Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
2021-05-24YingyinXuYuanhuiLiYuxiaoLuXiaoinFengGuotingTianQinghongLiu
Yingyin Xu, Yuanhui Li, Yuxiao Lu, Xiaoin Feng, Guoting Tian, Qinghong Liu,*
a Soil and Fertilizer Research Institute, Sichuan Academy of Agricultural Sciences, Chengdu 610066, China
b Department of Vegetables, College of Horticulture, China Agricultural University, Beijing 100193, China
c Department of Environment and Chemical Engineering, Tangshan College, Tangshan 063000, China
d Institute of Biotechnology and Germplasmic Resource, Yunnan Academy of Agricultural Science, Kunming 650223, China
Keywords:Lepista sordida Polysaccharide Antioxidant activity Hepatoprotective effects
ABSTRACT A novel alkali-soluble polysaccharide from Lepista sordida (LSAP) mycelia with antioxidative and hepatoprotective activities was characterized.The weight-average molecular weight and number-average molecular weight of LSAP were 1.442 × 103 and 6.05 × 102 kDa, respectively.LSAP was consisted of glucose (57.9%), xylose (31.8%), and small amounts of rhamnose, arabinose, galactose, glucuronic acid, and galacturonic acid (1.2%-3.1%).The FT-IR and 2D NMR confi rmed that LSAP was composed of Xylp, Araf, 4-O-Me-α-D-GlcpA, (1→4)-linked β-D-Glcp, and (1→4)-α-D-GalA, and β-glycosidic linkages between these sugar units.LSAP displayed notable effects on 1,1-dephenyl-2-picryhydrazyl (DPPH) radical scavenging, hydrogen peroxide scavenging, lipid peroxidation inhibitory ability, reducing power and Fe2+ chelating property.These biological effects were further verifi ed by suppressing CCl4-induced oxidative liver damage in mice at doses of 100 and 200 mg/kg.Administration of LSAP in mice prior to CCl4 signifi cantly prevented the CCl4-induced elevation in serum alanine aminotransferase, aspartate aminotransferase, and hepatic malondialdehyde.Mice treated with LSAP demonstrated to increase activities in superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the liver.We also found out that LSAP prevented CCl4-induced oxidative liver histological alteration.LSAP may exert hepatoprotective effects against CCl4-induced damage through antioxidant mechanisms in model mice.
1.Introduction
Reactive oxygen species (ROS) are critical in molecular biology and medicine.ROS are natural byproducts of normal oxygen metabolism, but are involved in several pathological conditions such as cardiovascular disease, diabetes, aging, and carcinogenesis [1-3].The liver is a vital organ involved in metabolism and detoxifi cation, and the liver can suffer from oxidative stress due to elevated ROS levels.This can progress to subclinical icteric hepatitis to hepatic fibrosis, cirrhosis, and hepatocellular carcinoma [4].Carbon tetrachloride (CCl4) is a classical hepatotoxin that undergoes reductive dehalogenation by cytochrome P450 to form trichloromethyl-free radicals (-CCl3or CCl3OO-).Formation of these radicals initiates the process of lipid peroxidation and depletion of antioxidant enzymes in the hepatic parenchyma cells [5].A variety of antioxidants have been investigated in regard to protection the body from damaging oxidation reactions.There has been increasing interest in naturally occurring antioxidants in view of the adverse side effects associated with synthetic antioxidants [6].
Mushrooms are produced by at least 14 000 and perhaps up to 22 000 species.Many mushrooms contain bioactive compounds, typically polysaccharides or triterpenes.Current studies suggest that mushrooms have great potential for the production of useful bioactive metabolites, and they are a prolific resource in drug development [7].Unfortunately, relatively few mushrooms have been molecularly profiled, and much work remains to fully utilize mushrooms.Lepista sordidais a species of mushroom in the northern hemisphere and that forms so-called fairy rings [8].However,L.sordidahave not been domesticated, and studies on their bioactive compounds have begun only recently.Miao et al.[9]purified 4 polysaccharides from the fruit body ofL.sordidaand demonstrated their effects against human laryngocarcinoma.Zhong et al.[10]explored the anti-aging activities of mycelial polysaccharide fromL.sordida.
Here, we purified and characterized an alkali-soluble polysaccharide fromL.sordidamycelia (LSAP), further evaluated its antioxidant and hepatoprotective effect usingin vitrobiochemical tests and a mouse model ofin vivoCCl4intoxication.Our findings demonstrate for the first time the strong antioxidant and hepatoprotective effects of LSAP, and suggest LSAP has great potential as a raw material for functional food additives because of these effects.
2.Materials and methods
2.1 Materials and reagents
Diagnostic kits for activity assays of alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).Linoleic acid, ascorbic acid, DPPH, and monosodium salt of (2-pyridyl-)-5,6-diphenyl-1,2,4-triazine-4’4’’-disulfonic acid (ferrozine) were obtained from Sigma-Aldrich Co.(Steinheim, Germany).Bifendate (dimethyl 7,7’-dimethoxy-[4-4’-bibenzo[d][1,3]dioxole]-5,5’-dicarboxylate) was obtained from Beijing Union Pharmaceutical Factory.All other chemicals were of analytical grade and obtained from Beijing Chemical Co.(Beijing, China).
2.2 Purification of L.sordida polysaccharide
TheL.sordidaculture was grown in a 250 mL flask containing 50 mL medium (lactose 40 g/L, KH2PO40.5 g/L, MgSO40.5 g/L, soybean meal 10 g/L, distilled water) at 24 °C on a rotary shaker (160 r/min) for 29 days.The mycelia were harvested by centrifugation (8 000 ×g, 4 °C, 30 min).After the mycelia were washed three times with distilled water, and then dried to a constant weight at 60 °C.
The dried mycelia were crushed into powder with a disintegrator.The powder (100 g) was extracted twice with ethanol (1 L) to remove the lipids.After the residue being dispersed in water (1:18,m/V) using ultra-sonication for 10 min, put in water bather at 90 °C for 3 h.The supernatant (water-soluble polysaccharides) was discarded after centrifugation (9 000 ×g, 4 °C, 10 min).The residue was washed with distilled water and centrifugated at 9 000 ×gfor 10 min, to remove the water-soluble polysaccharide adhering on the surface of powder.Then the residue was extracted with 0.5 mol/L NaOH (1:18,m/V) at room temperature for 2 h to let the LSAP released.After centrifugation (9 000 ×g, 4 °C, 10 min), the supernatant was precipitated with ethanol (1:5,V/V) at 4 °C for 6 h.The precipitate was collected by centrifugation (9 000 ×g, 4 °C, 10 min) and deproteinatedviathe Sevage method [9].The deproteinated supernatant was extensively dialyzed against distilled water (MWCO 3500 Da) to remove the small molecular compounds (e.g.flavonoids and polyphenols) and lyophilized to yield crudeL.sordidapolysaccharides [11].
CrudeL.sordidapolysaccharides as described above were dissolved in distilled water, centrifuged, and supernatant was applied to a 6 × 24 cm column of DEAE sepharose (GE Healthcare; Sweden).The column was eluted successively with distilled water and 1 mol/L NaCl at flow rate of 10 mL/min.The unbound fraction eluted with distilled water following lyophilization was subjected to gel filtration on a Superdex 75 HR 10/30 column (GE Healthcare; USA) using AKTA purifier (Amersham Biosciences; Sweden) at flow rate of 0.5 mL/min.Fractions were collected with an automated fraction collector and monitored by phenol-sulfuric acid method at 490 nm [11].The major fraction (termed LSAP) was collected and lyophilized (Fig.1).
2.3 Structural characterization of LSAP
2.3.1 Molecular weight
Weight-average (Mw) and number-average (Mn) of molecular weights on LSAP were determined by gel permeation chromatography (Agilent 1200; USA) using an UV detector at 280 nm on a PL-gel (10 mm) Mixed-B column (i.d.7.5 mm), calibrated with polystyrene standards.4 mg LSAP was dissolved in 2 mL distilled water, filtered, and 20 μL solutions were injected by automatic sampler.The column was operated at room temperature, with flow rate 0.5 mL/min [12].
2.3.2 Monosaccharide composition
Carbohydrate analysis of LSAP sample was performed using a high-performance anion-exchange chromatography (HPAEC) system (model ICS3000, Dionex; USA) with pulsed amperometric detector and anion exchange CarboPac PA-1 column (4 × 250 mm).The remaining sugars associated with LSAP were studied by hydrolysis with dilute sulfuric acid by the standard method of the National Renewable Energy Laboratory (CO, USA), with calibration using standard solutions ofL-rhamnose,L-arabinose,L-glucose,L-galactose,D-galacturonic acid,D-glucaric acid, andD-xylose [12].
2.3.3 FT-IR spectroscopy
FT-IR spectrum of LSAP was collected using an FT-IR microscope (model iN10, Thermo Nicolet Corp.; Madison, WI, USA) equipped with liquid nitrogen-cooled MCT detector.Spectrum was recorded from 4 000 cm-1to 650 cm-1with 4 cm-1resolution and 128 scans [12].
2.3.4 Two-dimensional heteronuclear single quantum coherence nuclear magnetic resonance (2D HSQC NMR) spectroscopy
2D HSQC NMR spectrum was collected on an Avance 400-MHz spectrometer (Bruker) fitted with 5-mm gradient probe with inverse geometry (proton coils closest to sample).LSAP (40 mg) was dissolved in 0.5 mL H2O-d6, and the central solvent peak at δH4.79 ppm was used as internal reference.Standard Bruker implementations of 2D (gradient-selected, 1H-detected HSQC) NMR experiment was used for structural characterization and assignment authentication [12].
2.4 Analysis of antioxidant activities using in vitro systems
2.4.1 DPPH radical scavenging activity
The activity was measured with the method reported by Kao et al.[13]with slight modifications.LSAP (0-5 mg/mL, 200 μL) was mixed with 600 μL DPPH solution (0.004% in methanol), shaken thoroughly, left for 30 min in the dark, and absorbance was measured at 517 nm using a UV-visible spectrophotometer (Thermo Scientific; USA), with ascorbic acid as reference compound.Assays were performed in triplicate.DPPH radical scavenging activity was calculated as:
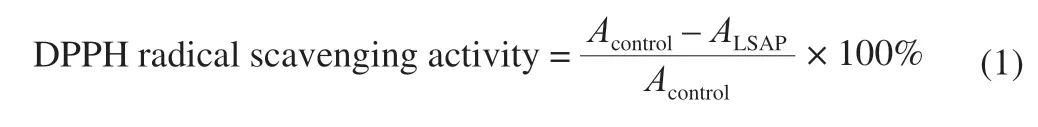
whereAcontrolis absorbance of control (water used instead of LSAP), andALSAPis absorbance of LSAP.
2.4.2 Hydrogen peroxide (H2O2) scavenging activity
The ability to scavenge H2O2was measured as Wang et al.[14]with minor modifications.The 200 μL phosphate buffer (0.1 mol/L, pH 7.4), 40 μL H2O2solution (0.3%), and 100 μL LSAP solution (0-5 mg/mL) were mixed together, left for 10 min, and absorbance was measured at 234 nm.H2O2scavenging activity (%) was calculated in the same way as described in Equ (1).
2.4.3 Reducing power
Reducing power of LSAP was measured by the method of Tseng et al.[15].LSAP solutions at various concentrations (each 1 mL) were mixed with 1 mL 0.2 mol/L phosphate buffer saline (pH 6.6) and 1 mL 0.1% (m/V) K3Fe(CN)6solution, incubated at 50 °C for 20 min.Next, 1 mL 10% (m/V) trichloroacetic acid solution was added and centrifuged at 3 000 ×gfor 10 min.1 mL of resulting upper layer was combined with 1 mL distilled water and 0.2 mL 0.3% FeCl3solution, and absorbance was measured at 700 nm, with ascorbic acid as positive control.Higher absorbance of reaction mixture indicated greater reducing power.
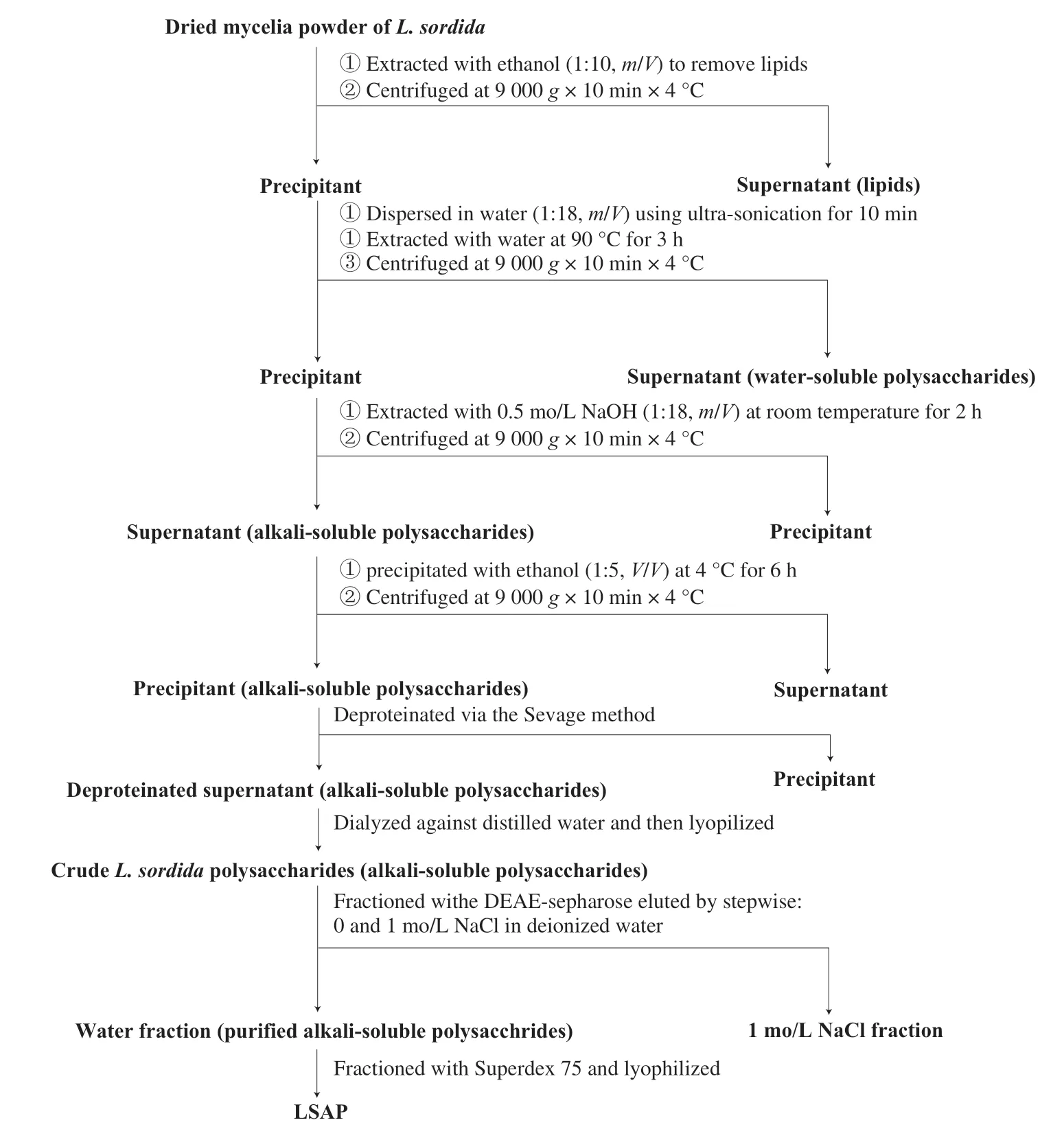
Fig.1 Scheme for LSAP from L.sordida.
2.4.4 Inhibitory effect on lipid peroxidation
The inhibitory effect on lipid peroxidation was determined in accordance with the conjugated diene method using a few modifications [16].LSAP (0-5 mg/mL, in 25 μL distilled water) was mixed in a test tube with 500 μL 10 mmol/L linoleic acid (Sigma-Aldrich Chemical Co.; St.Louis, MO, USA) emulsion in 0.2 mol/L sodium phosphate buffer, and left in the dark for 15 h at 37 °C to accelerate oxidation.1.5 mL of 60% methanol in distilled water was added, and absorbance was measured at 234 nm against a blank.Inhibitory effect on lipid peroxidation (%) was calculated in the same way as described in Equ (1).
2.4.5 Ferrous (Fe2+) ion chelating activity
Chelating effect on Fe2+ions was measured by the method of Lu et al.[17]with some modifications.LSAP powder (0-5 mg/mL in 200 μL distilled water) was mixed with 550 μL distilled water and 10 μL of 2 mmol/L ferrous chloride, and reaction was initiated by addition of 40 μL of 5 mmol/L ferrozine (Sigma-Aldrich).The reaction mixture was left for 10 min at room temperature, and absorbance was measured at 562 nm, with ethylenediaminetetraacetic acid disodium salt (EDTA·2Na) as reference.Fe2+chelating activity (%) was calculated in the same way as described in Equ (1).
2.5 Animal experiments
Male Kunming mice (18-22 g) from Beijing HFK Bioscience Co.(Beijing) were used for CCl4experiments.Prior to experiments, animals were acclimated for 1 week with ad lib access to food and water, temperature (25 ± 2) °C, humidity (55 ± 5)%, and 12 h light/12 h dark cycle [17].All experiments were performed in accordance with regulations of Experimental Animal Administration issued by the State Committee of Science and Technology, P.R.C.(IACUC No.CAU20080420-5).
2.6 In vivo hepatoprotective activity
Mice were allocated randomly into 5 groups (I-V), each withn= 7: Group I, normal control; Group II, administered CCl4only; Group III, CCl4+ bifendate (200 mg/kg); Group IV, CCl4+ LSAP (100 mg/kg); Group V, CCl4+ LSAP (200 mg/kg).Animals in Group I and II were given a single dose of distilled water (0.2 mL, i.g.) daily.A positive control Group III was treated with CCl4+ bifendate (200 mg/kg in distilled water, i.g.).Groups IV and V were pretreated with CCl4+ LSAP (100 or 200 mg/kg, i.g.) once per day for 10 days.On day 11, animals in all groups except I were treated with a CCl4/olive oil mixture (0.2%, 12 mL/kg, i.g.) 2 h after the last administration, while Group I received olive oil alone [17].All animals were sacrificed by anesthesia 16 h after this final treatment.
2.7 Biochemical assays
Serum was obtained by centrifugation (664 ×g, 10 min) of collected blood.Serum ALT and AST activities were measured using commercial diagnostic kits, with results expressed as international units per liter (IU/L).
Livers were removed, weighed, and homogenized in cold physiological saline (10%,m/V).Homogenate was centrifuged (461 ×g, 10 min), and supernatant was subjected to MDA, SOD, and GSH-Px analyses using commercial diagnostic kits.MDA, SOD, and GSH-Px activities were normalized relative to protein, with results expressed respectively as U/mg protein, nmoL/mg protein, and U/mg protein.
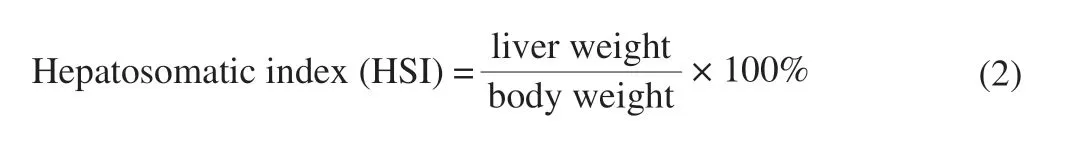
2.8 Histopathological studies of liver
2.8.1 Light photo-microscopy
Fresh liver samples were cut rapidly into 5 mm pieces, which were then fixed in 10% formalin solution at room temperature for 24 h.Fixed tissues were dehydrated by ethanol gradient, embedded in paraffin, and cut into 5-6 μm sections.The sections were placed on slides, stained with hematoxylin-eosin (H&E), and examined on a light photomicroscope (magnification 20 ×).
2.8.2 Transmission electron microscopy (TEM)
Liver tissues were fixed in 2% glutaraldehyde overnight at 4 °C and then in 1% osmium tetroxide buffer for 2 h.Slides were dehydrated by ethanol gradient, infiltrated with propylene oxide, and embedded in polymer resin (Spurr).Sections were stained with Sato’s triple lead citrate solution and examined by TEM (model JEM-1230, JEOL; Tokyo, Japan) at 80 kV.
2.9 Statistical analysis
Experiments were performed using a completely randomized design, and results were expressed as means ± SD (standard deviation).Data were analyzed by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests, using the SPSS software program, V.20.0.Differences between means withP< 0.05 were considered statistically significant.
3.Results
3.1 Physicochemical properties of LSAP
3.1.1 Molecular weight and monosaccharide composition
Mw and Mn values of LSAP were 1 442 and 605 kDa, respectively, measured by gel permeation chromatography.The polydispersity (Mw/Mn) of LSAP was calculated as 2.384.
The monosaccharide composition of LSAP is presented in Table 1.The predominant monosaccharide component was glucose (57.9%) followed by xylose (31.8%); rhamnose, arabinose, galactose, glucuronic acid, and galacturonic acid (1.2%-3.1%) were minor monosaccharide components.These results indicated that this polysaccharide was a potential xyloglucan.
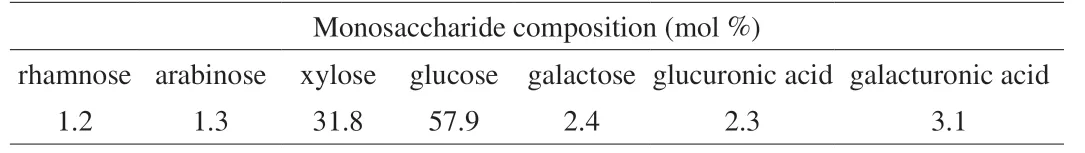
Table 1 Monosaccharide composition of LSAP.
3.1.2 FT-IR Spectrum
The FT-IR spectrum of LSAP is shown in Fig.2.The spectra shows 11 characteristic peaks, including a broad stretching intense peak characteristic of a hydroxyl group at 3 408 cm-1, a weak C—H stretching band at 2 927 cm-1, The bands at 1 647 and 1 414 cm-1were attributed to the antisymmetric and symmetric stretching vibrations of the C=O in the carboxyl group, and the bands at 1 037 cm-1were due to the stretch vibrations of C—O—C and C—O—H.Additionally, the small peaks at 897 and 798 cm-1indicate the existence ofα-glycosidic bonds [18,19].
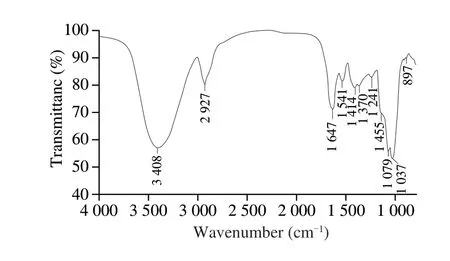
Fig.2 FT-IR spectrum of LSAP.
3.1.3 2D HSQC NMR spectrum
In the 2D HSQC NMR spectrum of LSAP (Fig.3), 6 cross-peaks of 5.34/99.9, 3.46/73.0, 3.66/72.8, 3.60/76.7, 3.88/70.0 and 3.68, 3.85/60.9 ppm were assigned to H-1/C-1, H-2/C-2, H-3/C-3, H-4/C-4, H-5/C-5 and H-6(a), H-6(b)/C-6 of the →4)-β-D-Glcp-(1→ residue.1H/13C chemical shifts at 5.08/101.3, 3.54/71.2, 3.92/73.4, 3.75/73.4, 3.80/71.3 and 3.84/68.8 ppm were assigned to H-1/C-1, H-2/C-2, H-3/C-3, H-4/C-4, H-5/C-5 and H-6/C-6 of →6)-α-D-Glcp-(1→ units, and cross peaks at 4.90/98.2, 3.17/74.2, 3.43/75.5 and 3.49/68.2 ppm were assigned to H-1/C-1, H-2/C-2, H-3/C-3 and H-4/C-4 of →6)-β-D-Glcp-(1→ units.The cross peaks at 4.47/102.6, 3.30/73.2, 3.58/74.8 and 3.72/76.2 ppm were assigned to H-1/C-1, H-3/C-3, H-4/C-4 of →4)-β-D-Xylan-(1→, while those at 3.50/70.0, 4.17/69.1, 3.63/69.2 and 3.70/67.8 ppm corresponded to H-2/C-2, H-4/C-4, H-5/C-5 and H-6/C-6 of →6)-β-D-Galp-(1→ residues existed in LSAP [19,20].
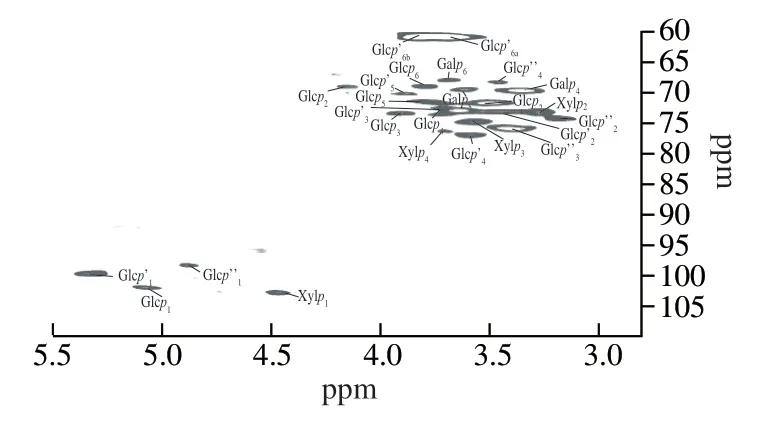
Fig.3 2D HSQC NMR spectrum of LSAP.Glcp: →6-)-α-D-Glcp-(1→; Glcp’: →4)-β-D-Glcp-(1→; Glcp’’: →6)-β-D-Glcp-(1→; Xylp: →4)-β-DXylan-(1→; Galp: →6)-β-D-Galp-(1→
3.2 In vitro antioxidant activity
3.2.1 DPPH radical scavenging activity
The DPPH radical scavenging activity of LSAP and ascorbic acid were shown in Fig.4A.The DPPH radical scavenging activity of LSAP was dose-dependent.At the highest concentration (5 mg/mL), LSAP (IC50=(2.75 ± 0.20) mg/mL) and ascorbic acid (IC50= (1.13 ± 0.06) mg/mL) scavenged 79.46% and 97.67% of the DPPH radicals, respectively.
3.2.2 Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging activity of LSAP was shown in Fig.4B.Compared with ascorbic acid (IC50= (0.77 ± 0.09) mg/mL),LSAP (IC50= (3.60 ± 0.39) mg/mL) showed moderate H2O2scavenging ability, and the percentage of H2O2scavenging increased from 12.83 to 62.23 for LSAP.More, LSAP manifested a dosedependent H2O2scavenging ability.
3.2.3 Reducing power
LSAP reduces the Fe3+/K3Fe(CN)6complex to Fe2+; consequently, the Fe2+could be monitored by measuring absorbance at 700 nm [21].Among 1-5 mg/mL, the concentration-dependent effect was obvious, and the ferric-reducing values of LSAP were from 0.24 to 1.14, respectively (Fig.4C).The data suggest that LSAP are strong antioxidants despite being inferior to ascorbic acid.
3.2.4 Inhibitory effect on lipid peroxidation
The inhibition of lipid peroxidation ability of LSAP was shown in Fig.4D.When tested at 5 mg/mL, LSAP and ascorbic acid elicited 77.59% and 98.00% inhibition of lipid peroxidation, respectively.The result indicated that the inhibition of lipid peroxidation ability of LSAP was dose-dependent.Moreover, LSAP (IC50= (0.69 ± 0.11) mg/mL)had less activity in inhibiting linoleic acid peroxidation compared with ascorbic acid (IC50=(0.04 ± 0.01) mg/mL).
3.2.5 Ferrous ion chelating activity
At a dosage of 5 mg/mL, LSAP and EDTA·2Na respectively chelated 46.79% and 99.76% of Fe2+ions (Fig.4E).The chelating effects increased with concentration.The Fe2+ion chelating activity of LSAP (IC50= (5.14 ± 0.64) mg/mL) was not as strong as EDTA·2Na (IC50= (0.57 ± 0.11) mg/mL).
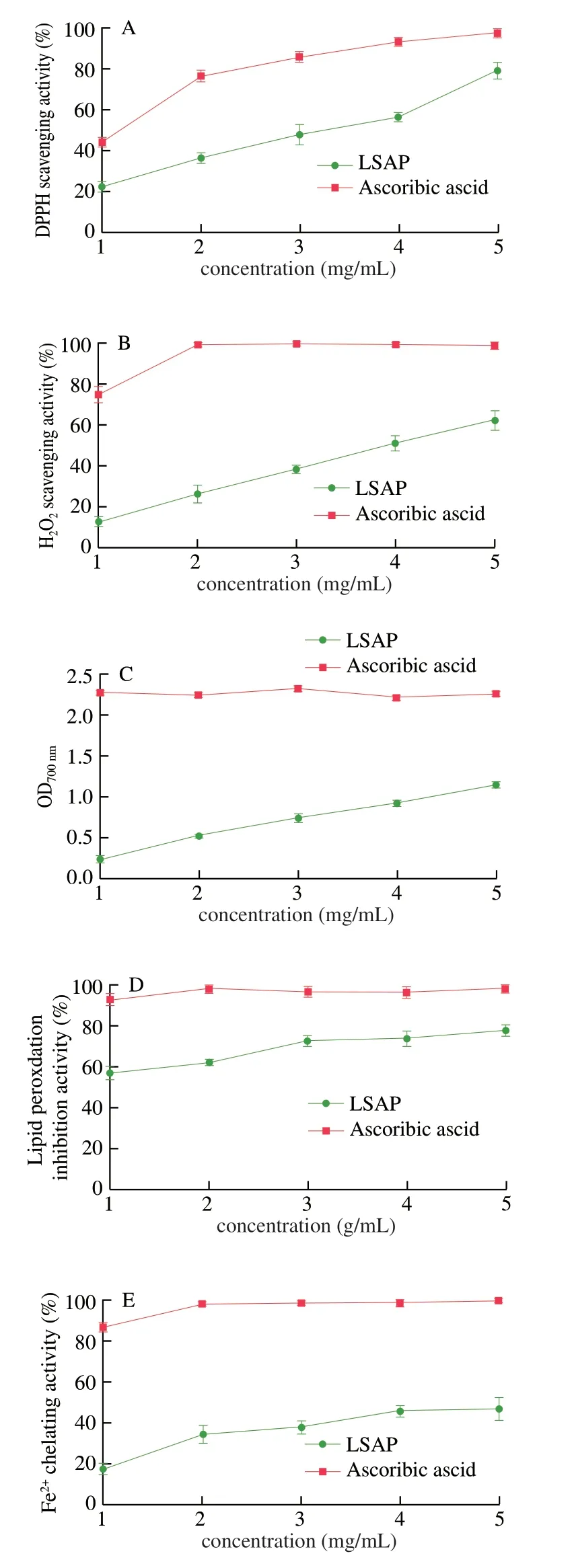
Fig.4 In vitro antioxidant effects of LSAP.(A) DPPH scavenging activity.(B) H2O2 scavenging activity.(C) Reducing power.(D) Inhibitory effect on lipid peroxidation.(E) Fe2+ ion chelating activity.Controls: ascorbic acid in A-D and EDTA·2Na in E.The values are presented as the mean ± standard deviation (n = 3).
3.3 Effects of LSAP on body weight, liver weight, and HSI in mice
The liver weight (P< 0.05) and HSI (P< 0.05) increased significantly after CCl4treatment versus normal control mice (Table 2).However, it could be mitigated by pre-treatment with LSAP.The results were similar to pre-treatment with the positive control bifendate.The HSI effects were dose-dependent.At higher doses (200 mg/kg),LSAP reached higher HSI values.There was a decrease in the body weight of CCl4treated groups (P< 0.05).These results agree with a previous study.Lu et al.[17]found the hepatoprotective effects of Huangshan Maofeng, a green tea produced in Anhui province, China.
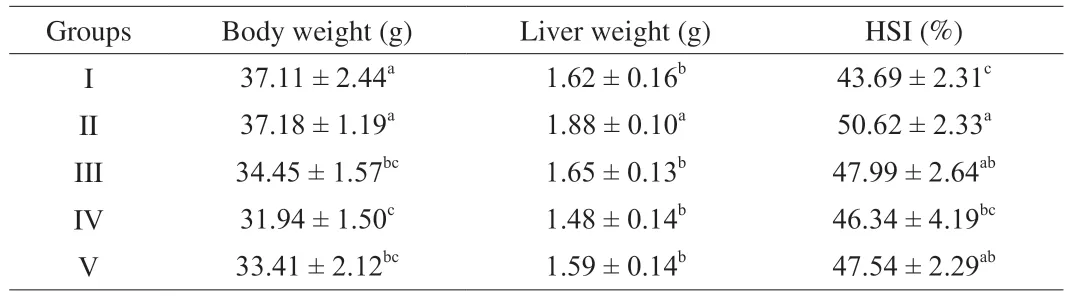
Table 2 Effects of LSAP on body weight, liver weight, and HSI in mice.
3.4 Effects of LSAP on activities of hepatic markers (ALT, AST) and serum enzymes (MDA, SOD, GSH-Px) MDA, SOD, and GSH-Px
CCl4treatment induced acute hepatotoxicity in mice.Serum activities of ALT and AST were significantly (P< 0.001) higher in the CCl4-only Group II ((813.76 ± 187.07) and (836.44 ± 205.56) IU/L, respectively) than in the normal control Group I ((148.27 ± 22.59) and (47.36 ± 8.13) IU/L) (Fig.5).
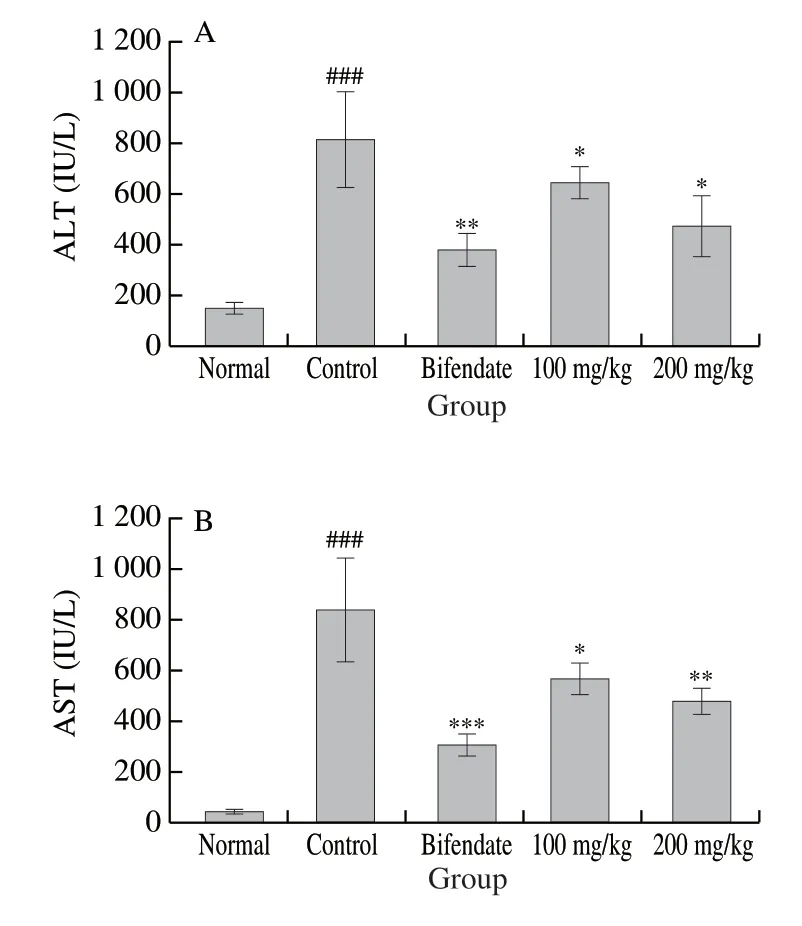
Fig.5 Effects of LSAP on the activities of serum ALT (A) and AST (B) activities in mice.Values are expressed as mean ± standard deviation (n = 7).###P < 0.001 for comparison with Group I.***P < 0.001, **P < 0.01, and*P < 0.05 for comparison with Group II.
The pretreatment with LSAP dose-dependently reduced the ALT and AST values that spiked with CCl4(P< 0.05 vs CCl4-intoxicated group).At 100 mg/kg, LSAP decreased the ALT and AST activities to (651.15 ± 65.67) and (582.32 ± 67.85) IU/L.At 200 mg/kg, LSAP had lower ALT and AST values of (472.84 ± 120.37) and (482.49 ± 48.04) IU/L, respectively.This was close to positive control bifendate (200 mg/kg), which had a value of (377.36 ± 66.35) and (299.49 ± 46.63) IU/L.
In the CCl4Group II relative to Group I, MDA level was significantly (P< 0.001) higher ((17.61 ± 1.93) U/mg protein vs.(5.88 ± 1.01) U/mg protein), whereas SOD and GSH-PX levels were significantly (P< 0.01) lower ((158.57 ± 34.16) U/mg protein vs.(237.83 ± 9.01) U/mg protein and (283.52 ± 16.56) U/mg protein vs.(447.35 ± 58.21) U/mg protein, respectively) (Fig.6).The protective effects of LSAP were also dose-dependent.The 100 mg/kg LSAP Group IV showed significantly (P< 0.05) lower MDA level ((12.08 ± 2.25) U/mg protein) and higher SOD ((241.45 ± 9.72) U/mg protein) and GSH-PX ((392.26 ± 61.55) U/mg protein) levels.For the 200 mg/kg LSAP Group V, values of MDA, SOD, and GSH-PX were (7.55 ± 2.00), (272.54 ± 10.99), and (455.10 ± 92.32) U/mg protein, respectively.These were fairly similar to corresponding values for the bifendate Group III, which were (8.82 ± 0.90), (235.14 ± 7.34), and (697.16 ± 61.01) U/mg protein.
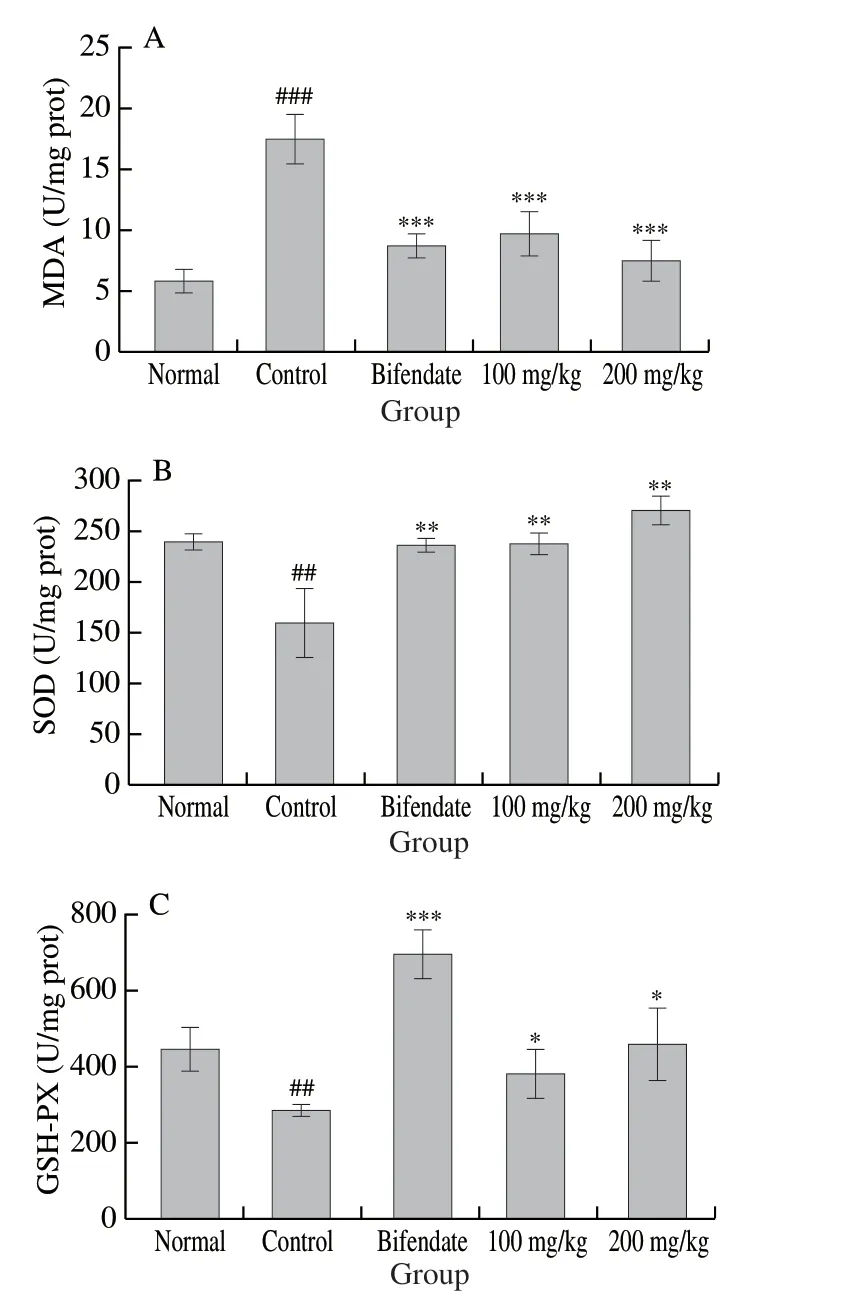
Fig.6 Effects of LSAP on activities of hepatic MDA (A), SOD (B), and GSH-PX (C) in mice.Values are expressed as mean ± standard deviation (n = 7).###P < 0.001 for comparison with Group I.***P < 0.001, **P < 0.01, and *P < 0.05 for comparison with Group II.
3.5 Histopathology examination
Liver histopathological studies were done to supplement the biochemical analysis [22].Normal hepatic cellular architecture is seen in Fig.7A that liver cells arranged radically from central vein.The administration of CCl4-induced extensive liver damage characterized by large vacuoles, inflammatory cells, and severe cellular degeneration (Fig.7B).However, bifendate pretreatment effectively protected the liver against CCl4-induced damage (Fig.7C).In addition, LSAP pretreatment ameliorated liver damage.It decreased the formation of large vacuoles and attenuated cellular degeneration (Fig.7D).
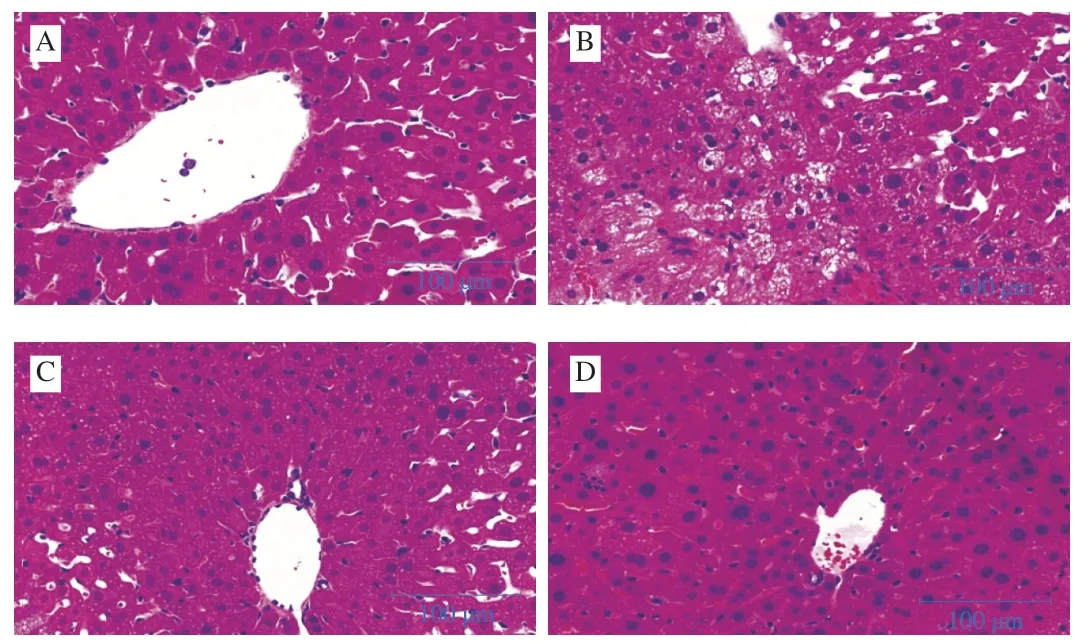
Fig.7 Protective effects of LSAP against CCl4-induced liver cell damage in mice (H&E staining; magnification × 20).A-D, Group I-IV.
TEM data with less lipid droplets of normal hepatocytes was shown in Fig.8A.TEM data of hepatocytes in the CCl4-intoxicated group (Fig.8B) showed that the liver cell was seriously damaged.There were many intracellular lipid droplets with a swelling and distortion of the mitochondria.The LSAP had hepatoprotective effects in CCl4-induced liver damage.The mitochondria, Golgi apparatus and nuclei appeared well preserved, and the number of lipid droplets decreased.The bifendate positive group had slight hepatic fibrosis (Fig.8C).The LSAP group had better ultrastructure of the nucleus (Fig.8D).These histopathological observations as well as the hepatic markers (AST and ALT) and serum enzymes (MDA, SOD and GSH- PX) confirmed the hepatoprotective effects of LSAP.
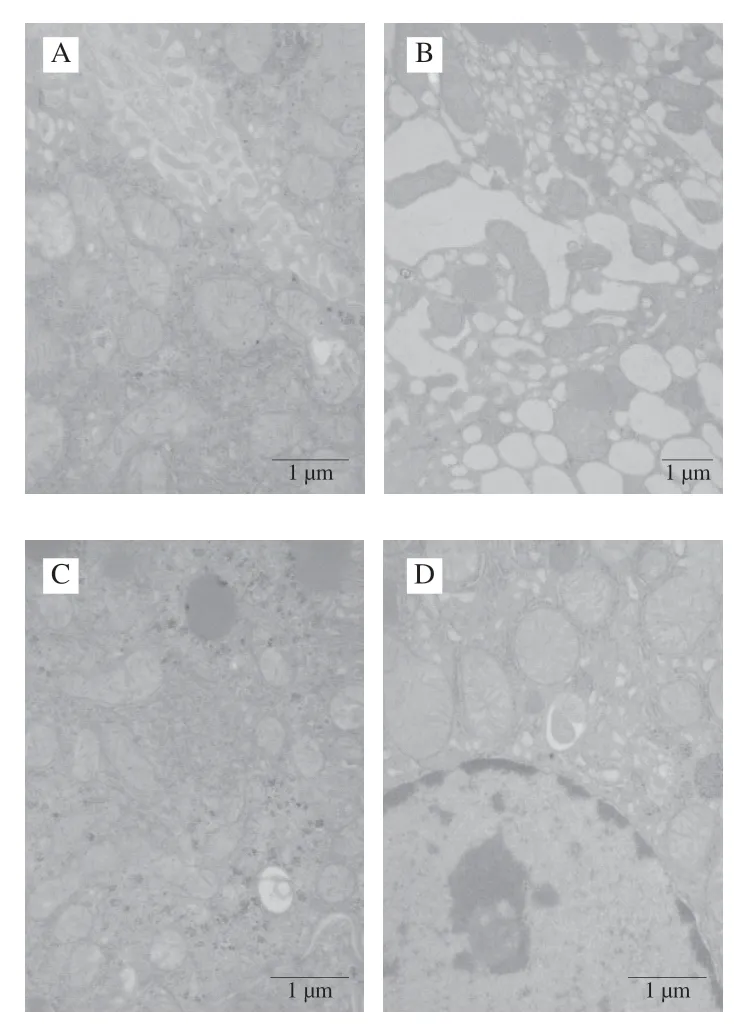
Fig.8 Effects of LSAP on ultrastructural pathology of liver in mice.A-D, Group I-IV.
4.Discussion
CCl4-induced liver damage has been used to screen hepatoprotective drugs since 1989.As one of the most commonly used hepatotoxins, CCl4is almost inevitable in studies of liver diseases [21].The CCl4-mediated hepatotoxicity comes from the cytochrome P450-catalyzed biotransformation of CCl4to the trichloromethyl free radicals (-CCl3), which are converted to the highly reactive trichloromethylperoxy radicals (CCl3OO-).These attack biomolecules to induce lipid peroxidation and depletion of the antioxidants caused by damage of the cell membrane and the organelles of the hepatocyte [5,21].In our study, we first demonstrated that LSAP had a dosedependent antioxidant effectin vitroand hepatoprotectvie effect in a mouse model with experimental CCl4-induced oxidative stress.
The DPPH assay was an efficient way to measure the free radical scavenging activities of antioxidants, and LSAP acted as an efficient hydrogen donor to DPPH radicals [23].Hydrogen peroxide can break DNA and oxidatively degrade most biological macromolecules such as lipids, proteins, carbohydrates, and nucleic acids [24].LSAP had moderate dose-dependent H2O2scavenging activity.A mycelial polysaccharide (CLSP) extracted fromL.sordidaby Zhong et al.[10]for an anti-aging assay, displayed a much lower antioxidant activity in DPPH and hydrogen peroxide scavenging compared with LSAP.We used linoleic acid and oxidized this to LOO- and LOS- in water to initiate lipid peroxidation.Here, LSAP manifested great activity in scavenging these lipid-derived radicals [25].LSAP also displayed a dose-dependent activity in reducing the power assay.This showed that they may act as hydrogen donors and react with free radicals to stabilize or terminate radical chain reactions [26].
Iron is a well-known pro-oxidant with high reactivity, and Fe2+chelation may involve an anti-oxidative reaction and retard metalcatalyzed oxidation [27].LSAP had a higher antioxidant activity but lower Fe2+chelating activity, which might be due to the different Fe2+chelating groups in their structure.Thus, its antioxidant activity was mainly notviametal-catalyzed oxidation.
The AST and ALT levels in serum have long been used as biochemical markers for liver damage [28].Enzymes leaking into the bloodstream from liver tissue and leading to remarkable heightened serum levels result from the damage of liver cells [29].Many studies have illustrated that administration of CCl4increased lipid peroxidation products (MDA, AST and ALT) activities, and hepatocellular necrosis with a decrease in antioxidative enzymes (SOD and GSH-PX) [30].
We preventively administered LSAP at dosages of 100 and 200 mg/kg lowered the activities of AST and ALT.LSAP helped to maintain the normal structure and function of hepatocytes.Also, MDA, SOD and GSH-PX recovered to near normal levels due to the treatment of LSAP.Therefore, we speculated that LSAP restored the innate antioxidant defense system by dampening the generation of free radicals induced by CCl4.The histopathological alteration and ultrastructural pathology induced by CCl4agreed with the serum assays and histology.
Hepatomegaly is enlargement of the liver and is usually associated with biliary disease, cancer, infiltrative diseases, hematological disorders, metabolic syndrome, and toxic/drug-related disorder.CCl4can oxidize P450 enzymes in the liver to produce hematological symptomsviametabolism [30].In our study, LSAP reduced HSI relative to the CCl4-only group.
The LSAP fromL.sordidademonstrated both higher antioxidant activity and hepatoprotective.Similar results were observed for LSAP of Chinese truffleTuber sinense[30].Elucidation of the basic structure of LSAP could contribute to our understanding of its practical utility.In contrast, molecular weights of 4 water-soluble polysaccharides fromL.sordidafruiting bodies reported by Miao et al.[9]ranged from 57 kDa to 156 kDa.Various molecular weights may come from the difference of source and extraction methods.As a potential xyloglucan, the dominant monomeric sugars of LSAP were glucose and xylose (up to 89.7%) as well as a small quantity of rhamnose, arabinose, galactose, glucuronic acid, and galacturonic acid.However, the polysaccharides from fruiting bodies ofL.sordidahad no xylose and were composed of glucose, arabinose, galactose, rhamnose, and mannose [9].The fraction extracted from culturedL.edodesmycelia displayed liver protection similar to xylan-like polysaccharides [31].The LSAP fromPlantago asiaticseeds having a similar molecular weight of 1 150 kDa relative to LSAP and mainly xylose composition also exhibited significant scavenging abilities [32].
Xyloglucan is an alternative to commercial polysaccharide which offers low cytotoxicity and hemolytic activity fromHymenaea courbarilvar.courbaril[33].The polysaccharides have a wide range of chemical structure and every kind of hepatoprotective polysaccharide varies from each other [33-35].More,β-xyloglucan is the predominant structural properties of LSAP, which may be the critical chemical base to play the hepatoprotective roles.
5.Conclusions
We obtained a LSAP fromL.sordidamycelia.Based on its chemical characteristics and anti-oxidative activities, LSAP offered a strong protection against hepatic damage due to acute CCl4exposure in mice as seenviathe activation of the hepatic antioxidant system and the restraint of lipid peroxidation.Our findings clearly suggest that LSAP can be utilized as an effective preventive agent for the treatment of oxidative stress-induced liver injury and is a natural functional food ingredient to enhance health.
Declaration of Competing Interest
All the authors declare no conflict of interest.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (Project No.2018YFD0400200) and the China Agriculture Research System (No.CARS-20-08B).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
- Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo
- The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense
- The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice
- Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice
- The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics
