Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
2021-05-24SudhaGovindanAnguJayaalJayasakthiShanmugamPrasannaRamani
Sudha Govindan*, Angu Jayaal, Jayasakthi Shanmugam, Prasanna Ramani
a Department of Biochemistry, School of Biosciences, Periyar University, Salem, India
b Department of Biochemistry, Kongunadu Arts and Science College, Bharathiar University, Coimbatore, India
c Dhanvanthri Lab, Department of Sciences, Amrita School of Engineering, Coimbatore, Amrita Vishwa Vidyapeetham, India
d Center of Excellence in Advanced Materials & Green Technologies (CoE - AMGT), Amrita School of Engineering, Coimbatore, Amrita Vishwa Vidyapeetham, India
Keywords:Hypsizygus ulmarius polysaccharide Antioxidant activity Hepatoprotective behaviour Ethanol consumption Liver damage
ABSTRACT Hypsizygus ulmarius polysaccharide (HUP) is a water-soluble polysaccharide obtained by hot water extraction, followed by precipitation and deproteinization.The characteristics of HUP, antioxidant activity and liver protection against alcohol-induced liver damage were studied.Structural characteristics indicate that the HUP is a pyran-type polysaccharide with a molecular weight of 2 076 Da.In antioxidant scavenging assay, HUP showed moderate DMPD radical scavenging activity, cupric ion reducing antioxidant capacity and inhibitory effect against lipid peroxidation in a dose-dependent manner.Regarding in vivo hepatoprotective activity, compared with the ethanol induction group, pre-treatment of low and high doses of HUP signifi cantly reduced the behaviours of serum enzymes, lowered the levels of hepatic oxidative stress markers, restored the levels of biochemical constituents, enhanced the levels of liver and serum enzymatic antioxidants and non-enzymatic antioxidants, and improved the serum lipid levels of alcohol-intoxicated rats.The hepatoprotective effect of HUP was comparable to positive control silymarin.Besides, HUP pre-treatment signifi cantly normalized the histopathological changes induced by ethanol.The results indicate that HUP could be used as a functional food and may protect the biological system from oxidative stress through its antioxidant activity, thus having a signifi cant protective effect on acute alcoholic liver injury.
1.Introduction
Alcohol consumption is becoming increasingly severe worldwide, and excessive utilization causes about 3.3 million global mortalities every year, according to the WHO survey report.Approximately 80% of alcohol is metabolized in the liver, which is a significant body part that takes part in digestion, synthesis of proteins, metabolism, and detoxifi cation [1,2].The most common consequence of alcohol consumption is an alcoholic liver disease (ALD), which has become a growing health problem for citizens worldwide.Excessive drinking alcohol may lead to fibrosis, hepatitis, cirrhosis, liver cancer, and progresses to liver failure [3].ALD also causes complications, such as atherosclerosis, hyperlipidemia, and even hypertension [4].The destruction of liver function triggered by alcohol-mediated oxidative stress leads to the release of liver enzymes such as aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) in the serum, peroxidation of lipids, loss of antioxidant function, and the production of reactive oxygen species (ROS) [5].More and more evidence show that oxidative stress plays a vital role in accelerating ALD by inducing changes in lipids, proteins, DNA and RNA content and its effects on cellular dysfunction [6].In the process of alcohol metabolism, it circulates into the microsomal ethanol oxidation system and is metabolized by the formation of ROS [7].The antioxidant system includes the primary enzyme antioxidants, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT), which protect the body from ROS mediation cell damage [8].Currently, steroids, vaccines and antiviral medications used to treat liver injury usually have limited efficacy and have many adverse reactions.Therefore, the development of effective treatments to protect society from acute alcoholic liver damage has received increasing attention.Natural polysaccharides with antioxidants and liver protection are needed as substitutes that may interfere with ALD.
In recent years, mushroom polysaccharides from fruit bodies, fermentation broth, mycelium, mushroom residues and spent mushroom compost have hepatoprotective [9,10], antihyperlipidemic [11], hypoglycaemic [12], anti-tumor [13], immunomodulatory [14], neuroprotective [15], anti-aging [16,17]anti-inflammatory and antioxidant [18]activities.Many researchers have demonstrated the hepatoprotective effect of fungal polysaccharides against ALD.According to the report of Zhao et al.[19], a modifiedCoprinus comatusmushroom polysaccharide was proved to reduce liver and serum lipid levels, significantly enhanced antioxidant enzyme activity, reduced the histopathological effects of alcohol-induced liver damage and has liver protection ability.Song et al.[20]reported the antioxidant and hepatoprotective effects of acidic and enzymatically hydrolysed mycelial polysaccharides ofPleurotus geesteranuson ALD in mice, which prevent oxidative stress and protect the liver by reducing lipid accumulation and alleviating the liver functions by histopathologic observation.Liu et al.[21]have shown that exopolysaccharide ofInonotus hispidusaltered the biochemical parameters of liver function and alleviated alcohol-induced liver damage from a histopathological perspective.Treatment with PSK-1b1 fromCoriolus versicolor(80 and 160 mg/kg/day) has a hepatoprotective effect on alcohol-induced liver injury in mice by reducing oxidative stress and modulating immunity [10].
Hypsizygus ulmarius(Bull.) (Lyophyllaceae, Agaricomycetes), a rare and delicious mushroom, is also known as “elm oyster” or “blue oyster” which is like oyster mushroom but has a different morphology and biological efficiency.The mushroom is a new fleshy species with enormous fruiting bodies and a light blue pinhead.It is one of the essential edible fungi in the world and a prevalent fungus cultivated in Asian countries.Greeshma et al.[22]reported that the ethanol extract extracted fromH.ulmariushas antioxidant, anti-inflammatory, and anti-tumour effects.So far, there is no report on the hepatoprotective effect ofH.ulmariuspolysaccharide (HUP) on ethanol-induced liver toxicity in rats.The purpose of this study is to evaluate the antioxidant activity and hepatoprotective effects of HUP on acute liver injury caused by ethanol.Besides, HPLC, FT-IR and NMR of HUP are also studied.
2.Materials and methods
2.1 Materials and chemicals
H.ulmariusfruiting bodies were provided by the Biological Centre of the Department of Horticulture, Hulimavu, Karnataka, India.H.ulmariuswas dried in a hot air oven (50 °C) and pulverized (40 mesh).Monosaccharide standards were purchased from Sigma (St.Louis, USA).Randox assay kit was used to estimate the total cholesterol, triglycerides, HDL-cholesterol (HDL-C), LDLcholesterol (LDL-C), total bilirubin, total protein and albumin.All other chemicals and reagents are of analytical grade and are commercially available.
2.2 Preparation of HUP
H.ulmarius(100 g) was ground into powder and soaked in 90% petroleum ether overnight to remove the lipid substances.The dried filtrate was extracted three times with hot water (1:25 (m/V) at 100 °C for 3 h.Then centrifuged at 3 000 r/min for 10 min and the filtrate was concentrated to 10% of the original volume by using a rotary evaporator, at 55 °C.Four times of the volume of ethanol (95%) was added to the resulting liquid, stirred vigorously and kept at 4 °C overnight.Then centrifuged at 8 000 r/min (20 min), collected the precipitate, re-dissolved in deionized water, and deproteinated with Sevags reagent (CHCl3:n-butyl alcohol = 4:1,V/V) [23].The partially purified fraction was subjected to dialysis, freeze-drying, and was named HUP.
2.3 Primary characterization of HUP
2.3.1 Determination of total carbohydrate, sulfate, protein and uronic acid content
Using glucose as the reference, carbohydrate was analysed by the phenol-sulfuric acid method [24].The protein content was evaluated by Coomassie Brilliant Blue (Bradford assay) with bovine serum albumin as the standard [25].According to the sulfuric acid-carbazole method, the content of uronic acid was determined with glucuronic acid as the standard [26].The barium chloride-gelatin technique was used to measure the sulfate content (using K2SO4as the standard) [27].
2.3.2 Chromatography and spectroscopy analysis
The monosaccharide component of HUP was determined by gas chromatography-mass spectrometry (GC-MS).10 mg of HUP was hydrolyzed with trifluoroacetic acid (2 mL, 2 mol/L) at 120 °C for 2 h and the product was reduced with NaBH4, derivatized withN-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA), and acetylated with acetic anhydride at 100 °C for 60 min.The resulting sugar alcohol acetate was analyzed on a Perkin-Elmer Clarus 690 AQ8 autosampler GC-MS instrument equipped with a PDA detector and RTX-5MS fused silica capillary column (30 m, 0.32 mm ID, 0.25 μm thickness).The resulting derivatives were separated at a ratio of 20:1 with 1 μL at a helium flow rate of 1 mL/min.The temperature program started at 65 °C (hold for 2 min), increased to 180 °C (hold for 2 min) at a rate of 20 °C/min, and then further increased to 280 °C at a rate of 5 °C/min for 10 min.Under the same conditions, standard monosaccharides (glucose (Glc), galactose (Gal), arabinose (Ara), mannose (Man), fucose (Fuc), ribose (Rib), rhamnose (Rha), xylose (Xyl), glucuronic acid (GluA) and galactuonic acid (GalA)) were also derivatized.Retention time (RT) and mass spectrometry determined the composition of monosaccharides from the library.
High-performance size-exclusion chromatography (HPSEC) was used to estimate the molecular weight (Mw) of HUP.The instrument was equipped with a refractive index detector (RID) and a Shodex SB-804 HQ column (300 × 8.0 mm, Showa Denko Corp., Japan).The temperature of the column was maintained at 45 °C.The mobile phase was ultra-pure water, the injection volume was 10 μL, and maintained at a constant flow rate of 0.9 mL/min.By plotting the logarithm of the molecular weight relative to the RT of the standard, different molecular weight dextran was used to build a standard curve, and the molecular weight was estimated.
HUP (1 mg) and KBr (100 mg) were mixed and then pressed into small balls for infrared spectroscopy analysis in the range of 4000-400 cm-1.A Thermo Scientific Nicolet 5700 InfraRed spectrophotometer was used to measure FT-IR spectrum.A 500 MHz NMR spectrometer (Bruker AVANCE III, Bruker Inc., Rheinstetten, Germany) was used for1H and13C NMR measurements, and the sample was dissolved in deuterated water.
2.4 Antioxidant activity
2.4.1 N,N-dimethyl-p-phenylendiamine (DMPD)/FeCl3 assay
The reduction of DMPD radical was carried out according to reported protocols [28].1 mL of 100 mmol/L DMPD (209 mg/10 mL of deionized water) was mixed with acetate buffer (100 mL, 0.1 mmol/L, pH 5.3) and ferric chloride (FeCl3) (0.2 mL, 0.05 mmol/L) to generate free radical, DMPD cation radical.Fresh reagents which are stable up to half-a-day at room temperature should be used.
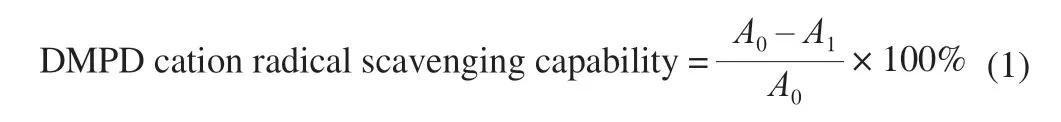
whereA0is absorbance at initial concentration,A1is DMPD cation radical absorbance at remaining concentration with HUP.
2.4.2 Cu2+ reduction assay
As previously reported by Karman et al.[29], the Cu2+reduction activity was measured.250 μL of CuCl2solution (0.01 mol/L), ethanolic neocuprine (0.0075 mol/L) and ammonium acetate buffer solution (1 mol/L) were added into the test tube; following which HUP (0.5-2.5 mg/mL) was added.It was further made-up to 2.0 mL with deionized water and incubated for 30 min in the absence of light.Absorbance measurements (450 nm) were carried out with distilled water as the blank.
2.4.3 Lipid peroxidation inhibition assay
Lipid peroxidation inhibitory activity was measured by the thiobarbituric acid reactive substance (TBARS) [30].HUP (1-5 mg/mL), rat liver homogenate (1 mL, 1%,m/V), FeCl2solution (50 μL, 0.5 mmol/L) and H2O2(50 μL, 0.5 mmol/L) were added in the given order and incubated (37 °C) for 1 h.Trichloroacetic acid (1.5 mL, 20%,m/V) was added to stop the reaction.Following this, thiobarbituric acid (1.5 mL, 0.8%,m/V) was added and incubated at 95 °C for 1 h.The tube was cooled in an ice bath and centrifuged (4 000 r/min, 10 min) after which the absorbance (532 nm) of the supernatant was measured.The positive control was used for comparison.
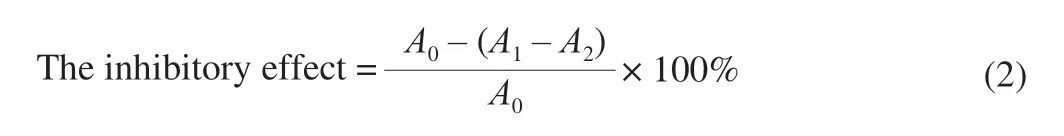
whereA0is control absorbance, use demineralised water rather than HUP,A1is sample absorbance,A2is the water absorption of the sample asA1, not mouse liver homogenate.
2.5 Acute toxicity studies
According to the Organization for Economic Co-operation and Development (OECD) guideline 423 [31], the acute oral toxicity study of HUP was carried out using female Wistar rats.A total of 24 female rats were randomly divided into 4 groups, each containing 6 rats.The control group only received normal saline, and the other test groups received increased doses of HUP to 250, 500, 750 mg/kg.Rats in all groups were given the same food and water under the same conditions.They were observed during the first 4 h and after that twice daily during the study period (21 days) for mortality, signs of toxicity and behavioural changes.Besides, weight changes, food and water intake were also recorded during the study.
2.6 Hepatoprotective effects of HUP in vivo
2.6.1 Experimental protocol
Approximate 150-200 g female Wistar rats were obtained from the small animal breeding station in Thrissur, Kerala, for research.All the rats were locked in cages to adapt to the following conditions: temperature: (22 ± 1) °C, humidity: 50%-55%, light/dark cycle: 12 h, water and feed: accessible always.The experiment was performed according to the experimental license agreement accepted by the Institutional Animal Ethical Committee (Reg No.659/02/a/CPCSEA).
The rats that have adapted to the environment in a week were randomly divided into 5 groups, namely (8 in each group): I.control group (NC), II.model control group (MC), III and IV.HUP treated group, V.positive control group (PC).During the tube feeding, the rats in the HUP treated groups received 200 mg/kg bw (HUP-L) and 400 mg/kg bw (HUP-H) respectively, while the PC group received Silymarin (50 mg/kg bw), and brine solution in the NC and MC groups.The gavage was performed daily for 7 days.Then, rats other than the normal control group orally took a 50% ethanol diluted in normal saline of 12 mL/kg bw 3 times every 12 h [32].After the last dose, the rats were fasted for 10 h and sacrificed by decapitation.
2.6.2 Preparation of serum and liver homogenate
The blood samples were centrifuged at 3 000 r/min for 10 min at 4 °C, immediately after collection, to provide serum which was preserved at -80 °C until the experiment.The liver tissue was taken out, washed immediately with cold 0.9% NaCl, homogenized in 10% Tris HCl buffer (0.1 mol/L; pH 7.4) and centrifuged at 3 000 r/min for 20 min at 4 °C.The supernatant was utilized for many biochemical analyses.
2.6.3 Determination of liver index
The body weight for each rat was recorded before sacrifice.The liver weight was measured immediately after sacrifice [33].
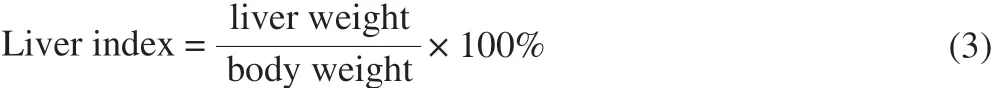
2.6.4 Biochemical assays
The activities of serum AST, ALT, ALP and lactate dehydrogenase were determined as per the reported procedures by Reitman et al.[34], King et al.[35], and King [36]et al.respectively.The total antioxidant capacity (TAOC) was determined by Benzie et al.[37].Total serum cholesterol (TC), triglycerides (TG), HDL-C, LDL-C, total bilirubin, total protein and albumin levels were measured according to the procedures described in the commercially available reagent kit.
The activities of liver and serum SOD, CAT, GSH-Px, glutathione reductase and glutathione-S-transferase were analysed as per the reported protocols of Daset et al.[38], Sinha [39], Ellman [40], Beutler [41]and Habig et al.[42], respectively.The hepatic protein content was determined by Lowry et al.[43].The levels of reduced glutathione and vitamin C in serum and liver were assessed by the method of Moron et al.[44]and Omaye et al.[45].The level of protein oxidation was identified in the liver by measuring the total protein carbonyl content according to the spectrophotometric method of Levine et al.[46].As described by Niehaus and Samuelsson [47], the lipid peroxidation reaction was estimated spectrally by measuring the reactivity of thiobarbituric acid (TBARS).The content of lipid hydroperoxides (LOOH) was determined according to the method of Jiang et al.[48].
2.6.5 Histopathological studies
The liver tissue was fixed in neutral formalin (10%), dehydrated in graded ethanol and immersed in paraffin wax.About 5 μm sections were stained with hematoxylin-eosin (H&E).These sections were analysed for histopathology using an optical microscope (YS100, Nikon, Japan).
2.7 Statistical analysis
The statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using SPSS software (version 20).All data were expressed as mean ± standard deviation (SD), andP< 0.05 was considered statistically significant.
3.Results and discussion
3.1 Characterization of HUP
HUP was prepared fromH.ulmariusby hot water extraction, ethanol precipitation to obtain partially purified polysaccharide.The chemical analysis results showed that HUP contained 76.98% total carbohydrate, 12.53% uronic acid, 11.29% sulfate and 4.66% protein (Table 1).
3.2 Chromatography and spectroscopy investigation
The monosaccharide arrangement of HUP was determined by comparing the retention time with that of standard sugars (Table 1, Fig.1A, 1B).HUP mainly contains 6 kinds of monosaccharides, including Xyl, Rha, Fuc, Man, Gal and Glc, and their mass percentages are 3.33%, 12.20%, 1.33%, 15.61%, 44.24% and 34.27%, respectively, indicating that Gal is the major monosaccharide followed by Glc in HUP.Yan et al.[49]reported that Glc, Gal and Man are the common sugars in edible mushroom.There were 2 peaks in the HPSEC elution profile of HUP, indicating that HUP having 2 fractions, is a small heterogeneous polysaccharide and molecular weight was estimated to be 2 249 and 1 867 Da (Fig.1C).The weight average molecular weight was 2 076 Da.This finding is consistent with the literature report that polysaccharides from wild Finnish mushroomCraterellus tubaeformisfractions consisted of two polysaccharide population with bimodal molecular weight [50].
Fig.1D shows the FT-IR spectrum of HUP, ranging from 500 cm-1to 4 000 cm-1.The characteristic broadband at 3 425 cm-1is because of O—H broadening vibrations [51], and weak absorption bands around 2 925 cm-1were assigned to C—H vibrations [52].The signal at 1 650 cm-1was ascribed to bound water [53].The strong peak at 1 650 cm-1originates from C=O stretching vibration, and the bond at 1 407 cm-1originated from the stretching vibration of —COOH indicating that there were carboxyl groups in HUP [54], which is in agreement with their uronic acid content (Table 1).The peak at 1 249 cm-1is because of the tensile vibrations of S=O, representing HUP contains sulphate [55].Furthermore, the band at about 1 000 cm-1may indicate that HUP is composed of pyran polysaccharides [56].A specific band at 1 200-1 000 cm-1called the “fingerprint” area is used to identify the primary chemical group like stretching vibrations of (C—O—H) side groups and the (C—O—C) glycosidic vibrations, representing that HUP is a polysaccharide.HUP is an anionic polysaccharide containing COO— and C=O groups as revealed by FT-IR analysis.
The NMR investigation demonstrates the linkage within the partially purified HUP fraction.The signals generated by the anomeric hydrogens typically appear in the range of 4.3-5.9 ppm and the hydrogens of theα-glycoside usually resonates from the protons of the correspondingβ-glycoside to the downfield of 0.3-0.5 ppm.Moreover, theα-anomeric protons (5.070 ppm) were further deshielded from theβ-anomeric proton (4.451 ppm) making these two anomers distinct (Fig.1E, 1F).The hydrogens present in the cyclic ring showed overlapping of signals between 3.260 and 4.144 ppm which are assigned to protons of C2 to C5 (or C6) carbons of the glycosidic ring.The overlapping of signals is due to the interaction of axial-equatorial hydrogens which always appears as multiplets in hydrogen NMR.These ring carbons appear between 60.730 and 76.243 in13C NMR.The initial structural analysis displays that the purified metabolite in this study is similar to polysaccharides from other fungi [9,10].
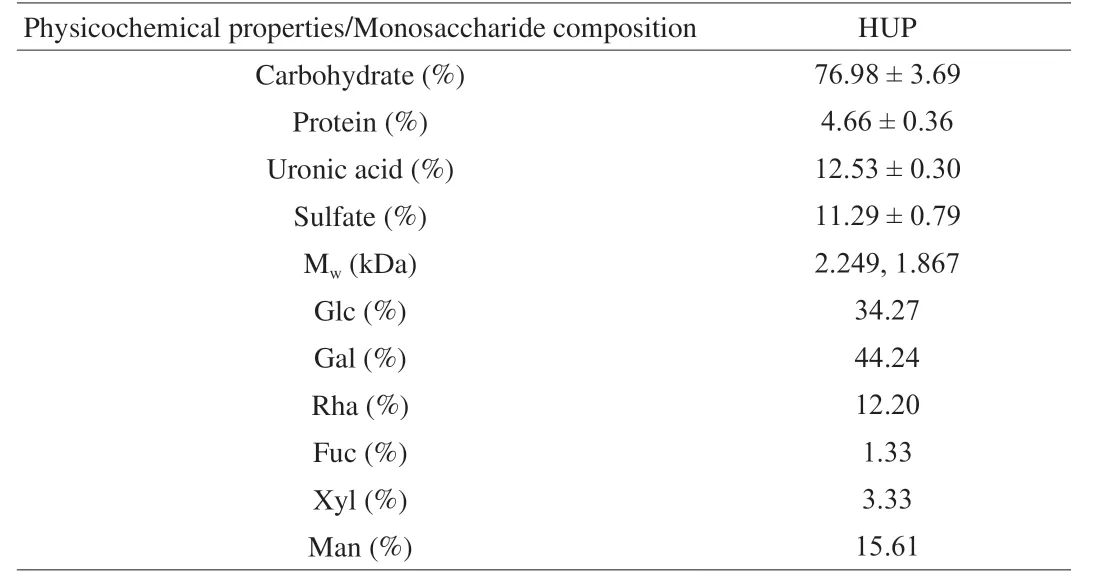
Table 1 Physicochemical properties, molecular weight and monosaccharide composition analysis of HUP.
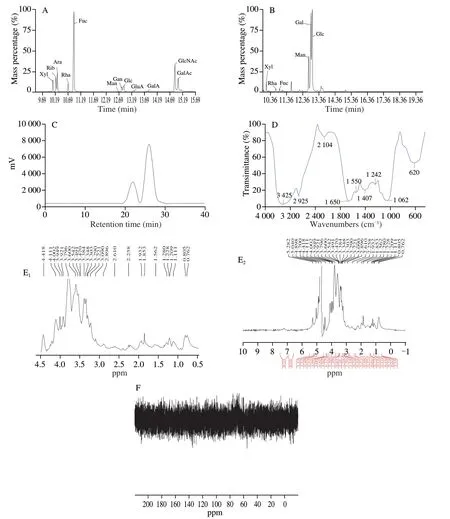
Fig.1 Characterization of HUP.(A) GC chromatograms of standard monosaccharides, (B) GC chromatograms of HUP (C) molecular weight, (D) FT-IR, (E) 1H NMR and (F) 13C NMR.
3.3 In vitro antioxidant activity of HUP
DMPD radical decolourization activity, cupric ion reducing capacity, and lipid peroxidation inhibition activity of HUP were tested, and the results are illustrated in Fig.2.HUP displayed dosedependent scavenging activity in all the scavenging assays in the tested concentrations.HUP expressed good scavenging activity on DMPD radical (21.09% to 70.76% at 0.5-2.5 mg/mL), moderate cupric ion reducing power (0.285 to 0.565 at 0.5-2.5 mg/mL) and lipid peroxidation inhibition effect (29.87% to 47.80% at 1-5 mg/mL).However, HUP exhibited less effect as compared to ascorbic acid in all assays.
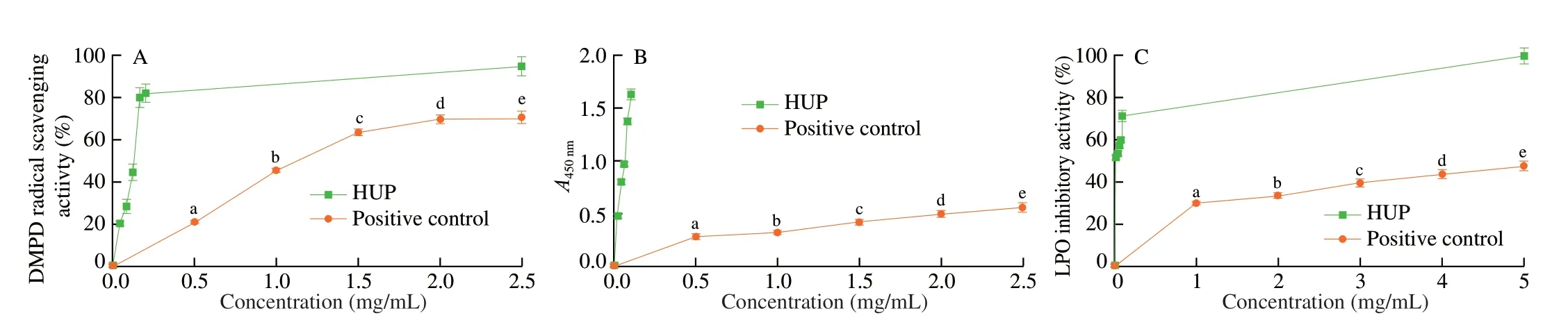
Fig.2 In vitro antioxidant activities of HUP.(A) DMPD radical scavenging assay, (B) cupric ion reducing power assay, (C) lipid peroxidation inhibition assay.Different letters indicate a significant difference (P < 0.05) between different concentrations of HUP.Data are expressed as mean ± SD (n = 3).
The DMPD assay is a method for measuring the ability of hydrogen donating antioxidants.The copper reducing capacity of polysaccharides is a vital indicator index of their antioxidant potential.Lipid peroxidation inhibition shows its protective effect on biological macromolecules and inhibits free radical chain reactions.Polysaccharides may play a role in scavenging hydrogen atoms or electrons in free radicals, and the largest electronic donor may be the hydroxyl or carboxyl groups of polysaccharides.Presence of electron-withdrawing groups such as carboxyl or carbonyl group in polysaccharide could lower the dissociation energy of O—H bond leading to release of a hydrogen atom.Besides, low molecular weight polysaccharides can have more reducing hydroxyl ends to react with free radical substances and have higher antioxidant activity.It has been determined that uronic acid residues significantly affect physiological, biological and pharmacological properties of polysaccharides.This is because the basic structure of biopolymers has changed, thereby changing their physical and chemical properties, resulting in characteristic biological activities and solubility behaviour.Probably, the antioxidant ability may be related to its chemical composition and structure (such as molecular weight, monosaccharide composition and conformation).
3.4 Acute toxicity studies
The results of acute toxicity test are depicted in Table 2.The variations in body weight have been utilized as an index of harmful reactions to medications and chemicals [57].During the 21 days treatment period, there was no substantial difference in general behaviour, body weight and relative organ weight between the 3 doses of HUP group and the NC group.Compared with the NC group, serum biochemical parameters did not change significantly, indicating that HUP is non-toxic.There was no change in AST, ALT and ALP activities, suggesting that HUP did change liver cell function and metabolism.There were no statistically substantial changes in haematological parameters between HUP-treated rats and control groups, indicating that HUP does not affect hematopoiesis.
3.5 Hepatoprotective effects of HUP
3.5.1 Effect of HUP on liver index
The liver index effectually reflects the degree of liver damage.In comparison to the normal control rats, the liver index of the alcoholinduced rats was significantly increased (P< 0.05), causing severe injury to liver tissue, leading to hepatomegaly.HUP-L and HUP-H significantly reduced the liver index (Fig.3).
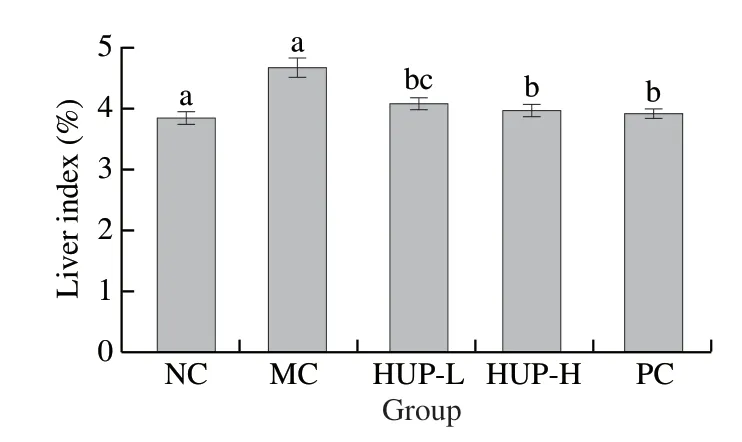
Fig.3 Effects of HUP on liver index in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
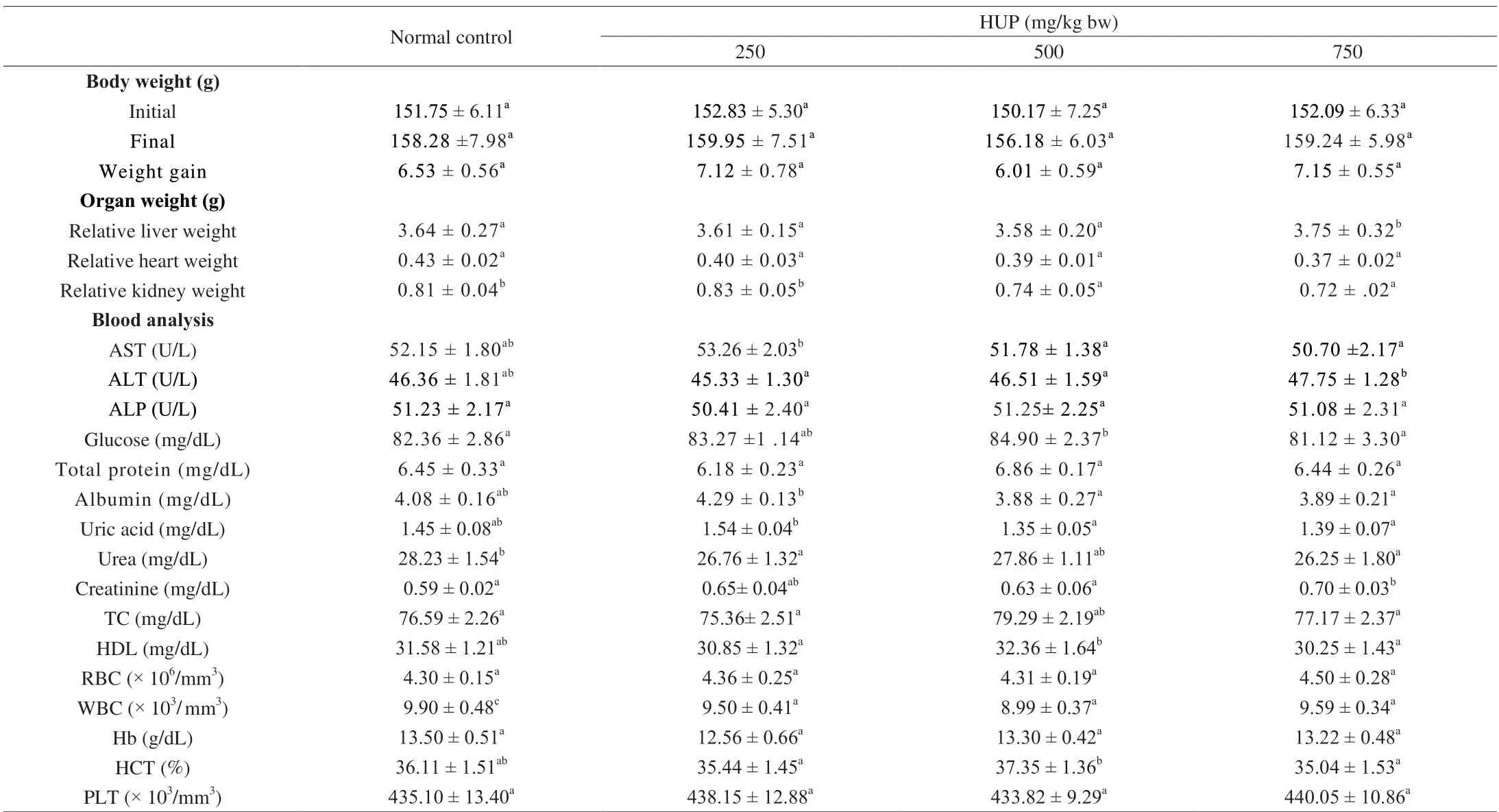
Table 2 Results of acute toxicity of rats fed HUP for 21 days.
3.5.2 Effect of HUP on serum marker enzymes and biochemical constituents
Fig.4 summarize the activities of marker enzymes (AST, ALT, ALP and LDH) in the serum of normal control and experimental groups.In this study, the significant (P< 0.05) increase in AST (1.9 folds), ALT (2.2 folds), ALP (2.5 folds) and LDH (2.2 folds) activities proved ethanol-induced liver cell damage.The administration of two different doses (200 and 400 mg/kg bw) of HUP significantly reduced the ethanol-induced elevation of hepatic marker enzymes in a dose-dependent fashion.HUP-H produced the maximum effect, which is comparable to silymarin (50 mg/kg bw).
Serum ALT, AST and ALP are the most reliable markers for diagnosing liver injury since they are cytoplasmic and released into the circulation after cell injury [58].The high concentrations of serum transaminase are a pointer of liver damage, and the increase in ALT is a more sensitive indicator.The increase in serum transaminase activity might be due to the release of these enzymes from the cytoplasm, which quickly enters the circulation after plasma membrane rupture and cell damage.Free radicals liberated by ethanol metabolism may have caused damaged liver cell membranes, causing cytoplasmic contents to leak into the systemic circulation.HUP pre-treatment reduced enzyme activity, indicating that it has the potential to escalate plasma membrane stability, thus conserving the structural integrity of hepatocytes.Aforementioned findings are supported by histopathological observations.These results are in accordance with the results ofPleurotus geesteranus[9]andCoprinus comatuspolysaccharide [19].Mushroom polysaccharides can effectively protect liver damage.
After ethanol injection, compared with the NC group, serum total bilirubin and nitric oxide levels were considerably increased (P< 0.05), while total protein and albumin levels decreased, suggesting liver toxicity.HUP-L, HUP-H and silymarin prevented ethanol-induced liver damage.
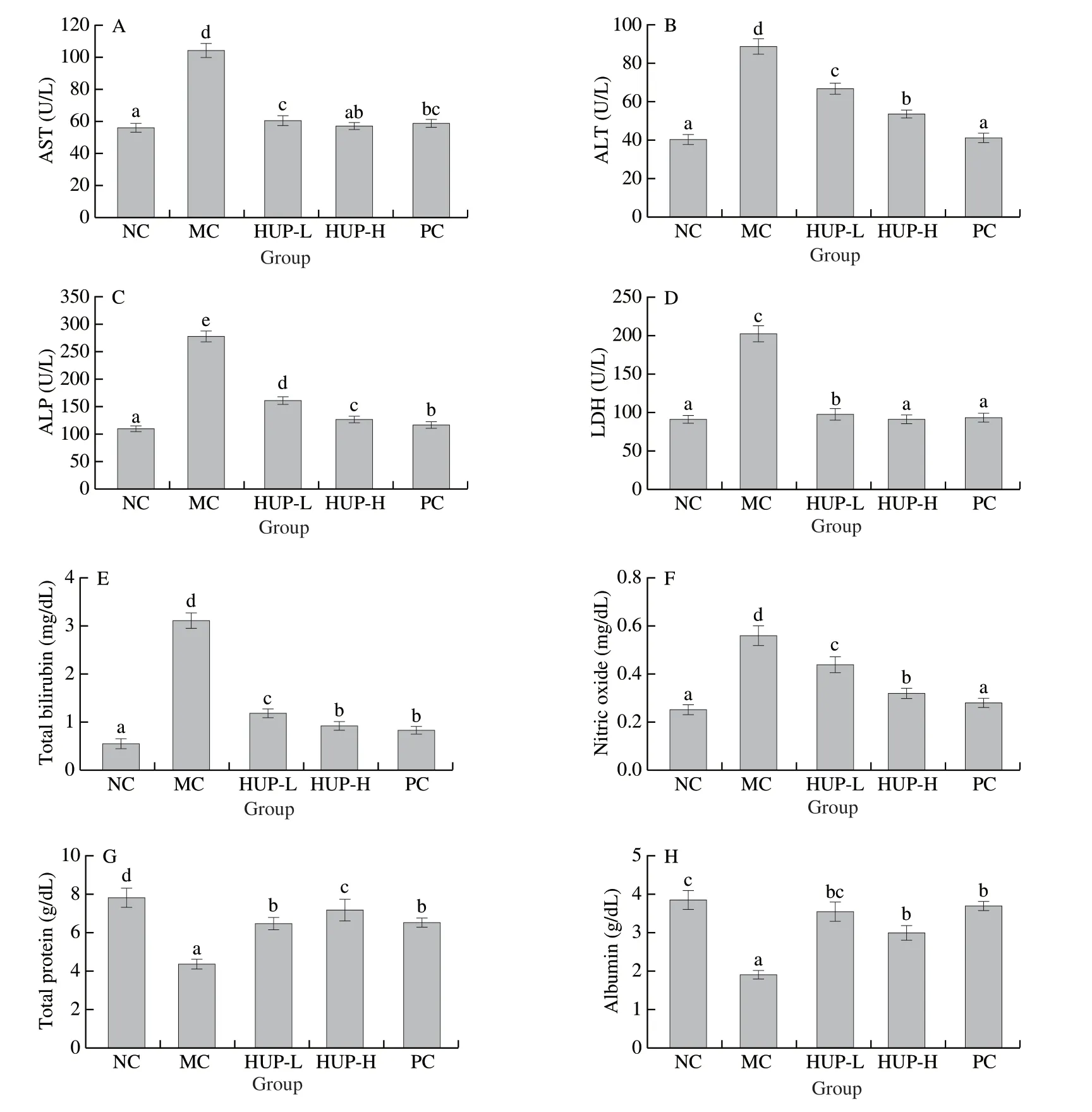
Fig.4 Effects of HUP on serum enzymes and biochemical indices.(A) AST, (B) ALT, (C) ALP, (D) LDH, (E) total bilirubin, (F) nitric oxide, (G) total protein and (H) albumin in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
Bilirubin has been used to assess chemical-induced liver damage.It is an important breakdown product of haemoglobin and is normally excreted in the bile.The best-known necrotizing agents (such as ethanol) cause sufficient damage to the liver parenchyma, resulting in a substantial increase in bilirubin level, which is one of the most sensitive tests for diagnosing liver disease.Any normal increase in serum total bilirubin level indicates hepatobiliary disease and severe liver cell dysfunction.Due to defects in protein biosynthesis in the liver, total protein levels will decrease under hepatotoxicity [59].Albumin is a key component of serum protein synthesized by the liver, is used to monitor liver function [60].A decline in serum total protein and albumin indicates decreased liver function.The nitrite/nitrate level is a reliable indicator of NO production.In this study, alcohol ingestion augmented NO levels, which may further lead to toxicity through the formation of peroxynitrite (an effective oxidant), that generates nitration of tyrosine and cause biological inactivation of essential proteins and enzymes [61].Stabilization of serum bilirubin, nitric oxide, protein and albumin levels by administering HUP and silymarin further shows the improvement of the functional status of liver cells.
3.5.3 Effect of HUP on hepatic enzymic antioxidants
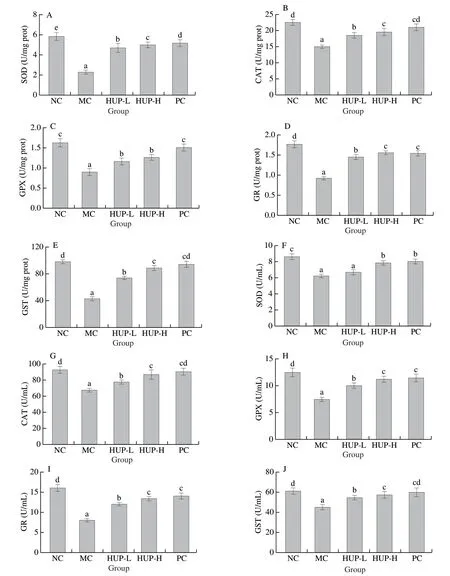
Fig.5 Effects of HUP on hepatic enzymic antioxidants (A) SOD, (B) CAT, (C) GPx, (D) GR, (E) GST and serum enzymic antioxidants (F) SOD, (G) CAT, (H) GPx, (I) GR, (J) GST in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
The human body has several mechanisms to prevent oxidative stress caused by free radicals which can be done by key antioxidant enzymes like SOD, CAT and GPx.In comparison to the normal control group, the activities of all enzymatic antioxidants in the ethanol-treated rats were significantly reduced (P< 0.05) (Fig.5), which indicates increased oxidative damage to the liver.In the HPU pre-treatment group (200 and 400 mg/kg bw), the activities of all enzymes increased significantly, indicating that the enzyme activities were restored.The serum and liver antioxidant enzyme activities in the HUP treatment group were expressed in a dose-dependent manner.The hepatic SOD, CAT, GPx, GR and GST activities in the HUP-400 group were the highest, which were 127.94%, 42.41%, 60.00%, 67.74% and 106.23% higher than those in the MC group.Serum SOD, CAT, GPx, GR and GST activities were 31.77%, 29.24%, 49.93%, 66.54% and 37.83% higher than the MC group.The recovery of these enzyme activities after HPU administration indicates that HPU may be an effective antioxidant, which might be related to the free radical quenching activity of antioxidants.HPU has phytochemicals such as phenols, flavonoids and polysaccharides, which can effectively scavenge free radicals.Similar results were reported fromPleurotus geesteranus[9]andCoprinus comatus[19]polysaccharide.
SOD, a vital defence enzyme, catalyse the disproportionation of superoxide to hydrogen peroxide (H2O2) and O2[62].CAT found in all aerobic cells breaks down H2O2into O2and H2O.GPx plays a major role in the reduction and detoxification of peroxides [63].GST plays a crucial role in the detoxification and excretion of xenobiotics [64].Enhanced antioxidant enzyme activity may be one of the primary mechanisms promoting liver protection.It is believed that scavenging free radicals play an essential role in the treatment and prevention of liver damage caused by alcohol.
3.5.4 Effect of HUP on hepatic non-enzymic antioxidants
Glutathione is the main antioxidant of the human body and the main non-protein thiol in the organism.It displays a vital part in synchronizing the innate antioxidant defense mechanism.It is a non-enzymatic antioxidant and with various enzymatic processes catalyses the reduction to hydrogen peroxide and hydroperoxide [65].Vitamin C, another major non-enzymatic antioxidant acts as a free radical scavenger and is most susceptible to free radical oxidation [66].T-AOC is a key indicator of antioxidant capacity and is associated with non-enzymatic antioxidant defence [67].Ethanol intoxication resulted in a reduction in serum and liver GSH, vitamin C and TAOC levels in comparison with NC group (Fig.6).However, rats pre-treated with HPU in the dose of 200 and 400 mg/kg before ethanol treatment restored the levels of non-enzymic antioxidants.A remarkable dose-dependent increase was observed with a maximum increase in 400-HUP group, reaching the same level as that of standard drug silymarin, suggesting that non-enzymatic antioxidant defence systems of rats were bolstered.
3.5.5 Effect of HUP on antioxidant stress markers
Oxidative stress, one of the crucial factors leading to the pathogenesis of the ALD is produced by increased free radical production and enhanced peroxidation of lipids [68].The protective effect of HPU on ethanol-induced oxidative stress markers is depicted in Fig.7.In this study, compared with the NC group, alcohol induction significantly increased liver lipid peroxide (LPO), hydroperoxide (HPO), and protein carbonyls (PCO).However, pre-treatment with HPU (200 and 400 mg/kg) inhibited the elevation of oxidative stress markers to near normalcy in a dose-dependent manner.The hepatic LPO, HPO and PCO levels were decreased by 50.45%, 29.25% and 39.09% in the HUP-400 group.The results show that HUP reduces alcohol-induced stress markers in rats, which further provides evidence that HUP has a hepatoprotective effect.
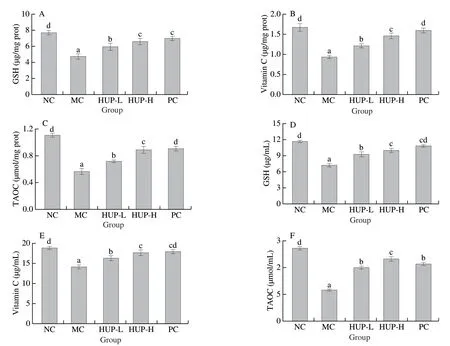
Fig.6 Effects of HUP on hepatic non-enzymic antioxidants (A) GSH, (B) vitamin C, (C) TAOC and serum non-enzymic antioxidants (D) GSH, (E) vitamin C, (F) TAOC in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
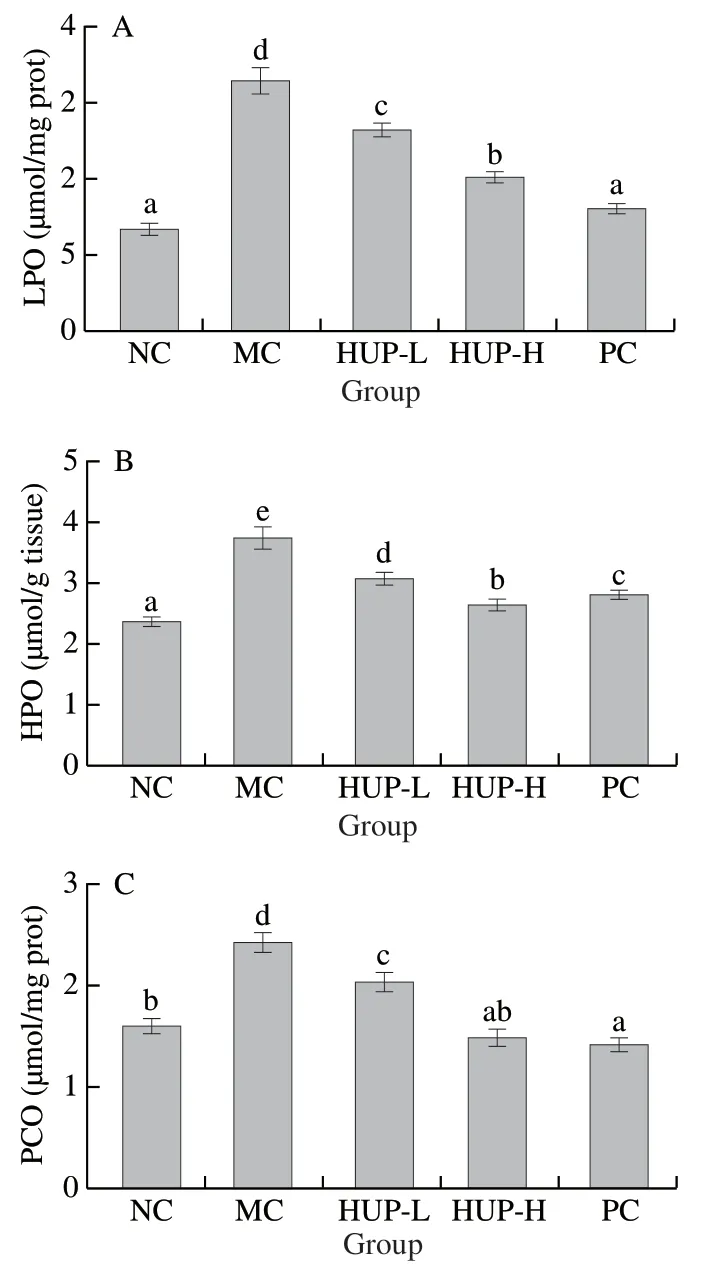
Fig.7 Effects of HUP on oxidative stress markers.(A) LPO, (B) HPO and (C) PCO in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
As an indirect index of oxidative stress, MDA, the final product of lipid peroxidation, is formed by the interaction of polyunsaturated fatty acid and ROS, and cause damage to the cell membrane.Hydroperoxides are unstable intermediates of lipid peroxidation byproducts formed from PUFA oxidation and cause membrane damage.The primary hallmark of oxidative stress is protein carbonylation.The most common protein oxidation products in biological samples are the carbonyl derivatives of arginine, lysine, proline, and threonine residues, which can be used as markers for oxidative stress in most types of ROS.The oxidative damage of protein is reflected in the increase of protein carbonyl content in poisoned rats by ethanol.Besides, MDA formed during lipid peroxidation can be incorporated into proteins by reacting with theα-amino group of lysine or the sulfhydryl group of cysteine to form carbonyl derivatives [69].HUP treatment restored oxidative markers to normal levels, which is due to the antioxidant activity of HUP.
3.5.6 Effect of HUP on serum lipid profile
As demonstrated in Fig.8, the levels of TC, TG, HDL-C, LDL-C and VLDL-C in ethanol intoxicated group were considerably increased (P< 0.05), while the levels of HDL-C were decreased, indicating that alcohol caused serum lipid metabolic disorder.This increase in serum LDL concentration induced by ethanol may be due to the production or failure of LDL receptors.HDL has a protective role in reverse cholesterol transport, inhibits LDL oxidation and neutralizes the atherogenic effects of oxidized LDL-C.The increase in LDL and VLDL leads to a decrease in HDL.This decrease may also be due to a decrease in the activity of lecithincholesterol acyltransferase activity [70].Treatment of HPU (200 and 400 mg/kg bw) resulted in a significant reduction in VLDL, a substantial decrease in LDL and an escalation in HDL-C.The effects of HUP were comparable to that of standard drug silymarin.Our results indicate that HUP can provide anti-hyperlipidemic activity against alcohol toxicity.
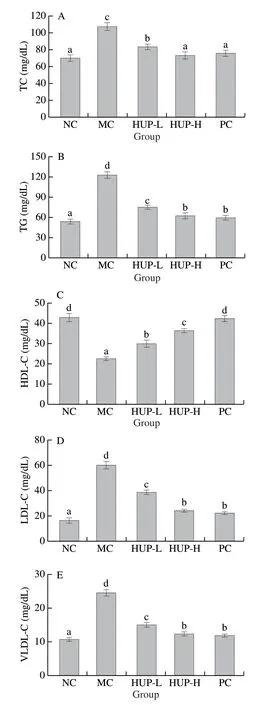
Fig.8 Effects of HUP on serum lipid levels of (A) TC, (B) TG, (C) HDL-C, (D) LDL-C and (E) VLDL-C in alcohol-induced rats.Data are represented as mean ± SD with n = 8 rats for each group.Bars with different letters are significantly different (P < 0.05).
ALD is clinically associated with severe dyslipidaemia and can be assessed by the levels of TC, TG, HDL-C and LDL-C [9].Enormous consumption of alcohol may lead to excess fat accumulation in the liver leading to alcoholic fatty liver (steatosis) [71].The liver lipid contents are increased by the high levels of TC and TG in the liver, by a mechanism in which HDL-C uses the “cholesterol transport” pathway for the transfer of TC from the liver to the surrounding tissues, leading to the development of liver disease.Besides, the LDL-C and VLDL-C influence irregular accumulation of atherosclerotic plaque formation on a blood vessel wall, leading to increased oxidation levels [72].In short, high levels of HDL-C and low levels of LDL-C and VLDL-C can transport alcohol from surrounding tissues to the liver, thereby catabolising it in the blood circulation.In the current work, the blood lipid level in the alcohol-induced rats improved significantly after feeding HUP, indicating that HUP can diminish alcohol-induced dyslipidemia.The above results are consistent with reports of Zhao et al.[19]and Song et al.[9]that mushroom polysaccharides can effectively reduce liver lipids caused by alcohol.
The hepatoprotective activity of polysaccharides is mainly related to its monosaccharide composition, and the proportion of monosaccharide is also crucial for biological activity.Generally, polysaccharides have a more complex composition of monosaccharides and have better hepatoprotective activity.The PSP-1b1 obtained from mycelium ofCoriolus versicoloris composed of 7 kinds of monosaccharides and has hepatoprotective activity, which may be attributed to the higher content of Glc, Gal and Man [10], which is consistent with this study.The molecular weight of polysaccharides is an important factor affecting biological activity.Four polysaccharide components (Abnp1001, Abnp1002, Abap1001 and Abap1002) with hepatoprotective activity were isolated fromAgaricus bisporus.The smaller molecular weights of Abnp1002 and Abap1002 show more effective liver protection by reducing serum enzymes [73].These reports are consistent with the present research that HUP is a lower molecular weight polysaccharide with hepatoprotective effect.The biological activity of polysaccharides is also related to the content of uronic acid.As one of basic components of polysaccharides, the content of uronic acid is directly related to the free radical ion scavenging activity [74].Three polysaccharides (PSWP, PSAP-1 and PSAP-2) were isolated from purple sweet potato tubers.Among them, PSWP (hot water extract) with the highest uronic acid content showed more substantial hepatoprotective effect than PSAP-1 (0.5 mol/L NaOH extract) and PSAP-2 (2 mol/L NaOH extract) [75].HUP has a higher uronic acid level than PSWP and has a liver-protective effect.Therefore, it can be concluded that high uronic acid content is beneficial to the hepatoprotective activity of polysaccharides.
3.5.7 Histopathological studies
Histopathological examination of the liver cortex was performed, and Fig.9 shows a photomicrograph of liver tissue stained with hematoxylin and eosin.The NC group showed regular liver cell morphology, clear cytoplasm, normal nucleus, distinct cell boundaries, and no fat steatosis.Histological examination of ethanol-treated rat liver showed damage to the entire liver parenchyma, showing vacuolation, typical hepatic cord loss, ballooning degeneration, fatty accumulation and necrosis of hepatocytes.Pre-treatment with HUP and silymarin significantly improved histopathological changes as revealed by milder necrosis, lymphocyte infiltration and liver fat change.At the pre-treatment concentration of HPU-400 group, hepatic architectures were like that of the NC group.This finding is consistent with the level of the enzyme markers.
4.Conclusion
HUP exhibited antioxidant, liver protective and lipid-lowering activities in ethanol-induced rats.The preliminary characterization showed that HUP is a pyran polysaccharide containing Gal (44.24%), Glc (34.27%), Man (15.61%) along with the small amount of Xyl (3.33%), Fuc (1.33%) and Rha (1.20%).The results ofin vivoexperiments show that HUP pre-treatment can effectively prevent ethanol-induced liver damage, which can be demonstrated by reducing serum marker levels (ALT, AST, ALP and LDH), liver oxidation markers (LPO, HPO and PCO), and significantly restoring liver and serum enzymic (SOD, GPx, CAT, GST and GR) and non-enzymic antioxidants (GSH, vitamin C and TAOC) in alcohol-intoxicated rats.Besides, we proved that HUP has moderate DMPD free radical scavenging activity, lipid peroxidation inhibition and iron-reducing ability.The liver protection mechanisms of HUP may be related to its ability to prohibit lipid peroxidation, retain antioxidant behaviour and decrease oxidative stress.This is the first evaluation report of liver protection activity from HUP.Therefore, the results show that HUP can protect the biological system from oxidative stress through its antioxidant activity, thereby having a substantial hepatoprotective effect on acute alcoholic liver injury in rats.Our work has preliminarily shown that HUP may be one of the essentially active substances responsible for protecting the liver.These findings indicate that HUP can be utilized as potentially non-toxic health nourishment and as a good liver protectant.However, further investigation is required to explore the underlying accurate molecular mechanisms, which is the basis of HUP’s protective effect on liver injury.
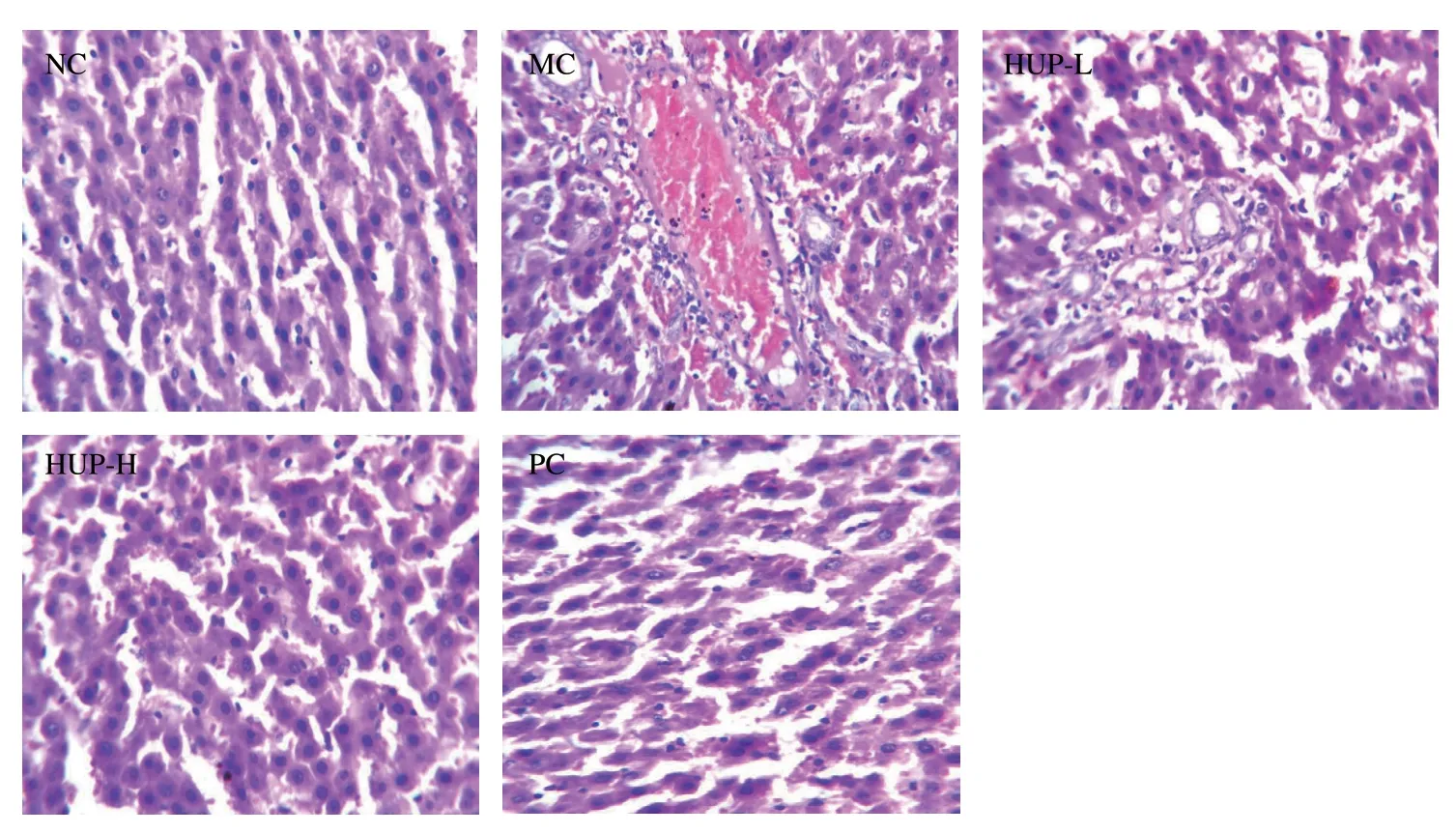
Fig.9 Effects of HUP on hepatic morphological changes in alcohol-induced rats (magnification × 250).
Conflict of Interest
The authors declare they have no conflict of interest.
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Amelioration of metabolic disorders by a mushroom-derived polyphenols correlates with the reduction of Ruminococcaceae in gut of DIO mice
- Immunomodulatory effects of polysaccharides from edible fungus: a review
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
