Progress in Light-weight High Entropy Alloys
2021-09-15CAIZhihaiGUOYuLIUJianLIUJunGUOJieDUXianHUANGShaofu
CAI Zhihai, GUO Yu, LIU Jian*, LIU Jun, GUO Jie, DU Xian, HUANG Shaofu
(1. National Engineering Research Center for Mechanical Product Remanufacturing,Army Academy of Armored Forces, Beijing 100072,China; 2. College of Mechanical Engineering, Anhui University of Science&Technology, Huainan 232000, China)
Abstract: High entropy alloys (HEAs) possess good mechanical properties and a wide range of industrial applications. In this paper, phase formation prediction theory, microstructure, properties and preparation methods of light-weight HEAs (LWHEAs) were reviewed. The problems and challenges faced by LWHEAs development were analyzed. The results showed that many aspects are still weak and require investigation for future advanced alloys, such as clarification of the role of entropy in phase formation and properties of HEAs,improved definition and different generations division of HEAs, close-packed hexagonal (HCP) phase structure prediction and corresponding alloy design and fabrication. Finally, some suggestions were presented in this paper including in-depth research on formation mechanism of multi-component alloy phase and strengthening of large-scale HEA preparation methods via technology compounding and 3D printing technology. Also, there is a need for more research on the in-situ preparation of HEA coatings and films, as well as developing LWHEAs with superior strength and elevated temperature resistance or ultra-low temperature resistance to meet the requirements of future engineering applications.
Key words: high entropy alloys (HEAs); light-weight; microstructure
1 Introduction
Traditional alloys often contain 1-2 principle chemical elements but their properties can be improved by adding small amounts of other elements. However,enthalpy-based alloys suffer from two disadvantages.First, preparing materials with superior comprehensive performances is usually challenging. Second, the space for performance improvement of such alloys is limited. Hence, traditional alloys are increasingly unable to meet the demands of modern technologies. For example, due to the demand from transportation and defense industries, the development of lighter and stronger materials is needed for the purpose of saving energy and raw materials. However, traditional Al, Mg and Ti alloys have gradually failed to meet this demand.In 2004, Prof. Yeh[1]and Prof. Cantor[2]proposed the concept of high entropy alloys (HEAs) separately and simultaneously by breaking through the traditional alloy design concept. Such alloys contain at least five main elements with atomic percentage of each element lying between 5% and 35%, as defined by Prof. Yeh, although this definition is still debatable. These alloys are also known as multi-component alloys. The mixing entropy (mainly refers to configuration entropy) of HEAs is much higher than that of traditional alloys. This results in four effects: high entropy thermodynamics,slow diffusion dynamics, lattice distortion in structure,and cocktail performance[3]. Hence, HEAs can easily form simple FCC or BCC solid solutions and nanoscale precipitates. They can also lead to better characteristics in terms of high strength, elevated hardness, excellent thermal stability, corrosion resistance[4], and wear resistance[5]. Therefore, high entropy alloys have gained increasing interest in both academia and industry. The number of literature reports on high entropy alloys in Elsevier, Springer and CNKI databases since 2015 is 11 330, 4 216 and 3 449, respectively. The number of reports per year is shown in Fig.1. It can be seen from the figure that the number of HEAs-related literature is generally increasing year by year (2015-2019).
Numerous HEAs have been developed after more than two decades. Such alloys can be roughly divided into face-centered cubic (FCC), body-centered cubic(BCC), close-packed hexagonal (HCP), amorphous,and multi-phase composite HEAs. In terms of physical properties and functions, these alloys could be mainly divided into: LWHEAs[6,7], high-temperature resistant HEAs[8-10], radiation resistance HEAs[11], and soft magnetic HEAs[12-14]. In terms of material patterns, HEAs can be fabricated as ingots, bars, powders, coatings,and films. Among alloys, LWHEAs, which are typically fabricated using the elements Al, Mg, Ti, Li, Be, Cu,Sc, Sn, Fe, Mn, Co, Ni and Zn, are the most utilized in light-weight equipment and construction of circular economy and society. Unfortunately, there are only a limited number of studies on LWHEAs until now. The number of literature reports on LWHEAs in Elsevier,Springer and CNKI databases is only 456, 205 and 8,respectively. However, the development trend is also increasing year by year (2015-2019), as shown in Fig.2. There is also no uniform and clear definition about the density of LWHEAs yet. Kuma[15]and Youssefet al[16]defined low density as less than 3 g/cm3. However, in other literatures[7,17,18], the material is considered LWHEAs as long as the density is less than 7 g/cm3. In this paper, the latter rule was followed and the development and research status of LWHEAs were reviewed by considering most the HEAs reported in the literature with densities of more than 7 g/cm3.[18]In fact, if only HEAs with density less than 3 g/cm3are defined as LWHEAs, there might be no more than 10 such literature reports so far. Furthermore, this reviewalso considered and analyzed the future problems in the development of LWHEAs and put forward some suggestions accordingly.
2 Research status of LWHEAs
Current research dealing with HEAs is mainly focused on component design, phase formation, transformation theory, preparation of HEAs, and analysis of microstructure and properties[19-24].
2.1 Components of LWHEAs
Unlike traditional alloys based on enthalpy principle, HEAs are unique alloy systems[25-28]. Numerous elements, such as Al, Fe, Co, Cr, Ni, Mn, Ti, Mg, Zn and Nb can be adopted for designing new HEAs, leading to tremendous diversification. Hence, many alloy systems with different characteristics have been successfully prepared, including AlCrNbTiVZr, FeCoCrNiMn, Al-FeMgTiZn, MgCaAlLiCu, and FeNiCoSiCrAlTi[28-31].However, inclusion of several kinds of elements in the alloys could also lead to more complex formation and transformation of phases, leading to the challenging construction of phase diagrams. The diverse optional elements also bring difficulties for designing proper HEAs. The composition of HEAs phase is not only hard to study by ‘trial-and-error’ traditional methods but also expensive. Hence, finding alloys meeting all requirements via checking each alloy composition by traditional repeated tests is quite challenging. Therefore, design of HEAs is mainly performed by phase formation prediction and simulation of transformation based on thermodynamics theory and empirical criteria. Note that phase formation of HEAs and prediction parameter model of phase formation (such as atomic radius differenceδ, mixing enthalpy ΔHmix,Ω,Λ, and valence electron concentrationVEC) have so far been extensively studied. These parameters can be calculated according to Eqs. (1) – (5)[32-35]:
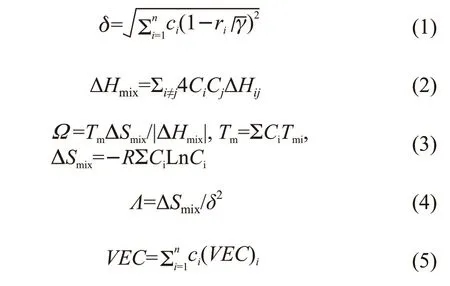
where is the weighted average atomic radius of all components,riis the atomic radius of componenti,ciis the atomic fraction of componenti, ΔHijis the mixing enthalpy of elementsiandj,Tmiis the melting point of alloy elementi,ΔSmixis the mixing entropy,Ris the gas constant, and (VEC)iis the valence electron number of atoms in componenti.
The above parameters and their combinations can be used to predict the phase formation of HEAs.Note that solid solution phase (SS) forming range was reported asδ< 6.2% and -12 kJ/mol <ΔHmix< 5 kJ/mol[32]. Whenδ> 3% and ΔHmix< 0 kJ/mol, intermetallics (IM) phases should form, and an amorphous phase would form atδ> 6.2% and -40 kJ/mol < ΔHmix<-12 kJ/mol. Yang Xiao and Zhang Yong[36]noticed the formation of only SS phase whenΩ≥ 1.1 andδ< 3.6%.By comparison, SS + IM phases formed when 1.1 ≤Ω≤ 10 and 3.6% ≤δ≤ 6.6%. Metallic glass (MG)phase (also called amorphous phase) could be formed atΩ≤ 2 andδ≥ 5%. Furthermore, single disordered solid solution phase would be induced atΛ> 9.6. For 2.4 <Λ< 9.6, multi disordered solid solution phases will be formed, and finally, intermetallic compounds would be generated atΛ< 2.4. Other studies showed the formation of FCC structure SS atVEC> 7.8, BCC structure SS atVEC< 6.8, and BCC + FCC structure SS at 6.8 <VEC< 7.8.
Commonly used simulation methods include first-principles density functional theory (DFT)[37],molecular dynamics (MD)[38], and calculation of phase diagram (CALPHAD)[39]. Note that DFT is particularly used to predict the physical properties of alloys but MD is often used to predict their thermodynamic properties.CALPHAD phase diagram calculation method could be utilized to directly calculate the minimum Gibbs free energy at given temperature and pressure. Then, the equilibrium phase of multi-component system could be predicted according to binary and ternary systems data,using thermodynamic theory and database[40].
Xieet al[41]carried out molecular dynamics simulations to identify the initial growth process of AlCo-CrCuFeNi HEA thin films. Their simulations showed the significant impact of the number of elements and differences in atomic size on the atomic configuration.At low Al contents (2% and 3%), crystal layer grew,while pure amorphous film was observed for the highest proportion (39%) of the atom which deviates the most from the mean calculated for the equimolarity.On the other hand, they were able to predict the evolution trend from solid solution to bulk metallic glass by calculating parameters of HEAs likeδandΩ. The results were found to be in good agreement with simulations using current molecular dynamics. Tianet al[42]employed the first principle to calculate phase stability,elastic modulus, and theoretical tensile strength of HTS materials. They found BCC phase to be more stable than FCC phase. Nonget al[43]also used first-principle calculations to study common intermetallic structure,electronic and elastic properties of FeTiCoNiVCrMn-CuAl system. It was shown that FeTi, Fe2Ti, AlCrFe2,Co2Ti, AlMn2V and Mn2Ti phases may occur during formation processes of alloys. Further studies revealed phases with high shear and elastic moduli as excellent strengthening phases of HEAs to improve their hardness characteristics. The effect of partial density of states on bonding mode was also studied along withp-dhybrid strength to clarify the underlying mechanism for the elastic properties of such compounds. Widomet al[38]demonstrated the feasibility of Monte Carlo hybrid simulation combined with molecular dynamics in the first principle environment. Such methods were more efficient than traditional molecular dynamics since they could exchange chemical species without limitation of rate correlated with barrier. This combined route could especially suit simulation of HEAs with free substitution of multiple chemicals. Chemical order was balanced in solid state using Monte Carlo method, which cannot be achieved with traditional molecules.
CALPHAD has successfully been used to predict the formation of HEAs phases. For instance, Zhanget al[44,45]calculated binary and multi-component phase diagrams to explain phenomena observed during preparation of HEAs, as well as to describe the development prospects of alloys design assisted with phase diagram calculations. They used possible phase diagram calculations to explain the influence of various elements on phase stability and FCC/BCC phase transition, as well as provide useful guidance for the development of novel HEAs. Raghavan[46]utilized CALPHAD method to predict phase formation of large number of mixtures containing FCC, BCC or FCC + BCC phases. The results revealed the usefulness of CALPHAD route for predicting BCC phase formation when compared to FCC phase.
So far, most studies dealing with HEAs are focused on the development of HEAs with good mechanical properties, while low-density HEAs have not been well explored[15,47-49]. Since HEAs are often composed of five or more elements, the introduction of elements with densities above 5 g/cm3like Fe, Co and Ni could form alloys with better properties. On the other hand,the density could be reduced by increasing the content of lighter elements while maintaining the mechanical properties. In AlNbTiBZr alloy, this could be achieved by increasing the aluminum content. Another way is to add other light elements. Sunet al[50]used CALPHAD to predict the phase structures of HEAs containing at least two light elements, and compared the results with experiments. They demonstrated that applicability of the traditional CALPHAD method would depend on the manufacturing process of HEAs. Factors, such as solute retention and energy defect should be considered when preparing HEAs by non-equilibrium method. Special attention should be paid to rapid solidification process,where silicon-containing HEAs intermetallic compounds predicted by traditional CALPHAD calculation method will be restrained by solute closure. In terms of the influence of light elements, such as aluminum, titanium, silicon, alkali and alkaline earth metals on phase formation of HEAs, some studies revealed the facile incorporation of magnesium or other alkali and alkaline earth metals into various intermetallic compounds in HEAs prepared by traditional casting method. However, such alloys could dissolve into solid solution through non-equilibrium processes, such as mechanical alloying. In sum, non-equilibrium process was found effective for incorporating light elements, such as Si or alkali and alkaline earth metals into HEAs.
CALPHAD method has been successfully used to predict phase stability of multi-component HEAs systems and a key role in the design of HEAs. However,thermodynamic databases of CALPHAD method still suffer from reliability. For HEAs, CALPHA databases have been calculated on the basis of existing binary and ternary thermodynamic systems since thermodynamic databases of multi principal component alloys are still not completely established. Hence, obtained data can only be regarded as semi-quantitative results.Some studies suggested that phase formation of alloys can be predicted by CALPHAD method[51]. In addition, predicted results were found quite different from the experimental data. CALPHAD method could still provide reference for HEAs design, and experiments of multicomponent alloy systems can be implemented conveniently once complete thermodynamic databases were built. On the other hand, several studies showed good consistency between phase diagram calculations with the experimental data.
Stepanovet al[52]employed Thermo-Calc software and TTTI3 database to simulate equilibrium phase diagram of Al0.5CrNbTi2V0.5alloy (Fig.3). They found that phase composition of the alloy corresponded well to predicted equilibrium phase diagram. Senkovet al[17,53]calculated the non-equilibrium and equilibrium solidification phase diagrams of NbTiVZr, NbTiV2Zr,CrNbTiZr and CrNbTiVZr HEAs using Pandat software and PanTi database. The comparison between the experiment and simulated phase diagrams indicated the feasibility of PanTi thermodynamic binary and ternary systems in predicting the phase equilibrium but could not accurately predict volume of phases in the alloy. In addition, rapid screening of large number of alloy systems by CALPHAD modeling was proposed.
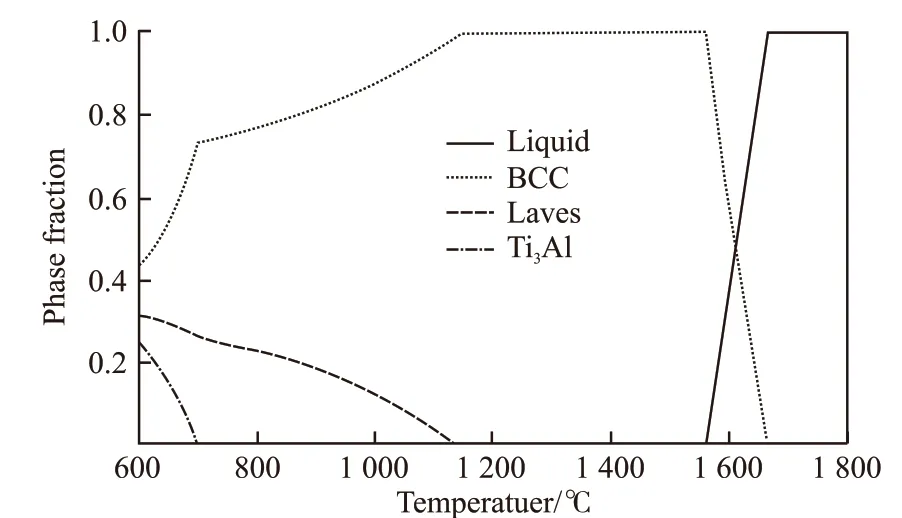
Fig.3 Simulated equilibrium phase diagram of Al0.5CrNbTi2V0.5[52]
Sanchezet al[54]used Thermo-Calc software and TCAL5 database to calculate the equilibrium phase diagrams of Al40Cu15Mn5Ni5Si20Zn15, Al45Cu15Mn-5Fe5Si5Ti5Zn20, Al35Cu5Fe5Mn5Si30V10Zr10, and Al50Ca-5Cu5Ni10Si20Ti10HEAs. The comparison of the observed results with predicted phase diagrams suggested that formed phases in the first three alloys were consistent with simulated equilibrium predictions. However,phase formed in Al50Ca5Cu5Ni10Si20Ti10alloy was only partially consistent with the prediction, showing the applicability of TCAL5 database. The experimental results provided a way for the development of new alloys.
The TCNI8 database contains complete binary data of Al, Cr, Nb, Ti, V and Zr elements, very important for the design of LWHEAs. Fenget al[18,55]successfully calculated Al-Cr-Nb-Ti-V-Z system using CALPHAD method and TCNI8 thermodynamic database[56].They also successfully predicted the formation of single BCC phase in AlNbTiV, NbTiVZr and AlCr0.5NbTiV,as well as predicted formation of L21+ BCC two-phase region in phase diagram by reducing the Ti content.The composition and phase transition temperature of L21phase (837 ℃) predicted by CALPHAD thermodynamic model agreed well with the experimental results.CALPHAD modeling of various high-temperature HEAs with BCC phase based on this database also corroborated the experimental observation results[57-62].CALPHAD has also been employed to predict the crystal structures of formed intermetallics of AlCrNbTiV,CrNbTiZr and CrNbTiVZr during solidification process, and the data were found to be consistent with the experiments[63]. Meanwhile, Al1.5CrFeMnTi LWHEAs were found to contain BCC, L21and C14 laves phases,in which L21phase showed the same distribution as cast BCC phase. The size, shape, coherence and spatial distribution of L21phase can be changed by annealing at 750 and 850 ℃. The interface energy between L21and BCC phases was very small with low lattice mismatch. These features should be advantageous for L21phase during nucleation. However, the database did not include the thermodynamic description of all constituent triples of Al-Cr-Nb-Ti-V-Z system, except for Al-Cr-Ti system. Therefore, such database would require further improvement.
Meanwhile, special databases of HEAs have been researched and developed to solve problems of conventional databases that are unable to accurately predict the formation of alloy phases. For example, Yanget al[64]used PanHEA database to calculate the phase structures and chemical compositions of Al0.75Co-CrFeNi and Al1.5CoCrFeNi alloys at 1 200 ℃. They showed that Al1.5CoCrFeNi alloy mainly consisted of disordered BCC (A2) + ordered B2 phase. For Al0.75Co-CrFeNi alloy, the disordered FCC + ordered B2 phase was identified as the main phase, in which B2 ordered phase was rich in Al and Ni elements along with certain dissolution of Co, Cr and Fe elements. The chemical composition of ordered phase B2 in both alloys was the same. CALPHAD method was used to predict not only the nature of phases but also the mole fraction of each phase. The results suggested that mole fraction of disordered phase in Al0.75CoCrFeNi alloy was three times that of ordered phase. By comparison, the mole fraction of disordered and ordered phases in Al1.5CoCrFeNi alloy was equivalent. Yurchenkoet al[65]calculated the equilibrium phase diagrams of AlNbTiVCrxand AlNbTiVZrxby means of Thermo-Calc software and TCHEA2 database. Compared to the experimental data,the calculated phase diagrams were only found good for predicting the correct trend of alloy phase formation but could not accurately predict phase transformation temperature and its chemical composition. Hence, high entropy databases still require further improvement and perfection.
Several studies have shown that reliable HEAs database should optimize binary and ternary components in the entire composition and temperature range.The reliability of such databases would be based on empirical rules, phase diagram checking, and density functional theory (DFT) for modeling. Gaoet al[66]effectively combined phase diagram check, CALPHAD modeling, DFT calculations and first-principle molecular dynamics to propose hundreds of new single equivalent molar components, such as Dy-Er-Gd-Ho-Lu-Sc-Sm-Tb-Tm-Y, Mo-Nb-Re-Ta-Ti-V-W, Mo-Nb-Ta-Ti-V-W and Ba-Ca-Eu-Sr-Yb quaternary high order equimolar alloy systems.
2.2 Microstructure and properties of LWHEAs
Liet al[67]prepared a new type of Mgx(MnAlZn-Cu)100-x(with atom percentagex= 20, 33, 43, 45.6,and 50) light alloys by induction melting process. The structure and properties of the alloys were analyzed and the polyphase structure was mainly HCP and Al-Mn dihedral quasicrystal phases. The densities of the alloys according to composition ratio of magnesium ranged from 2.20 to 4.29 g/cm3. The hardness of the alloys was found to be high at room temperature (178-429 HV)with relevant compressive strength ranging from 400 to 500 MPa.
Some reported studies suggested that the microstructure and mechanical properties of Al0.5CrNbTi2V0.5HEAs annealed at 1 200 ℃ for 24 hours could be controlled by annealing heat treatment[52]. In casting state,the alloys showed BCC phase structures but formed relatively small Laves phase with uniform distribution in BCC matrix after annealing. The precipitation of Laves phase grains increased the compressive yield strength of the alloys from 1 240 to 1 340 MPa at room temperature and the ductility of the alloy declined.
Chenet al[68]prepared BeCoMgTi and BeCoMg-TiZn equal molar ratio alloys by mechanical alloying method. The XRD patterns of the alloy powders obtained at different grinding times are displayed in Fig 4. No crystalline solid solutions and intermetallic compounds were formed during ball milling process but only amorphous phase was gradually formed until complete amorphization. The direct formation of amorphous phase was mainly due to the large difference in size between the atoms.
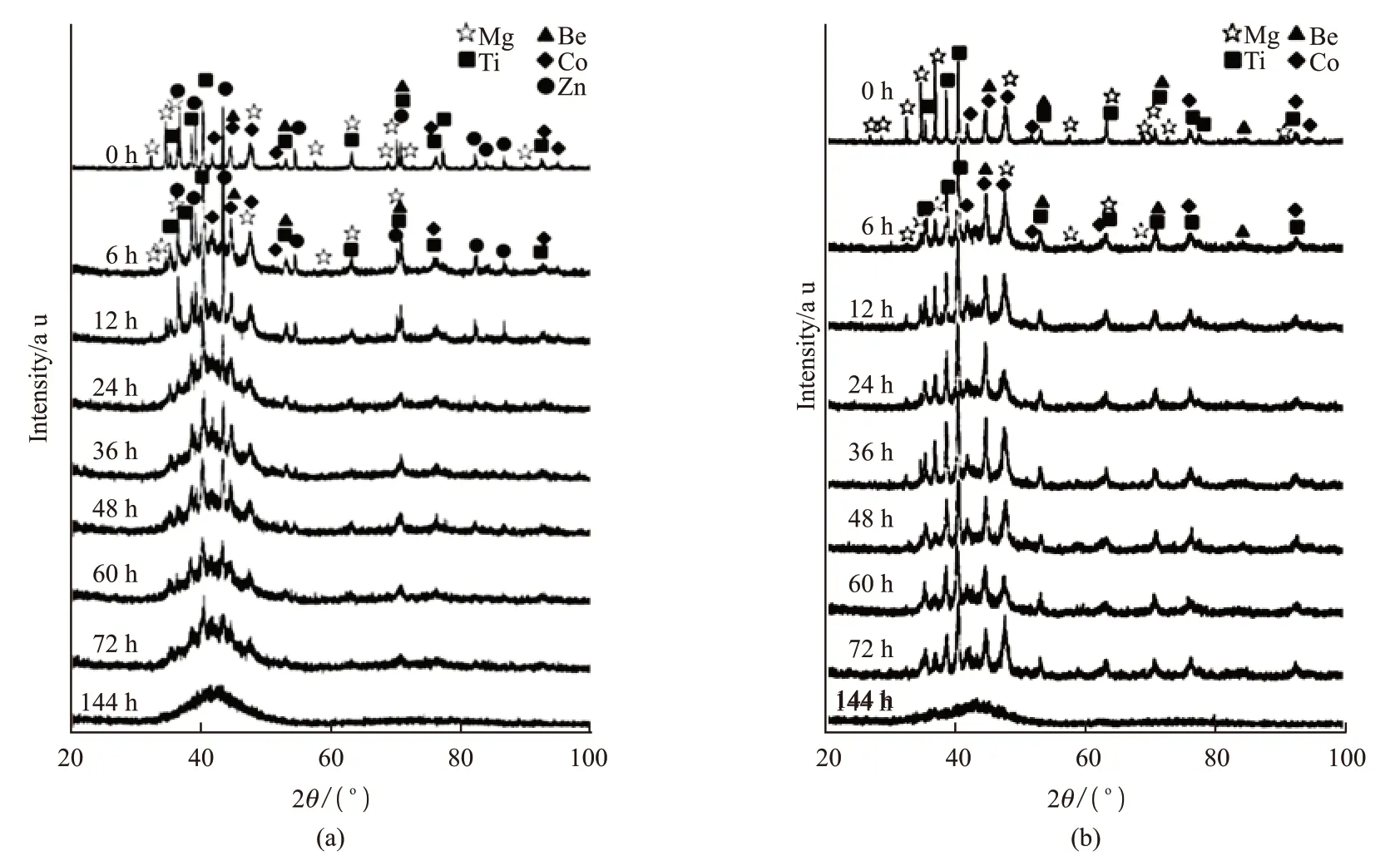
Fig.4 XRD patterns of alloy powders milled with different time: (a) BeCoMgTi alloy and (b) BeCoMgTiZn alloy[68]
For fabrication of light-weight structural materials for aerospace, Yanget al[69]studied low-density alloys Al-Li-Mg- (Zn, Cu, and Sn) by induction melting method and characterized the microstructures under cast conditions. They found the microstructures of the alloys to contain equal Mg2Sn and Li2MgSn (Fig.5).The stability of disordered solid solution phase was generally lower than that of competitive order intermetallic compounds for alloys composed of low-density materials, such as Al, Mg, and Li. To gain a better understanding of the factors affecting the phase stability in such alloys, the effects of factors, such as mixing entropy (△Smix), mixing enthalpy (△Hmix), atomic size difference (δ), electronegativity (△χ) and valence electron concentration (VEC) on phase formation were all analyzed. The atomic size differenceδwas found to play a key role in phase transition. Largerδvalues can promote the formation of amorphous phases or ordered intermetallic compounds. By contrast, smallerδvalues would lead to easy formation of random solid solutions.The standard solid solution formation of multicomponent alloy was identified asδ≤ 6.6%. In addition to smaller δ values, absolute value of △Hmix(-22 - 5 kJ/ mol) and lower △χ (< 0.175) can effectively promote the formation of solid solutions.
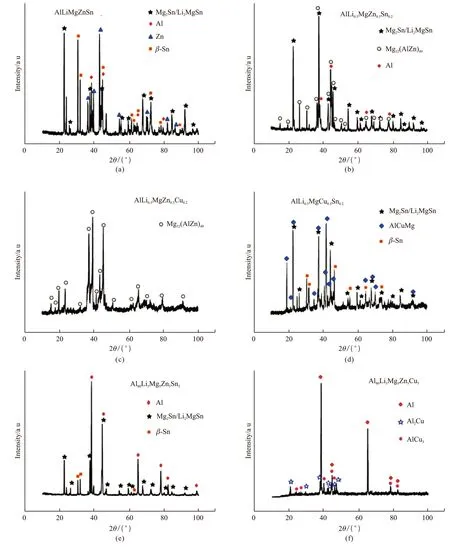
Fig.5 XRD patterns of low-density multicomponent alloys[69]: (a) AlLiMgZnSn; (b) AlLi0.5MgZn0.5Sn0.2; (c) AlLi0.5MgZn0.5Cu0.2; (d) Al-Li0.5MgCu0.5Sn0.2; (e) Al80Li5Mg5Zn5Sn5; (f) Al80Li5Mg5Zn5Cu5
Youseffet al[16]prepared a new type of Al-20Li20Mg10Sc20Ti30HEAs by mechanical alloying. The microstructures and properties of such alloy after ball milling were identified as single-phase FCC with grain size of 12 nm, density of 2.67 g/cm3, hardness of 6.1 GPa, and strength around 2-3 fold higher than that of nano-Al crystal (even higher than that of metal glass VIT1). The structures and mechanical properties of Alx-NbTiVZr (x= 0, 0.5, 1, and 1.5) alloys have also been studied[70]. The results showed solidified alloys contained BCC and Laves (C14) phases. After homogenization annealing, NbTiVZr alloy displayed single BCC phase but Laves (C14) phase and Zr2Al particles were present in BCC matrix of alloys containing Al element.The volume fraction of the second phase increased with Al, and the microhardness rose accordingly. For example, the microhardness of solidified NbTiVZr and Al1.5NbTiVZr alloys were estimated to 379 and 619 HV, respectively. The density of alloys enhanced with Al element content, from 6.49 g/cm3for NbTiVZr to 5.55 g/cm3for Al1.5NbTiVZr.
The phase structures of light-weight AlxCrFeMn-Tiyalloys (x= 1, 1.5, 2, 3 and 4;y= 0.25 and 1) prepared by arc melting have been previously studied[18].The results showed the presence of not only BCC phase but also Fe2AlTi type L21structure (Fig. 6).In addition to BCC and Laves phases present in Al3CrFeMnTi0.25and Al4CrFeMnTi0.25alloys, complex phases (such as, Al8Cr5and Al58.5Cr10.3Fe31.2) have also been identified. The reason for this is the low heat of formation between aluminum, titanium and transition metals, as well as their strong attractive interactions.These features could easily lead to the formation of very stable intermetallic compounds. Therefore, the synthesis of single-phase HEAs containing Al and Ti would still be challenging. For instance, Duet al[30]prepared MgCaAlLiCu HEAs by casting method. They found the alloys to crystallize in single-phase tetragonal with compressive strength reaching up to 910 MPa.On the other hand, the LWHEAs Al20Be20Fe20Si15Ti35prepared by Tseng[71]exhibited hexagonal Laves phase similar to Fe2Ti and MgZn2phases, with presence of low contents of Al2(Ti, Fe) and Si3Ti5phases. The negative enthalpy of a mixture containing Ti-Be, Ti-Al and Ti-Si was found much higher than that of Ti-Fe in binary TiFe2. Also, high concentrations of Be, Al and Si were expected to promote the hardening of the solid solution in matrix phase. Therefore, the overall hardness of the alloy reached as high as 911 HV, which was higher than that of quartz. The density of the alloy reached 3.91 g/cm3. At 700 and 900 ℃, the alloy also showed high-temperature oxidation resistance, better than that of Ti-6Al-4V. Therefore, the material can be used as light-weight, wear resistance, and high-temperature resistance alloy. Varalakshmet al[72,73]prepared CuNiCoZnAlTi and AlFeTiCrZnCu based HEAs with good composition uniformity by mechanical alloying method. The formation of nano solid solutions with BCC crystal structure was observed in all alloys with grain size around 10 nm. The hardness of AlFeTiCrZn-Cu solid solution was recorded as 2 GPa, and hardness and compressive strength of CuNiCoZnAlTi were 7.55 and 2.36 GPa, respectively. The excellent strengths of the alloys were attributed to solution strengthening and its nanocrystalline characteristics. Mauliket al[74]also reported LWHEAs design could use high entropy effect to adjust the bulk composition and maximize solubility(or ductility, oxidation resistance,etc) of alloys by selecting light-weight elements (such as Ti, Al, Mg,etc)as main elements.
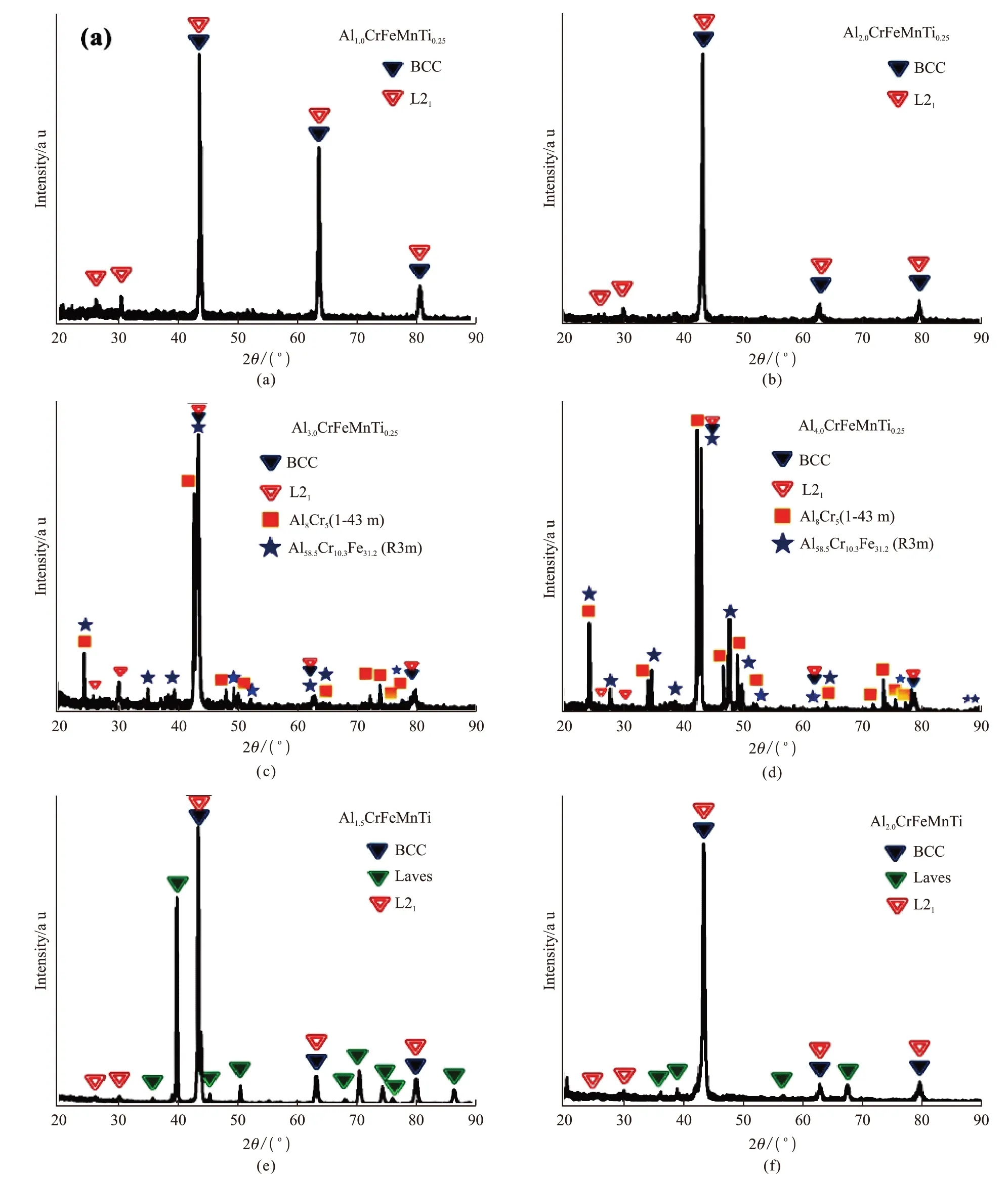
Fig.6 XRD patterns of a series of newly-designed light-weight HEAs[18]: (a) Al1.0CrFeMnTi0.25; (b) Al2.0CrFeMnTi0.25; (c) Al3.0CrFeMnTi0.25; (d)Al4.0CrFeMnTi0.25; (e) Al1.5CrFeMnTi and (f) Al2.0CrFeMnTi
As the original intention of LWHEAs research was to develop new light structure materials, most of the available research content is focused on their mechanical properties such as hardness, yield strength and plasticity, as discussed above. In fact, wear and corrosion failure is also the main failure mode of mechanical equipment and parts. Hence, tribological properties and corrosion resistance are also important research areas for engineering materials, although these two aspects of research account for a small proportion so far, as shown in Fig.7. Also, Fig.7 reveals that the studies on fatigue property, high temperature creep, high temperature resistance and oxidation resistance of LWHEAs are scarce so far. Chuanget al[75]designed and prepared a series of AlxCo1.5CrFeNi1.5TiyHEAs with different Al and Ti contents. The phase and microstructure were investigated, and adhesive wear tests were performed to study the wear mechanism, compared with the conventional wear-resistant steels SUJ2 and SKH51. The results showed that the amounts of Al and Ti strongly affected the phase and microstructure, particularly the content and morphology of the hardη-(Ni, Co)3Ti phase. The wear resistance of Co1.5CrFeNi1.5Ti and Al0.2Co1.5CrFeNi1.5Ti alloys was at least two times better than that of conventional wear-resistant steels with similar hardness, due to their excellent anti-oxidation property and resistance to thermal softening. Unfortunately,the mechanism of the excellent anti-oxidation property and resistance to thermal softening was not explained.Chenet al[76]produced Al0.6TiCrFeCoNi HEAs coating on ATSM A572 steel using high-velocity-oxygen-fuel(HVOF). The microstructure, phase composition and hardness were characterized. Also, the wear behavior against Al2O3counter body at different temperatures(room temperature, 300 ℃ and 500 ℃) was investigated. The results showed that the coating was very dense and consisted of lamellae. The phase structure consisted of two BCC phases: one was rich in Fe and Cr, and the other was rich in Al and Ni. However, there was no explanation on the specific phases. Hardness of the coating was 789±54 HV0.1. The wear of the coating was mainly caused by abrasion at all temperatures. The role of fatigue wear increased with the increase in temperature. However, tribo-reaction played an important role at 500 ℃.
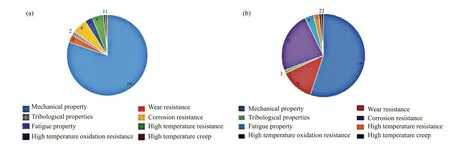
Fig.7 Statistical analysis chart of LWHEAs research literatures from 2015-2019: (a) Elsevier data (b) Springer data
In previous research[77], AlSiTiCrFeCoNiMo0.5and AlSiTiCrFeNiMo0.5coatings were prepared by plasma spray method, and their microstructure and wear resistance in both as-sprayed state and 800 ℃treated state were analyzed, compared with bearing steels SUJ2 and hot-die tool steel SKD61. It was found that the major phase of both AlSiTiCrFeCoNiMo0.5and AlSiTiCrFeNiMo0.5coatings was BCC structure with a severe lattice distortion. Both the coatings possessed a similar wear resistance with SUJ2 despite their smaller hardness than SUJ2 in the as-sprayed state, indicating that the microstructure of such alloys was superior to the wrought microstructure of SUJ2 and SKD61 steels.In the 800 ℃ treated state, the wear resistance of the two coatings was superior to that of SUJ2 and SKD61 steels as the hardness of the coatings increased to about Hv 900-1 000. Guoet al[78]fabricated TiN particles reinforced CoCr2FeNiTix(x= 0, 0.5, 1) HEA coatings by laser cladding on 904L stainless steels. The microstructures, microhardness, wear resistance and corrosion resistance were tested and analyzed. The results indicated that the phase structures of the coatings were composed of FCC plus TiN and a few Laves phases.The microstructure of the coating with no Ti consisted of columnar crystal while irregular dendritic and granular TiN ceramics as well as a few Laves phases were present in the Ti-containing coating. The hardness and wear resistance of the Ti-containing coating were much better than those of the substrate, more than 3 times of hardness and approximately 1/3 of wear mass whenx= 1. However, excessive Ti content could produce TiN particles and Laves phases, forming corrosion micro-cells and increasing corrosion tendency.
In another literature report[79], the microstructure,hardness, and corrosion properties of as-cast Al0.5Co-CrFeNi alloy, as well as Al0.5CoCrFeNi alloys aged at temperatures of 350, 500, 650, 800, and 950 ℃ for 24 h, were investigated. The results revealed that the microstructure of as-cast Al0.5CoCrFeNi comprised an FCC solid solution matrix and droplet-shaped phases(Al-Ni rich phases). After aging between 350 and 950℃, the microstructure transformed to FCC+ BCC solid solution with a matrix, droplet-shaped phases (Al-Ni rich phase), wall-shaped phases, and needle-shaped phases (Al-(Ni, Co, Cr, Fe) phase). This was because the aging process induced a spinodal decomposition reaction which reduced the amount of the Al-Ni rich phase in the aged microstructure and increased the amount of the Al-(Ni, Co, Cr, Fe) phase. However,there was no explanation on the specific components of the droplet-shaped phases, wall-shaped phases, and needle-shaped phases. The hardness of the Al0.5Co-CrFeNi alloy increased after aging, and the maximum hardness occurred in the aging temperature range of 350-800 ℃. Both the as-cast and aged specimens were considerably corroded when immersed in a 3.5% NaCl solution. Moreover, the Al-Ni rich phase was a sensitive zone, because Cl-ions preferentially attack it.
Qiuet al[80]cladded mixed Al, Cr, Fe, Cu and Co powder on Q235 steel to prepare HEAs coating by laser cladding method. Then, the microstructure and corrosion resistance property of the coating were researched.The results showed that the coating had excellent corrosion resistance compared to the substrate, because the content of rich Cr phase in the alloy was more than the passive critical alloy composition (18% Cr), resulting in its good corrosion resistance. Along with the increase in scanning speed, alloy corrosion resistance performance showed an enhancement initially and then deteriorated. The authors opined that the change in scanning speed likely induced the corresponding change in the size of grain, dendrite spacing, precipitation phase and ordered domains. When the scanning speed was too fast, the convection increased and the cladding layer surface was rough, resulting in poor corrosion resistance. However, no corresponding verified microstructure analysis was provided in this study. In addition, as the material was just a mixture of Al, Cr,Fe, Cu and Co powders, severe elemental segregation in the prepared coating was inevitable, which was detrimental for the corrosion resistance in our opinion.
Leeet al[81]studied the influence of Al concentration on the microstructure and corrosion of AlxCrFe1.5MnNi0.5alloys in H2SO4+ NaCl solutions. It was observed that an increase in the Al content increased the corrosion current density (icorr) values of this HEA in 0.5 M H2SO4. The microstructure of this HEA changed from FCC to FCC+BCC, and to BCC finally.The authors proposed that the FCC-type microstructure was more corrosion-resistant than the BCC-type microstructure. However, the opposite conclusion was presented in another literature report[82]. Thus, it is unclear how effective the addition of Al is for the corrosion resistance of HEAs. The effects of Al on the corrosion mechanism of Al-containing CCAs are not yet well clarified, as mentioned in another study[83], in which the effects of Ti, Cu, Cr, Mo, Ni, B and Sn on the corrosion resistance of HEAs were summarized and reviewed.
Yuet al[84]researched the corrosion resistance of as-cast and various tempered AlCoCrFeNiTi0.5HEAs in 3.5% NaCl solution. The results showed that the corrosion resistance of all tempered alloys was better than that of as-cast alloy. This was because a lot of defects such as vacancy, dislocation and grain segregation existed at the interface between dendrite, eutectic structure and grain boundary during alloy solidification. Tempering is beneficial to release the defects and the strain energy at the interface of eutectic structure and grain boundary, and the best temperature was 700°C. When the tempering temperature was greater than or equal to 800 °C, the corrosion resistance decreased again due to the obvious microstructure change resulting in the formation of new defects. However, this explanation is a bit confusing, as there was no explicit description of new defects, and the dendrite grain became smaller and element segregation became weaker when the tempering temperature was up to 800 ℃. In addition, the research results of Xieet al[85]revealed that the corrosion resistance of AlFeCrCoCu alloy was the best when tempered at 800 ℃, while the ideal tempering temperature was 700 ℃ for NiCrCoTiV alloy[86].Thus, proper heat treatment can improve the corrosion resistance of HEAs.
In addition, lightweight structure materials with high specific strength are always needed in the aerospace industry. Hence, the high temperature oxidation resistance is important to be researched. Butleret al[87,88]fabricated a series of AlNiCoCrFe HEAs, and studied their oxidation resistance mechanism. They found that several oxidation products such as Cr2O3,Al2O3, NiCr2O4and AlN were formed after 50 h high temperature oxidation of Alx(NiCoCrFe)100-x(x=8, 10,12, 15, 20, 30) HEAs in air at 1 050 ℃. For the alloy with low Al content (x=8, 10), two layers of oxidation products were formed, including inner layer composed of discontinuous Al2O3and precipitated AlN, and outer cladding layer of Cr2O3. For the alloys withx=12,15,the compositions of inner layer changed to semi-continuous precipitates gradually, and spinel NiCr2O4was formed in outer layer except Cr2O3. With the further increase in Al content, the inner layer was composed of continuous Al2O3and small amount of AlN, and the outer layer was Cr2O3. Increasing the Al content promoted the high temperature oxidation resistance of the alloy, mainly attributed to the formation of continuous Al2O3film with high Al content. According to a previous report[77], both AlSiTiCrFeCoNiMo0.5and AlSiTi-CrFeNiMo0.5coatings prepared by plasma spray method possessed good oxidation resistance at 1 000 ℃,though it deteriorated at 1 100 ℃. Two oxidation layers were formed composed of titanium oxide top layer and chromium oxide inner layer. Since titanium oxide could not provide good protection, it is believed that the oxidation protection was mainly due to chromium oxide.However, other research revealed that the sluggish diffusion effect of HEAs would hinder the formation of unstable transient oxides, and then promote the oxidation resistance[19,26,89]. However, as the light elements are always the main elements of LWHEAs, which are also relatively active, they would affect the corrosion resistance and high temperature oxidation resistance of the HEAs. Thus, it was difficult to obtain LWHEAs by considering the mechanical properties, corrosion resistance and high temperature oxidation resistance at the same time. No such alloy was reported so far.
According to the above studies, although the HEA is a new type of alloy, its strengthening mechanism and performance improvement methods are actually the same as those of traditional alloys. Adding light elements such as Al, Mg, Li, Ti,etc.or increasing the content of these elements can reduce the density of HEAs. The corrosion resistance of HEAs can be improved by adding Cr, Mo, Ti,etcor increasing their contents. Adding ceramic particles or forming hard phase can improve the wear resistance of alloy.Transformation from FCC to BCC can be induced to improve the strength and hardness of the alloy, and the plasticity of the alloy is improved on the contrary. The main strengthening mechanism of high entropy alloy is solution strengthening, and fine grain strengthening and dispersion strengthening are equally effective. In fact,the reason why most of the processing methods and technologies can improve the performance of alloy is the result of these strengthening mechanisms, just like heat treatment adopted in previous work[47,52,84-86].
2.3 Preparation methods of LWHEAs
The preparation methods of HEAs can mainly be divided into liquid forming, powder metallurgy,and vapor deposition. Liquid forming methods mainly consist of melting and smelting processes, such as vacuum arc melting, vacuum induction melting, and laser cladding. Vacuum arc melting is the most common processing method to produce rod, strip, and block HEAs.The main method of powder metallurgy is based on mechanical alloying to obtain nanocrystalline or amorphous particles with uniform structure and component distribution. Vapor deposition is a surface modification technology, mainly based on magnetron sputtering to produce HEAs films. Though HEAs may contain many elements, their preparation processes are not very different from those of traditional alloys. Table 1 summarizes the main LWHEA systems in terms of crystal structure, density, and preparation process.
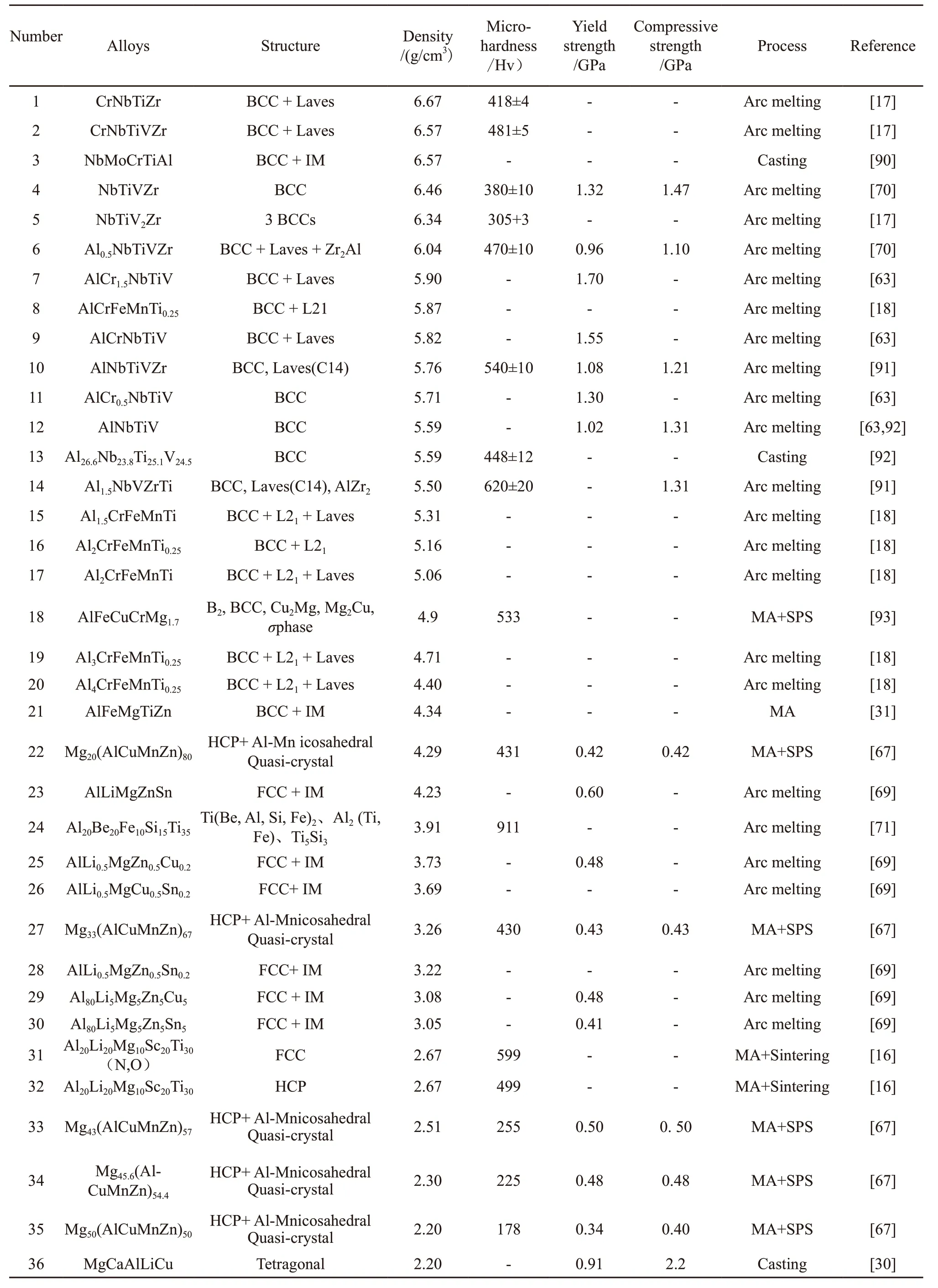
Table 1 Density, preparation method, crystal structure and mechanical properties of typical lightweight high-entropy alloys in recent years
2.3.1 Mechanical alloying
Mechanical alloying (MA) is based on mixing powders of various elements to prepare HEAs according to designed composition proportion. The mixture is subjected to high energy ball milling to allow powder particles to go through impact, shear, compression,friction, and other forces to yield HEAs powder. Note that MA can not only yield single-phase FCC or BCC phase HEAs powder but could also lead to BCC and FCC dual-phase HEAs powder. Compared to traditional process, mechanical alloying is the most commonly employed method to prepare HEAs powders. This is not only due to high yield and simple processing but also because powders that are difficult to mix under normal circumstances can be readily synthesized.However, mechanical alloying suffers from several disadvantages, such as long milling time, unstable microstructure of fabricated HEA powders, poor sphericity of particles, and powders that could easily be polluted.In addition, the fabricated powder needs to be used in combination with other HEA preparation technologies.2.3.2. Vacuum arc melting
Vacuum melting is mainly employed for the preparation of HEAs[94,95]. The process consists of placing pure metal in a crucible according to a certain design proportion. After vacuuming the chamber, argon gas is passed through, followed by arc discharge melting. After melting and mixing of all alloys evenly,they are cast into certain shapes. Vacuum melting has been adopted to prepare HEAs at melting temperatures reaching 3 000 ℃. Hence, most alloy elements of LWHEAs can be melted, benefiting the removal of volatile impurities and some gases. In addition, the crystal structure of ingots can be improved while being purified. However, the shape and size of samples are limited by the crucible, constituting the main disadvantage of vacuum arc melting.
2.3.3. Vacuum induction melting
Vacuum induction melting consists of melting metal in crucible under electromagnetic induction heating. The filling sequence and adjacent relationship between element blocks or powders are very important in successful process. In other words, raw material blocks with lower melting points should be placed at the bottom of the crucible to facilitate melting of raw materials with high melting points. On the other hand, element blocks that are easy to combine with each other should be separated to prevent the formation of refractory compounds. To avoid oxidation of raw materials and alloys after smelting, full vacuuming (or charge argon followed by several times vacuuming) before electric smelting should be implemented. Meanwhile, the electromagnetic suspension coil can be used for suspension smelting to improve the purity of HEAs by preventing contamination of alloy liquid by elements present in the crucible, constituting the biggest advantage of vacuum induction smelting.
2.3.4 Magnetron sputtering
Magnetron sputtering is a thin film preparation method that uses high-energy particles to bombard the surface of materials, so that surface atoms or particles can be separated from the material surface to finally deposit on the substrate. Magnetron sputtering is often used to prepare HEAs films. Sputtering methods include balanced magnetron, unbalanced magnetron,reactive magnetron, intermediate frequency magnetron, and pulse magnetron sputtering. The advantage of magnetron sputtering is its suitability for a wide range of materials. However, it is still limited by complexity of equipment and high preparation cost[96-98], as well as poor stability of the microstructure of the prepared HEAs film.
In addition to the above-mentioned methods, Liet al[99]successfully synthesized AlxCoCrFeNi LWHEAs by combustion method. These HEAs were produced through some highly exothermic aluminothermic reactions and could be separated from the ceramic ingot by ultra-high gravity. This new preparation method can greatly shorten the processing cycle and reduce energy consumption during production process of high-performance materials.
3 Challenges of future LWHEAs development
Theoretically, the definition of HEAs is still under debate. Until now, no uniform and clear definition about the density of LWHEAs, and no accurate prediction criterion for phase formation have been provided.Most current phase formation criteria cannot be used to accurately predict phase formation. The number of phase formation does not conform to Gibbs phase law.The sluggish diffusion effect contradicts the Gibbs-Andam and Einstein-Stokes equations. It is controversial because it is difficult to measure. Both Paul[100]and Miracle[51]concluded that the diffusion coefficient of Co-Cr-Fe-Mn-Ni HEAs was larger than that of traditional alloy by using the methods and data from previous literature[89], in which contrary conclusion was obtained. These theoretical aspects restrict the development of HEAs in terms of material design, preparation,and engineering applications.
The current HEAs design to predict phase formation is mainly guided by simulation and calculation methods, such as CALPHAD simulation, density functional theory calculation, and initial molecular dynamics simulation. However, the determination of optional elements and their contents in practical design depends mainly on research experience. In fact, no complete technologies or methods for practical design of HEAs are currently available.
The LWHEAs must contain a number of light elements. However, most light elements, such as Al, Mg and Li can easily react with the environment, making the synthesis process more challenging. Meanwhile, the melting points of light elements, such as Mg and Li are low and their vapor pressure is high. They also can explode, making the formation process of LWHEAs more difficult.
Table 1 reveals that current preparation methods of LWHEAs are mainly based on arc melting and mechanical alloying. Also, most prepared HEAs were powders or small volume ingots. Composition segregation may easily occur during smelting preparation process. However, no mature technology and method for preparation of large-size HEAs for engineering application has so far been reported, such as homogenization and large dimensions ingot, bar and wire.
4 Future development of LWHEAs
HEAs based on entropy design concept are characterized by high entropy. However, current HEAs formation criterions have few criterions related to mixed entropy. Hence, it is important to clarify the role of entropy in phase formation and properties of resulting HEAs. This could be clarified by multi-component alloy phase formation. Current research theories of Gibbs phase law, Gibbs-Andam equation and Einstein-Stokes formula are all developed for traditional single master alloys. Based on design concept of enthalpy, the influence of configuration entropy on solidification and formation process of single principal component alloys can be ignored. In addition, the system components can be distinguished as solvent and solute. On the other hand, in multi-principal component alloy systems, the influence of configuration entropy is significant and cannot be ignored. No obvious distinction between the solvent and solute can be made in terms of composition. Therefore, phase transformation and element diffusion behaviors of multi-principal component alloys during solidification process are inevitable and quite different from those of traditional alloys. In-depth studies of phase equilibrium conditions of multicomponent alloys should be performed to clarify the diffusion and migration mechanisms of elements under phase equilibrium conditions of multicomponent alloys. The solid solution strengthening and deformation mechanisms of HEAs should be clarified to improve relevant alloy research theories, such as Gibbs phase law, Gibbs-Andam equation and Einstein-Stokes formula, as well as establish and improve the multi-component alloy databases required in order to provide guidance for design of novel HEA materials.
Among phase formation prediction models, very few HCP phase prediction models exist. Also, few HCP phase structured HEA systems have been designed and prepared. Hence, prediction models of HCP phase structures should be established along with design and preparation of corresponding high entropy alloys.
On the other hand, although many studies have focused on HEAs, the fundamental governing laws remain unclear. Some studies have reported the structure and properties of HEAs in view of disputes related to definition of HEAs. As shown in Fig.8, the development of HEAs could be divided into two generations.Also, the characteristics of each generation HEAs and traditional alloys have been summarized and compared(Table 2). Thus, aspects related to the development of HEAs, the definition of HEAs, generation of HEAs,and structure and property characteristics of each generation would require future in-depth study.
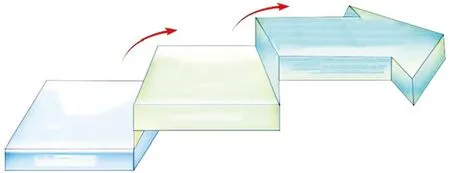
Fig.8 The evolution of alloys[101]
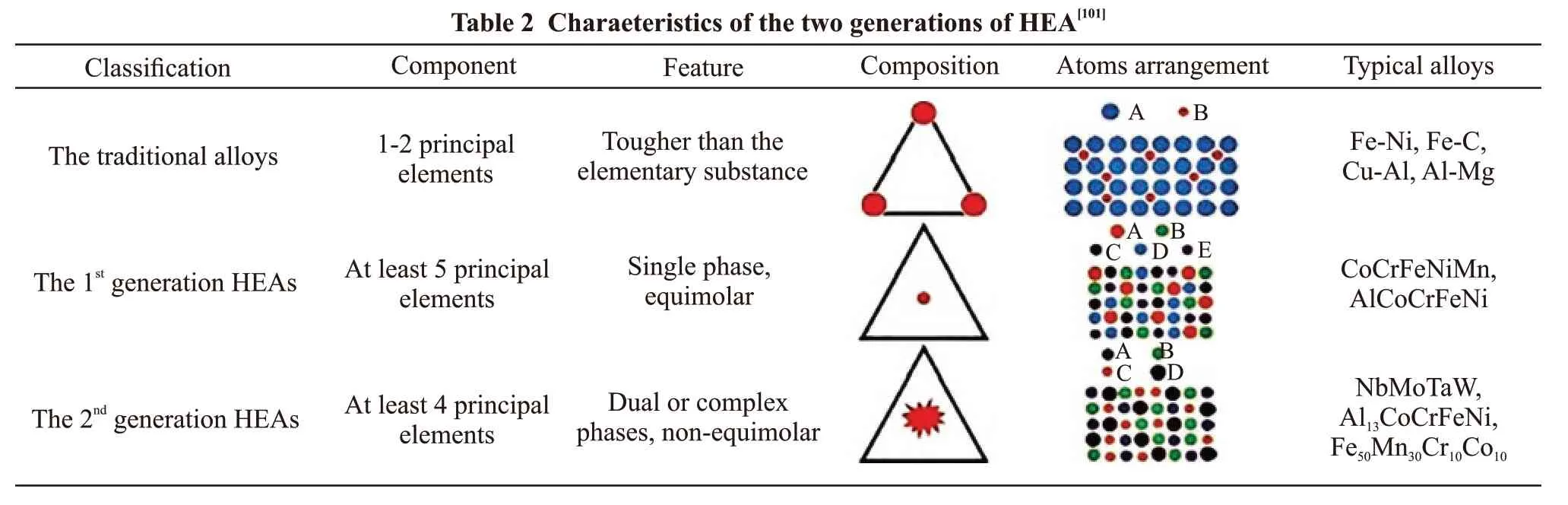
?
Applications are the ultimate goal of newly developed materials. Engineering preparation methods are conducive to reduce the cost and promote the applications of materials in various fields, as well as clarification of related theories. Current preparation methods of HEAs do not actually meet the needs of engineering applications. Therefore, development of new preparation methods could solve these problems. To this end,strengthening the combination of forming technologies,such as powder metallurgy combined with microwave sintering, casting and spray forming could lead to better materials. Meanwhile, the integrated forming of HEAs based on 3D printing technology is important in future developments, in which composition segregation problem can be solved during solidification process.
In addition,in-situpreparation of HEAs coatings and thin films on substrate surfaces should be developed to promote the application of HEAs in equipment remanufacturing. Remanufacturing is an efficient method to prolong the life of equipment and save resource and energy, and it is better to promote the development of recycling economy.
LWHEAs with superior strength, high-temperature resistance or ultra-low temperature are future development trends. Light-weight is highly desirable for equipment in various industries. With the demand for social development, the adaptability requirements of equipment under extreme conditions, such as ultra-low or ultra-high temperature environment become mandatary. Therefore, the performance of materials under extreme conditions has gained increasing attention.Hence, high strength, elevated temperature resistance or ultra-low temperature materials like LWHEAs are future desirable materials for many applications fields.
5 Conclusions
HEAs are alloys composed of many elements.Basic theories explaining the characteristics of HEAs are still lacking and more work is required to clarify the differences and change in phase stability behavior and properties of different HEAs. Meanwhile, despite the large number of possible components in HEAs, only a few are proved useful. However, HEAs are alloys based on entropy design concept, which is different from traditional enthalpy design concept to form new interesting materials. It is a revolution in the concept and method of material design. It is believed that more and more HEAs will emerge with the improvement of the basic theory and preparation technologies of HEAs to meet the development needs of human society. Particular attention should be paid to LWHEAs for their wide usage in various fields.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Poly(dopamine)-assisted Bioactive Coating on the Surface of Porous Poly (Ether Ether Ketone) to Promote Osteogenic Differentiation of rBMSC
- Influence of Heat Treatment on Microstructure and Mechanical Properties of Plasma Sprayed FeCrMoCBY Amorphous Coatings
- Lamella Multiple Grained Structure Making 2205 Duplex Stainless Steel with Superior Strength and Ductility
- Synthesis and Characterization of Polyaniline/MgTiO3 Composite with Excellent Thermal and Electrochemical Performance
- Synthesis and Characterization of Hyperbranched Epoxy with Terminal Ally Group and Its Application of Toughen Bismaleimide
- Loose Sand Cemented by Microbial Cementitious Material:Composition, Microstructure and Mechanical Properties
