osteoinductive activity.
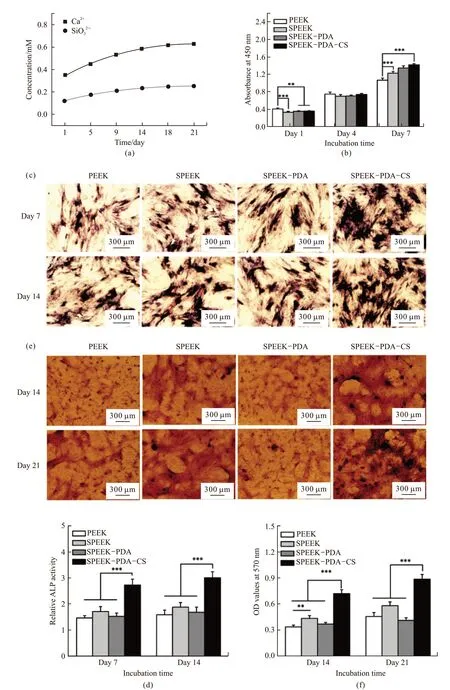
Fig.4 (a) Release profiles of Ca2+ and SiO32- from SPEEK-PDA-CS in PBS within 21 days; (b) Cell viability of rBMSCs on the surface of different samples at different time intervals; (c, d) ALP staining and quantitative analysis of ALP activity of rBMSCs on the PEEK with different coatings after co-cultured for 7 and 14 days; (e, f) Alizarin red staining and quantitative analysis of rBMSCs on the surface of the PEEK with different coatings after co-cultured for 14 and 21 days. (** represents p<0.01; *** stands for p<0.001)
Studiesin vitroindicate that cell viability of rBMSCs was significantly improved on the surface of SPEEK-PDA-CS than that on the PEEK surface.Meanwhile, the CS coating remarkably promoted osteogenic differentiation of rBMSCs on the surface of SPEEK-PDA-CS, including the increased ALP activity and deposition of calcium nodules.
3.6 Expression of osteogenic differentiationrelated genes
To further evaluate osteogenic differentiation of rBMSCs on the surface of SPEEK-PDA-CS, the expression of several osteogenic differentiation-related genes, including COL1, RUNX2, OPN, and OCN, was analyzed through RT-PCR detection at different intervals (7 and 14 days). As shown in Fig.5, the expression of COL1, RUNX2, OPN, and OCN in the SPEEK-PDA-CS group was significantly higher than that in the PEEK group, indicating the higher level of osteogenic differentiation. In the early stage (after co-cultured for 7 days), the rBMSCs on the SPEEK-PDA-CS group exhibit higher level of COL1 (p<0.001) and RUNX2(p<0.001) which are considered as the early markers for osteogenic differentiation. The expression of OCN(p<0.001) is also significantly increased in the SPEEKPDA-CS group than that in other groups in the later stage (after co-cultured for 14 days), which is the later marker for osteogenic differentiation.
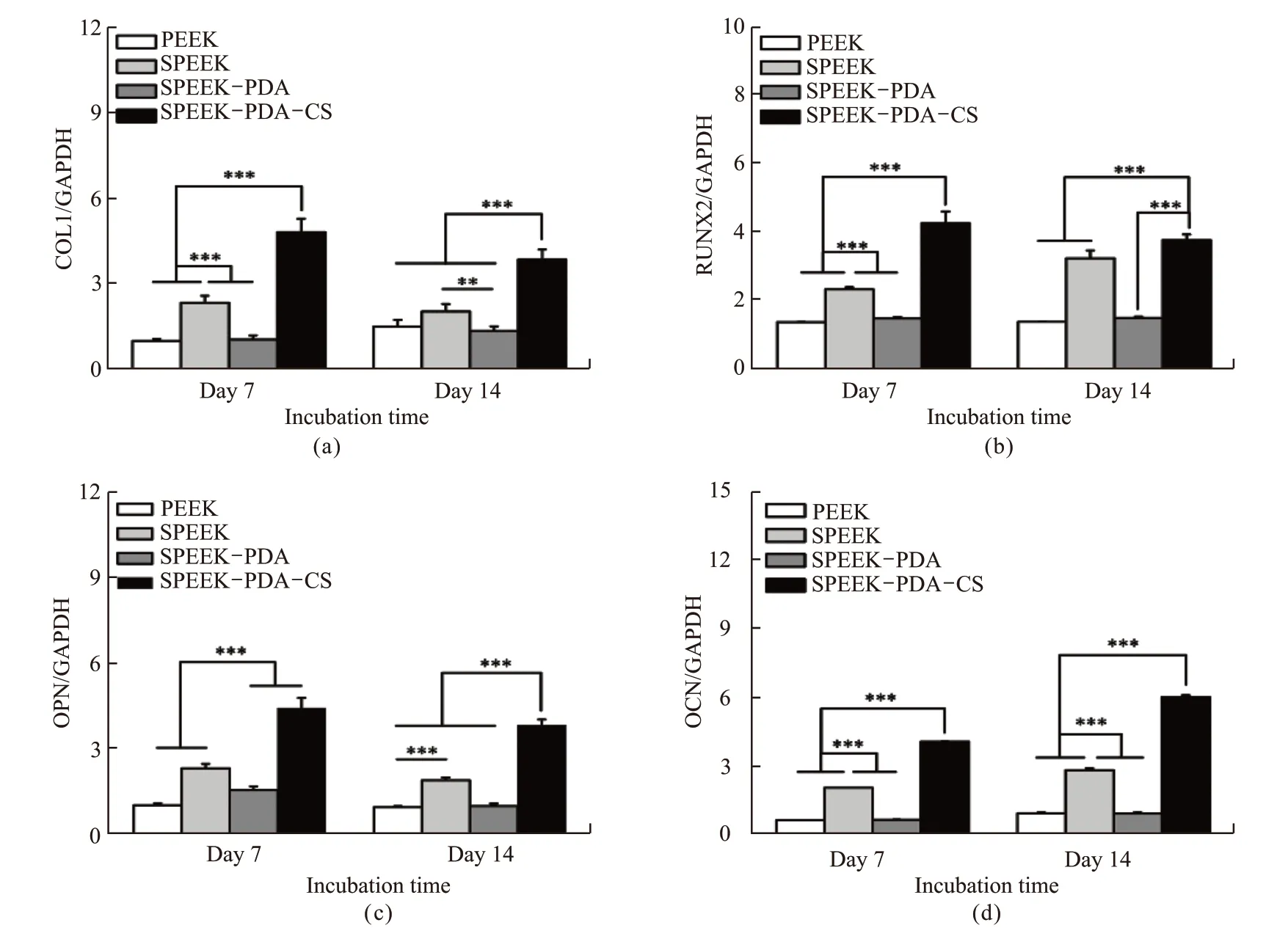
Fig.5 RT-PCR detection of osteogenesis-related gene expression of rBMSCs after co-cultured with the samples for 7 and 14 days: (a) COL1;(b) RUNX2; (c) OPN; (d) OCN. The mRNA level was the relative mRNA level which was normalized by house-keeping gene GAPDH (** represents p<0.01; *** donates p<0.001)
The up-regulated COL1 and RUNX2 genes are important transcription factors during osteogenesis which are responsible for collagen secretion and osteogenic differentiation, respectively[39,40]. The expression of OPN gene is relevant to the maturation stage of osteogenesis, and the expression of OCN is considered as a marker for the bone formation[41]. These results are agreed with the increased ALP activity and deposition of calcium nodules. Although the precise mechanism on the up-regulated expression of osteogenic differentiation related genes of rBMSCs needs to be further investigated, it is speculated that Ca2+and SiO32-released from SPEEK-PDA-CS could play important roles to promote the proliferation and osteogenic differentiation of rBMSCs[42-45]. It is interesting that the expression of osteogenic differentiation-related genes in the SPEEK group was higher than that in both the PEEK group and the SPEEK-PDA group, implying that the sulfonation treatment is beneficial to stimulate osteogenic differentiation. Therefore, these results indicate that SPEEKPDA-CS significantly promoted osteogenic differentiation of rBMSCs.
4 Conclusions
A facile modification strategy has been developed through deposition of the bioactive CS layer on the porous surface of SPEEK with the assistance of PDA. The porous surface provides anchoring space for integration between the implants and newly formed bone. SPEEKPDA-CS displayed higher bioactivity than PEEK to significantly promote the proliferation and osteogenic differentiation of rBMSCs, which includes the increase of ALP activity and formation of calcium nodules, the expression of osteogenic differentiation-related genes(COL1, RUNX2, OPN, and OCN). These results pave the way to extend clinical applications of bioactive PEEK-based implants for bone tissue repair and orthopedic surgery.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Influence of Heat Treatment on Microstructure and Mechanical Properties of Plasma Sprayed FeCrMoCBY Amorphous Coatings
- Lamella Multiple Grained Structure Making 2205 Duplex Stainless Steel with Superior Strength and Ductility
- Progress in Light-weight High Entropy Alloys
- Synthesis and Characterization of Polyaniline/MgTiO3 Composite with Excellent Thermal and Electrochemical Performance
- Synthesis and Characterization of Hyperbranched Epoxy with Terminal Ally Group and Its Application of Toughen Bismaleimide
- Loose Sand Cemented by Microbial Cementitious Material:Composition, Microstructure and Mechanical Properties
