Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo
2021-05-24RuolinHouXinLiuXiaopingWuMingfengZhengJunshengFu
Ruolin Hou, Xin Liu,*, Xiaoping Wu, Mingfeng Zheng, Junsheng Fu*
a The Third People’s Hospital Health Care Group of Cixi, Ningbo 315000, China
b College of Food Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China
c Mycological Research Center, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China
d College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China
Keywords:Auricularia auricula Edible fungus Melanin Therapeutic Liver damage
ABSTRACT This study explored the therapeutic effects of Auricularia auricula melanin (AAM) on alcoholic liver damage in vitro and in vivo.Human normal liver L02 cells were pre-treated with ethanol and then treated with AAM to explore the therapeutic effect of AAM on ethanol-induced hepatocyte injury.The results show that AAM signifi cantly elevated the cell viability, ameliorated the cell morphology, reduced the ROS and increased the GSH/GSSG of ethanol-pretreated L02 cells.Then, mice were administered with ethanol to induce acute alcoholic liver damage, and administered with AAM to further study the therapeutic effect of AAM on alcoholic liver damage in mice.As a result, AAM reduced the levels of ALT, AST, TG, and MDA, increased the levels of ADH, SOD, and CAT in liver damage mice.The therapeutic effect of AAM may be related to inhibition of CYP2E1 expression and activation of Nrf2 and its downstream antioxidase.The research enriched the bioactivity of AAM and provided some ideas for the development of melanin-related health foods.
1.Introduction
As a traditional edible fungus,Auricularia auriculahas a history of thousands years.A.auriculahas been consumed all over the world, for its high nutritional value and unique taste, especially in East Asian countries such as China, Japan and South Korea.Modern pharmacological research indicated thatA.auriculadisplays various biological activities such as hypoglycemic [1,2], antitumor [3], antioxidant [4]and immunomodulatory activities [5].Melanin is one of the main components ofA.auriculaand plays an important role in its biological activity.However, there are few systematic studies on thein vitroandin vivoactivities ofA.auriculamelanin (AAM).
Melanin is a negatively charged brown or black biomacromolecule [6,7].As a common polymer pigment in nature, melanin exists in plants, animals and microbes [8,9].Studies have confirmed that natural melanin has great potential in the fields of physics, chemistry and biology for its good anti-radiation [10], adsorption of heavy metals [11], anti-oxidation [12], and antibacterial activity [13].
Alcoholic beverages have become a part of people’s lives worldwide.However, heavy or long-term drinking will lead to a range of diseases, such as alcoholic liver disease (ALD).In recent years, the incidence of ALD is increasing year by year and has become the second leading cause of liver damage after viral hepatitis [14].Alcohol is mainly metabolized by cytosolic alcohol dehydrogenase (ADH) and catalase (CAT) in the liver.Cytochrome P450 2E1 (CYP2E1), an enzyme of the microsomal ethanol oxidizing system (MEOS), is also involved in alcohol metabolism when the concentration of alcohol is too high [15].Under the action of CYP2E1, a large amount of reactive oxygen species (ROS) will be produced [16].ROS can lead to protein denaturation, enzyme inactivation, RNA and DNA damage, then lead to hepatocyte damage and abnormal liver function [17].Nuclear factor E2 related factor 2 (Nrf2) is an important transcription factor which can protect cells from oxidative stress by enhancing their antioxidant capacity.Nrf2 binds to cytoplasmic kelch-like epichlorohydrin-associated protein 1 (Keap1), and the expression of Nrf2 is maintained at a low level in normal circumstances.Under oxidative stress, Nrf2 is dissociated from Keap1, following which it enters the nucleus and binds to the antioxidant response elements (ARE), thereby inducing the expression of phase II detoxifying enzyme and antioxidant enzyme, and reducing the harm of alcohol to the liver [18].However, excessive intake of alcohol produces large amounts of harmful free radicals that endogenous oxidants are insufficient to remove, leading to oxidative stress and liver damage.
In this research, we explored the therapeutic effect of AAM on acute alcoholic liver damagein vitroandin vivo.The results enriched the bioactivity of AAM and provided some ideas for the development of melanin-related health foods.
2.Materials and methods
2.1 AAM samples
The fruit bodies ofA.auriculawere brought from a supermarket in the locality (Fuzhou, Fujian Province, China).The melanin was prepared and purified according to the methods previously reported with some modifications [4,19].The specific method was as follow: fruit bodies ofA.auriculawere pulverized and passed through a 55 mesh standard sieve.The 1.25 mol/L NaOH solution was added to theA.auriculapowder at a liquid to solid ratio of 30:1, and extracted at 25 °C under ultrasonic conditions of 200 W for 60 min.After extraction, theA.auriculapowder was removed by centrifugation at 9 000 r/min for 3 min.The supernatant was adjusted to pH 1.5 with HCl and heated in 80 °C water bath for 10 h to remove proteins and carbohydrates.The supernatant was removed by centrifugation at 9 000 r/min for 5 min and the precipitate was washed to neutral with distilled water.The precipitate was successively washed with chloroform, ethyl acetate, 95% ethanol, 75% ethanol, distilled water for three times to remove lipids, and the AAM was obtained by centrifugation at 9 000 r/min for 3 min.The purified melanin was lyophilized and then water soluble melanin was obtained according to the methods of Yang et al.[20]and Zhang et al.[21], and the specific method was as follows: purified melanin was dissolved in a 0.1 mol/L NaOH aqueous solution before adjusting the pH to 7 under strong sonication using aqueous 0.1 mol/L HCl solution.After dialysis and freeze-drying, a water-soluble melanin was obtained.
Due to the existence of organic free radicals, electron paramagnetic resonance (EPR) spectrum is an effective method to identify melanin [22].The EPR spectrum of water-soluble melanin obtained by the above-mentioned method was taken in a Bruker A300 EPR Spectrophotometer (Bruker, Germany).The EPR spectrum showed a single slightly asymmetric line with a G-factor close to 2.003 0 (Fig.1), which established that the material derived fromA.auriculawas melanin.
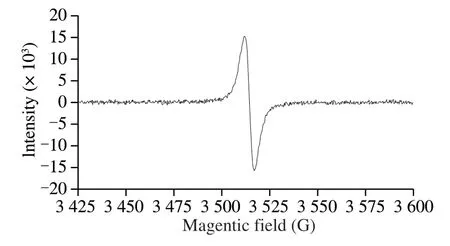
Fig.1 EPR spectra of AAM.
2.2 Chemicals and reagents
Human normal hepatic L02 cells were purchased from Fuheng Biological Technology Co.(Shanghai, China); thiazolyl blue tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St.Louis, USA); ROS assay kit and reduced glutathione/oxidized glutathione (GSH/GSSG) assay kit were obtained from Beyotime Institute of Biotechnology (Jiangsu, China); fetal bovine serum (FBS) was obtained from Gibco (Grand Island, New York, USA); RPMI 1640 culture medium was brought from Solarbio Technology Co.(Beijing, China); bifendate was purchased from Beijing Union Pharmaceutical Factory (Beijing, China); detection kits of aspartate aminotransferase (AST), activities alanine aminotransferase (ALT), triglyceride (TG), malondialdehyde (MDA), superoxide dismutase (SOD), CAT and ADH were obtained from Jiancheng Bioengineering Institute (Nanjing, China); TRIzol Kit was purchased from Invitrogen (Carlsbad, CA, USA); reverse transcription kit and SYBR green qPCR kit were brought from Transgen biotech (Beijing, China); other reagents were analytical grade purchased from Sinopharm Co.(Shanghai, China).
2.3 Cell culture and treatment
The cell experiment was performed according to the method previously described [13].Human normal hepatic L02 cells were cultured in RPMI-1640 medium containing 10% FBS, at 37 °C in an incubator with 5% CO2and 95% humidity.In order to reduce the effect of serum, cells were cultured in RPMI-1640 medium containing 0.5% FBS for 12 h before any treatment.AAM was dissolved and filtered through a 0.22 μm membrane before cell treatment.Cells were pre-treated with alcohol and then co-incubated with AAM.
The cell viability was measured by a MTT assay.After the cells were well treated, previous medium was removed, 90 μL of fresh medium and 10 μL of 5 mg/mL MTT were added to 96-well plates, and cells were cultured at 37 °C for 4 h.Then, the medium was removed and 200 μL of dimethyl sulfoxide (DMSO) was added to each well.The absorbance was determined by Varioskan Flash microplate reader at 490 nm (Thermo Scientific, USA).To study the effect of melanin on the morphology of L02 cells, XDS-3 inverted microscope (Optika, Italy) was used to observe the morphology of cells.The intracellular ROS level was measured according to the instructions of the kit.Intracellular ROS can form fluorescent dichlorofluorescein (DCF) with the fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH-DA).After the cells being well treated, the cell culture medium was removed and 10 μmol/L DCFH-DA was added.The cells were incubated for 20 min in a 37 °C cell culture incubator, and then washed with serum-free medium for 3 times to completely remove the DCFH-DA outside the cells.The Axio Scope A1 fluorescence microscope (ZEISS, Germany) was used to evaluate the fluorescence intensity of the sample at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
The GSH/GSSG ratio of the cell was measured according to the instructions of the kit.The well treated cells were collected and the protein was removed by deproteinization reagent, then the sample was subjected to two cycles of rapid freeze-thaw using a liquid nitrogen and a 37 °C water bath.The sample was placed in an ice bath for 5 min, and centrifuged at 10 000 r/min for 10 min at 4 °C.The total GSH was calculated by measuring the absorbance of the supernatant at 412 nm.The GSH scavenging auxiliary solution was added to the sample and reacted at 25 °C for 60 min, and used for determination the content of GSSG.The GSH/GSSG ratio was determined according to the following formula.
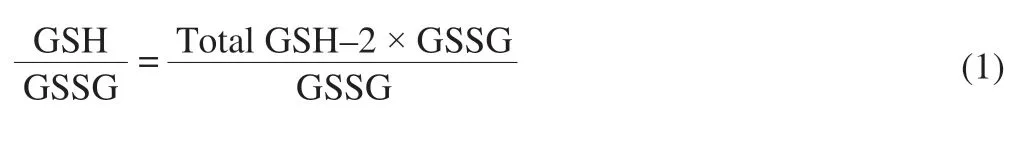
2.4 Animal care and experimental design
Experiments were carried out on the basis of the regulations of the Experimental Animal Welfare and Ethics Committee, Chinese Association for Laboratory Animal Sciences.Male C57BL/6 mice (20-25 g) were purchased from Beijing HFK Bioscience Ltd.All mice were grown in a specific sterile clean room at 21-25 °C, with free access to sterile standard diet and distilled water.
After one week of adaptation to standard conditions, all mice were randomly divided into 6 groups (10 mice in each group), including control group (CON), model group (MOD), low-dose AAM group (L-AAM, 50 mg/kg body weight (BW)), middle-dose AAM group (M-AAM, 100 mg/kg BW), high-dose AAM group (H-AAM, 200 mg/kg BW), and bifendate active control group (AC, 100 mg/kg BW).All mice were given 12 mL/kg BW of 50% ethanol by gavage to form an acute alcoholic liver damage model, except the CON group was given the same volume of distilled water.After 4 h of alcohol administration, AAM treatment group was given different concentrations of AAM once a day for 7 days, bifendate group was given bifendate by gavage, CON group and MOD group were given the equal volume of distilled water.After the last gavage, all mice were fasted for 12 h, then the mice were anesthetized and sacrificed to collect liver and blood.The blood was centrifuged at 3 500 r/min for 10 min to separate the serum.The serum and liver tissue were stored at -80 °C.
2.5 Liver index and serum biochemical analysis
The liver index of mice was calculated according to the following formula.
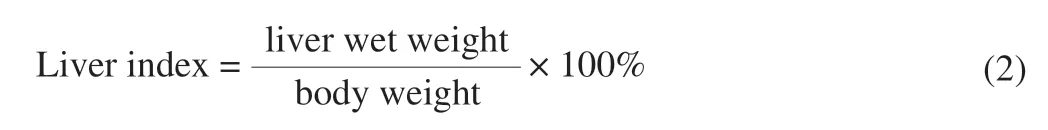
The ALT, AST and TG in serum were measured with corresponding kits.
2.6 Hepatic biochemical analysis
Liver tissue was homogenized with 9 volumes of normal saline, and then centrifuged at 3 000 r/min for 15 min.The supernatant was used to determine the activity of SOD, CAT, MDA, and ADH with corresponding kits.
2.7 Hepatic histopathological analysis
After the mice were sacrificed, the liver tissues were taken out and immediately fixed with 10% neutral formaldehyde solution.The fixed tissues were embedded in paraffin and cut into 5 μm sections, then stained with hematoxylin and eosin (H&E).The tissue morphology was observed under Nikon-E100 optical microscope (NIKON, Japan).
2.8 mRNA expression analysis
Total RNA of liver was isolated with trizol reagent and reversely transcribed into cDNA with corresponding kit.Subsequently, the obtained cDNA was subjected to qPCR analysis with SYBR®Green mix and a CFX96TM real-time PCR system (Bio-Rad, CA, USA).The primer sequences were as follows:

2.9 Statistical analysis
Statistical analysis was performed with SPSS software (version.13.0; SPSS, chicago, IL, USA).All data was presented as mean ± standard deviation.One-way analysis of variance (ANOVA) was used for comparison among groups,P< 0.05 andP< 0.01 were statistically significant.
3.Results
3.1 Effects of AAM on ethanol- induced damage in L02 cells
The effect of AAM on cell viability was detected by MTT assay.As shown in Fig.2A, the viability of L02 cells gradually decreased with the increase in the concentration of ethanol.When the ethanol concentration was 200 μmol/L, the cell viability was 52.72%.Thus, 200 μmol/L ethanol was used for modeling in subsequent experiments.As shown in Fig.2B, compared with the CON (without ethanol and melanin), AAM treatment alone had no significant difference in cell viability, even at a concentration of 1.6 mg/mL, which indicated that AAM was nontoxic to normal cells.Compared with the MOD that only incubated with ethanol for 24 h, AAM treatment dose-dependently inhibited the decline of cell viability caused by alcohol.When the AAM concentration was 1.2 mg/mL, the cell viability reached 96.77%, indicating that AAM significantly ameliorated the ethanol-induced liver injury.
The effect of AAM on cell morphology was also studied by morphological observation.As shown in Fig.2C, in the CON (without ethanol and melanin) and AAM CON (1.2 mg/mL AAM only) groups, the cells grew by static adherence and in a fusiform or polygonal shape.In the MOD (ethanol only), the cells gradually contracted and became spherical, separated from the surrounding cells, and the number of adherent cells gradually decreased over time.Compared with the MOD, AAM significantly ameliorated the ethanol-induced cell morphological changes.
3.2 Effects of AAM on ethanol-induced oxidative stress in L02 cells
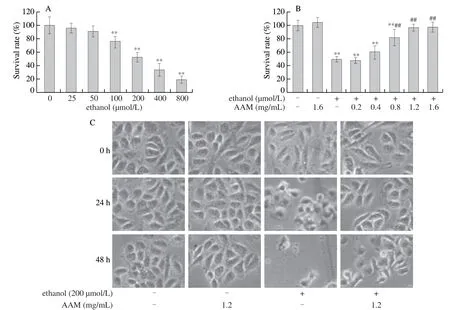
Fig.2 AAM attenuated ethanol-induced L02 cell injury.(A) L02 cells were treated with different concentrations of ethanol for 24 h, and the cell viability was measured by MTT assay.(B) L02 cells were pre-treated with ethanol for 1 h followed by co-incubated with different concentrations of AAM for 24 h, and the cell viability was measured by MTT assay.(C) L02 cells were pre-treated with or without ethanol for 1 h followed by co-incubated with concentrations of AAM for different time, the effect of AAM on cell morphology was observed by an inverted microscope.Data were shown as mean ± SD (n = 4).In ethanol group, - means without ethanol treatment, + means pre-treated with ethanol.*P < 0.05, **P < 0.01, compared with CON.#P < 0.05, ##P < 0.01, compared with ethanol MOD.

Fig.3 Effects of AAM on the production of ROS and the ratio of GSH/GSSG of L02 cell.(A) L02 cells were pre-treated with ethanol for 0.5 h followed by coincubated with AAM for 3 h, and the level of ROS was detected by fluorescence microscopy.(B) L02 cells were pre-treated with ethanol for 1 h followed by co-incubated with AAM for 24 h, and the ratio of GSH/GSSG was detected according to the relevant kit.Data were shown as mean ± SD (n = 4).In ethanol group, - means without ethanol treatment, + means pre-treated with ethanol.*P < 0.05, **P < 0.01, compared with CON, #P < 0.05, ##P < 0.01, compared with ethanol MOD.
The effect of AAM on alcohol-induced hepatocyte oxidative stress was determined by measuring the intracellular ROS and the ratio of GSH/GSSG.As the Fig.3A showed, compared with the blank CON, L02 cells treated with AAM and without ethanol stimulation did not increase in ROS, indicating that AAM did not cause oxidative stress on L02 cells.The ROS levels of the cells increased obviously in the ethanol group, indicating that ethanol caused oxidative stress of cells.While AAM treatment dose-dependently inhibited the increase of ROS caused by ethanol, indicating that AAM alleviated the oxidative stress caused by ethanol.The result of GSH/GSSG ratio is shown in Fig.3B.The ratio of GSH/GSSG in ethanol group was significantly lower than the blank CON and the AAM CON (P< 0.01).This suggested that a large amount of ethanol treatment weakened the antioxidant capacity of the hepatocyte.Compared with the ethanol group, AAM obviously inhibited the decrease of GSH/GSSG ratio caused by ethanol (P< 0.01).The results indicated that AAM resisted the ethanol-induced oxidative stress by increasing the antioxidant capacity of cells.
3.3 Effects of AAM on liver index and serum biochemical index in mice
Liver index can partially reflect the degree of liver damage.As Fig.4A showed, in comparison with CON group (4.48 ± 0.07), the MOD group significantly increased the liver index of mice (5.07 ± 0.07,P< 0.01).Comparing with the MOD group, the AAM group and the AC group significantly inhibited the elevation of the liver index in acute liver injury mice (P< 0.01).Early acute liver injury was signified as elevated of serum activities such as TG, ALT and AST.As shown in Fig.4B, compared with the CON group ((0.82 ± 0.07) mmol/L), the TG level in the MOD group ((1.55 ± 0.14) mmol/L) increased by 89.02%.This indicated that heavy ethanol consumption result in the accumulation of TG.Compared to the MOD group, different concentrations of AAM significantly decreased serum TG levels ((1.36 ± 0.17), (1.22 ± 0.18), and (1.22 ± 0.19) mmol/L, respectively,P< 0.01), similar result was seen in the AC group.The results indicated that AAM reduced the accumulation of TG caused by alcohol.As shown in Fig.4C and 4D, compared with CON group (ALT was (19.20 ± 4.52) U/L, AST was (83.07 ± 2.09) U/L), the levels of ALT and AST in the MOD group (ALT was (31.05 ± 3.89) U/L and AST was (94.77 ± 6.33) U/L) increased by 61.72% and 14.08%, respectively.This indicated that a large amount of alcohol intake caused liver damages in mice.Compared to the MOD group, different concentrations of AAM significantly reduced ALT levels ((23.36 ± 4.08), (20.11 ± 7.53) and (19.82 ± 3.07) U/L, respectively,P< 0.05 orP< 0.01) and AST levels ((87.76 ± 4.41), (86.53 ± 2.77) and (85.51 ± 2.23) U/L, respectively,P< 0.01) in acute alcoholic liver injury mice, similar results were found in the AC group.The results have shown that AAM ameliorated the liver damage caused by alcohol.
3.4 Effects of AAM on alcohol metabolic enzymes and antioxidant enzymes in mice liver
ADH is a key enzyme involved in ethanol metabolism.As Fig.5A showed, compared with the CON group ((5.42 ± 0.74) U/mg protein), the ADH activity in MOD group ((6.93 ± 0.86) U/mg protein) increased by 27.86%, which indicated that the body can improve the ability of alcohol metabolism by enhancing the level of ADH.Compared with MOD, different concentration of AAM treatment increased the levels of ADH in alcoholic liver injury mice ((7.21 ± 0.24), (8.11 ± 1.18), and (7.95 ± 0.70) U/mg protein, respectively,P< 0.05), similar result was seen in the AC group.It suggested that AAM promoted the metabolism of alcohol in the liver by increasing ADH levels.
Liver antioxidant enzyme activity (CAT, SOD) and their lipid product (MDA) are commonly used as biochemical markers of early liver injury.As Fig.5B-5D showed, compared with CON group (SOD was (120.19 ± 5.30) U/mg protein, CAT was (46.52 ± 5.30) U/mg protein, and MDA was (0.71 ± 0.04) nmol/mg protein), the SOD level in MOD group decreased by 23.50% ((91.94 ± 2.88) U/mg protein,P< 0.01), CAT level decreased by 26.63% ((34.13 ± 2.70) U/mg protein,P <0.01), and MDA level increased by 35.21% ((0.96 ± 0.13) nmol/mg protein,P< 0.01).These results suggested that heavy drinking destroyed the antioxidant system in mice.Compared with the MOD group, different concentrations of AAM treatment significantly elevated SOD levels ((100.16 ± 3.32), (113.38 ± 6.33), and (115.82 ± 3.54) U/mg protein,P< 0.05 orP< 0.01), and CAT levels ((34.61 ± 1.74), (41.31 ± 2.48), and (41.93 ± 2.23) U/mg protein,P< 0.01 in M-AAM and H-AAM group), and decreased the MDA levels ((0.85 ± 0.11), (0.80 ± 0.10), and (0.80 ± 0.07) nmol/mg protein,P< 0.05 orP< 0.01) in acute alcohol-induced hepatic injury mice.The results have shown that AAM ameliorated alcohol-induced oxidative stress injury in mice by improving the antioxidant capacity of the body.
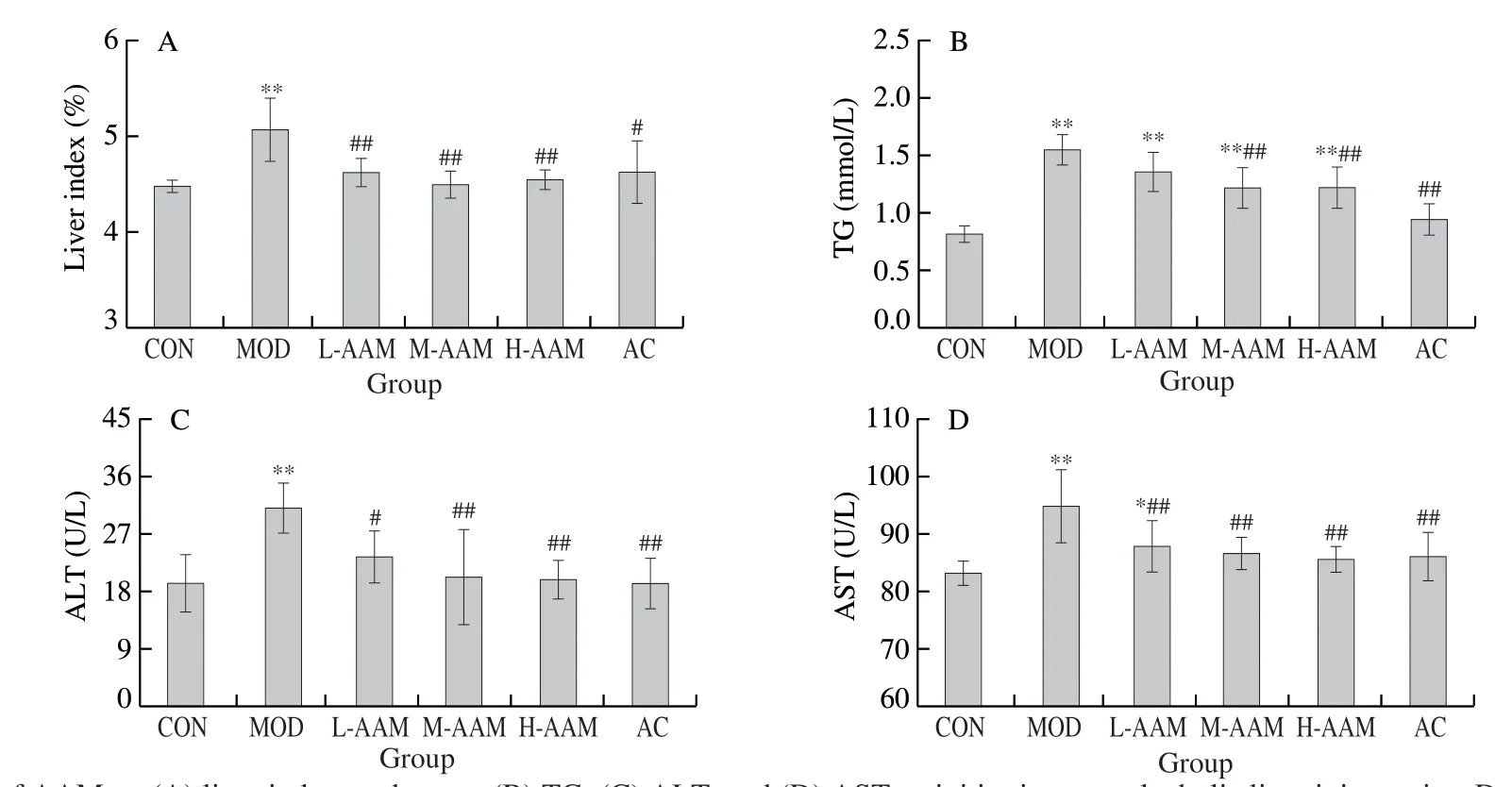
Fig.4 Effects of AAM on (A) liver index, and serum (B) TG, (C) ALT, and (D) AST activities in acute alcoholic liver injury mice.Data are presented as mean ± SD, *P < 0.05 and **P < 0.01 compared with normal group.#P < 0.05 and ##P < 0.01 compared with ethanol group.
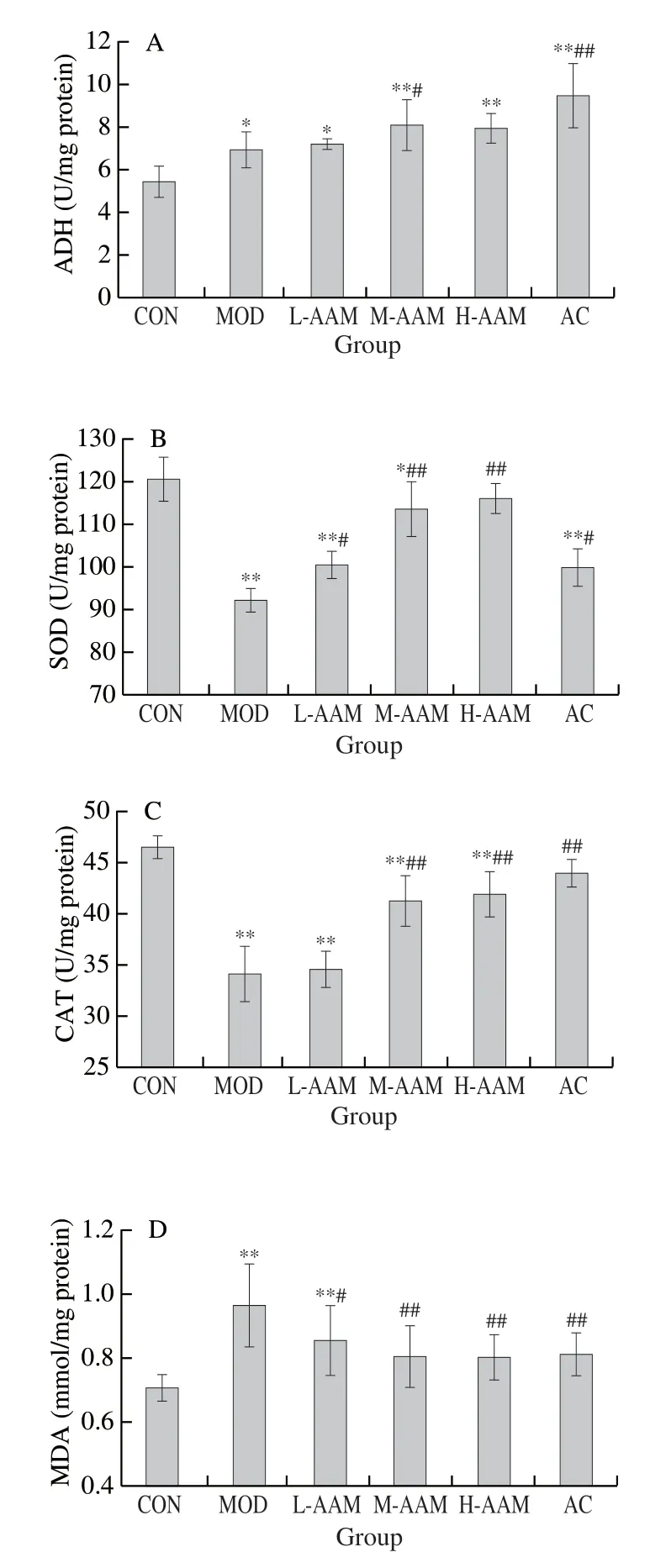
Fig.5 Effects of AAM on the hepatic (A) ADH, (B) SOD, (C) CAT, and (D) MDA activities in acute alcoholic liver injury mice.Data are presented as mean ± standard deviation, *P < 0.05 and **P < 0.01 compared with normal group.#P < 0.05 and ##P < 0.01 compared with ethanol group.
3.5 Effects of AAM on liver morphology in alcoholic liver injury mice
The effect of AAM on the pathological changes of liver tissue was further observed, and the results are showed in Fig.6.In the CON group, the outline of liver cells was clear and polygonal, and there were no infiltration of inflammatory cells in liver tissue.In contrast, inflammatory cell infiltration was found in the portal area of MOD group, which indicated that heavy drinking caused the inflammation in liver tissue.Compared with the MOD, the infiltration of inflammatory cells in L-AAM group was less, and almost no inflammatory cells infiltration in the M-AAM and H-AAM group.The morphology of liver tissue in AAM group and AC group was similar to that of the CON group.These results indicated that AAM can ameliorate the hepatic tissue morphology of mice with acute alcoholic liver injury.
3.6 Effects of AAM on the expression of CYP2E1 and Nrf2 related genes in liver injury mice
In this section, qRT-PCR was used to verify whether the therapeutic effect of AAM on alcohol-induced liver injury in mice is related to the inhibition of CYP2E1 expression, and the activation of Nrf2 and its downstream antioxidant enzymes.As shown in Fig.7A, compared with the CON group, the mRNA expression ofCYP2E1was significantly increased in the MOD group (P< 0.01), while different concentrations of AAM significantly reduced the mRNA expression ofCYP2E1(P< 0.01).As shown in Fig.7B, compared with the CON group, the mRNA expression ofNrf2was significantly decreased in the MOD group (P< 0.05), while the M-AAM group and the H-AAM group significantly increased the mRNA expression ofNrf2(P< 0.05).In addition, AAM elevated the expression of phase II antioxidant enzymes downstream of Nrf2.As shown in Fig.7C-7F, medium and high concentrations of AAM significantly increased the mRNA expression ofGclc,Gclm,CAT, andCu-Zn SODin alcoholic hepatic injury mice (P< 0.05 orP< 0.01).The results suggested that the therapeutic effect of AAM on acute alcoholic liver injury in mice may be related to the inhibition of the expression ofCYP2E1, and the activation ofNrf2and its related antioxidant enzymes.
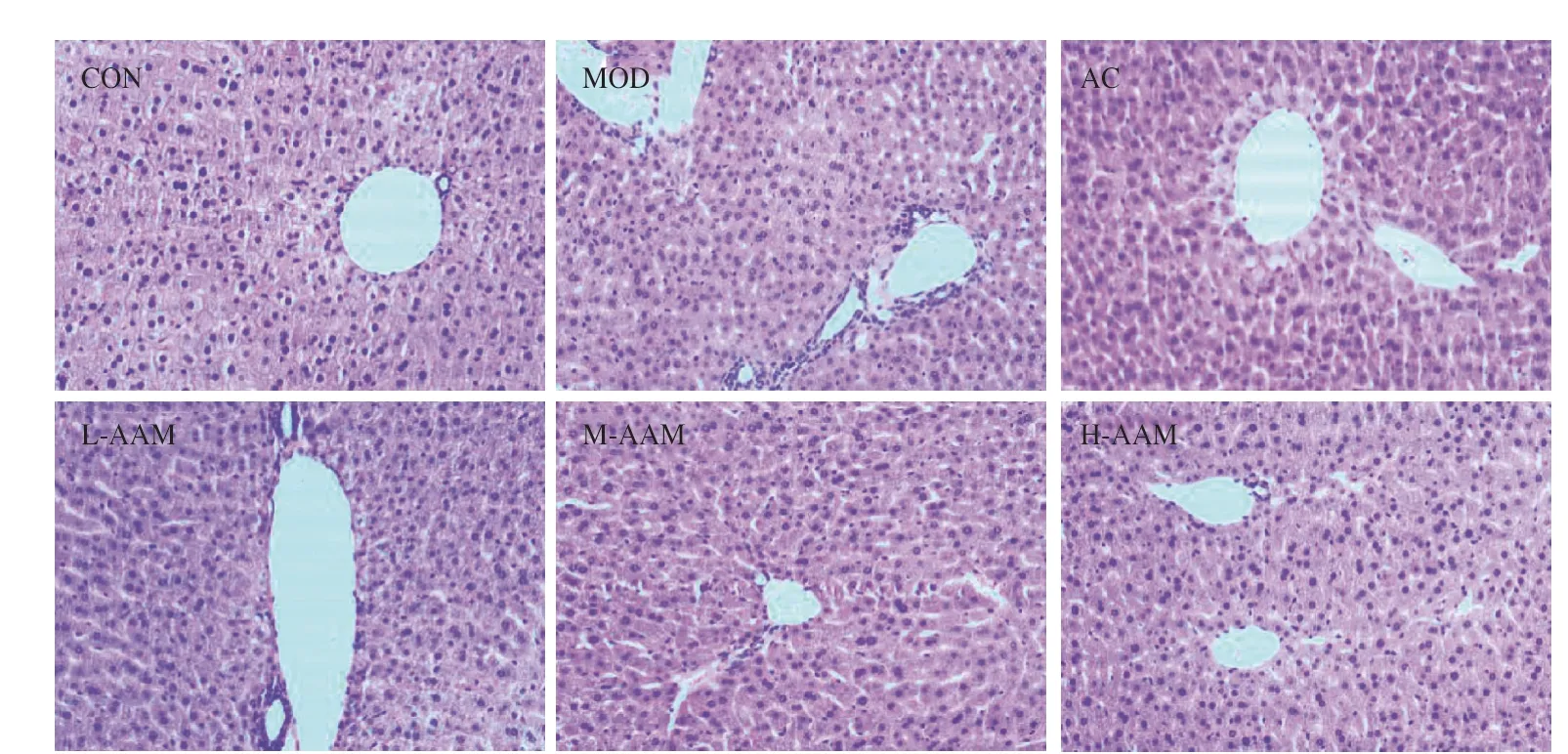
Fig.6 Effects of AAM on hepatic morphological changes in acute alcoholic liver injury mice (magnification, × 100).
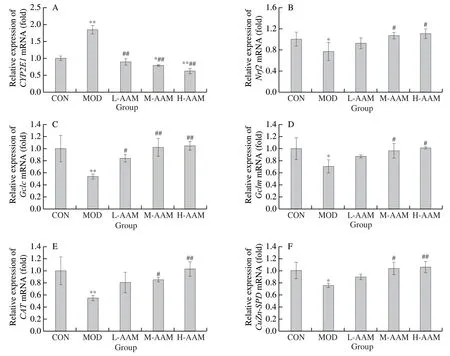
Fig.7 Effects of AAM on the mRNA expression of CYP2E1 and Nrf2-related antioxidant enzymes in acute alcohol liver injury mice.(A) The mRNA expression level of CYP2E1; (B) the mRNA expression level of Nrf2; (C) the mRNA expression level of Gclc; (D) the mRNA expression level of Gclm; (E) the mRNA expression level of CAT; and (F) the mRNA expression level of Cu-Zn SOD.Data are presented as mean ± SD, *P < 0.05 and **P < 0.01 compared with normal group; #P < 0.05 and ##P < 0.01 compared with ethanol group.
4.Discussion
A.auriculais a kind of edible fungus with high nutritional value, and melanin is one of its main active components.Previous studies have shown that AAM has goodin vitroantioxidant activity [23]and radiation protection activity [24].It can also inhibit the synthesis of bacterial biofilm [25].ALD is one of the most common liver diseases around the world.Therefore, research and development of effective methods for treating alcoholic liver injury have attracted more and more attention [26].In earlier research, our project team extracted the melanin fromA.auricula, analyzed its physicochemical properties, and its protective effects on alcoholic liver injury in mice [27].In the present study, we continued to explore the therapeutic effect of AAM on the liver damage that has already formed.In this study, the effect of AAM on human normal liver L02 cellswasinvestigated.We found that AAM had no toxicity to L02 cells, and it significantly inhibited the decrease of cell viability induced by alcohol.When the AAM concentration was 1.2 mg/mL, the cell viability reached 96.77% (Fig.2).AAM also obviously ameliorated the changes of hepatocyte morphology induced by alcohol.Furthermore, the effect of AAM on alcohol-induced L02 cell oxidative stress was determined by measuring the intracellular ROS and the ratio of GSH/GSSG.ROS are produced during the metabolism of ethanol.Alcohol exposure causes cells to produce too much ROS, leading to oxidative stress, which destroys cellular DNA, proteins and membrane lipids, ultimately leads to cell death [17].Glutathione is an antioxidant with a wide range of physiological effects, which plays an important role in maintaining the redox balance of cells.It is mainly synthesized and metabolized in liver and widely distributed in various organs of the body.Glutathione exists in two forms, GSH and GSSG.Under normal conditions, the concentration of GSSG was less than 5% of the total glutathione content in the cells.During oxidative stress, intracellular GSH depletion was accompanied by an increase in GSSG.Therefore, intracellular GSH/GSSG ratio well reflected the redox state of cells [28].Our results showed that AAM treatment dose-dependently inhibited the increase of ROS and the decrease of GSH/GSSG ratio caused by alcohol, which indicated that AAM resisted the alcohol-induced oxidative stress by increasing the antioxidant capacity of cells (Fig.3).
Next, we investigated the therapeutic effect of AAM on alcoholinduced liver injuryin vivoby determining the biochemical indexes of serum and liver, and observing the liver tissue sections of mice.Early acute liver injury was signified by elevated of serum levels such as TG, ALT and AST.TG will massively accumulate in the primary stage of ALD, which is a sensitive index reflecting fat metabolism [29,30].Serum ALT and AST are usually used as indicators for monitoring liver injury because they leaked from liver cells into the blood circulation system when liver injury occurs [29].The results indicated that that AAM significantly inhibited the increase of TG, ALT and AST caused by alcohol (P< 0.05 orP< 0.01), which indicated that AAM ameliorated the liver damage caused by alcohol (Fig.4).ADH exists in the liver cytoplasm, it can metabolize ethanol to acetaldehyde, and further to acetic acid [31].Oxidative stress is closely related to ethanol-induced liver damage.Antioxidant enzymes can protect the body from oxidative stress, and lipid peroxidation damage in tissues is often attributed to the depletion of antioxidant enzymes.For early liver injury, liver antioxidant enzyme activity (CAT, SOD) and their lipid product (MDA) are usually regarded as biochemical markers.Under the action of SOD, superoxide anion reacts with hydrogen ions to form hydrogen peroxide, and further converted to water under the action of CAT, which inhibits oxidative stressin vivo[32-34].The results of this study showed that AAM significantly increased ADH, SOD and CAT levels and decreased MDA levels in alcoholic liver injury mice (P< 0.05 orP< 0.01), which indicated that AAM ameliorated alcohol-induced oxidative stress by improving the antioxidant capacity of mice (Fig.5).Moreover, the morphological changes of liver tissues were observed by H&E staining (Fig.6).The results showed that AAM ameliorated the hepatic tissue morphology of mice with acute alcoholic liver injury.
CYP2E1 is a key member of the cytochrome P450 family, and is one of the main pathways of ethanol metabolism [35].CYP2E1 produces a large amount of ROS during the process of ethanol metabolism, which is considered to be the main contributor of oxidative stress [36,37].Nrf2 is an important transcription factor that mediates antioxidant protection [38].The activation of Nrf2 has been shown to play a key role in protecting against ethanol-induced liver injury [39,40].When oxidative damage occurs, Nrf2 transfers from the cytoplasm to the nucleus and activates its downstream phase II antioxidant enzymes to protect the body from oxidative stress.The results indicated that AAM inhibited the expression of CYP2E1 in alcoholic liver injury mice, and increased the levels of Nrf2 and its downstream antioxidant enzymes (Fig.7).This suggested that the therapeutic effect of AAM on alcohol-induced liver damage may be related to the inhibition of the expression of CYP2E1 and the activation of Nrf2 and its related antioxidant enzymes.
Conclusion
In summary, melanin, a natural pigment fromA.auricula, has an obvious therapeutic effect on alcohol-induced liver injuryin vitroandin vivo.The above results provided a theory basis for the application of AAM in the treatment of ALD.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
This work was financially supported by the Special Fund Project for Technological Innovation of Fujian Agriculture and Forestry University (CXZX2019055G), and the Science and Technology Project on Social Development of Cixi (CN2020027).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
- The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense
- The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice
- Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice
- The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics
