The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice
2021-05-24YngeLiuRonglongChenLnzhouLiRuitoDongHuiYinYwenWngAnhuiYngJininWngChngtinLiDiWng
Ynge Liu, Ronglong Chen, Lnzhou Li, Ruito Dong, Hui Yin, Ywen Wng, Anhui Yng, Jinin Wng, Chngtin Li*, Di Wng,*
a School of Basic Medical Sciences, Nanchang University, Nanchang 330038, China
b School of Life Sciences, Jilin University, Changchun 130012, China
c Department of Thoracic Surgery, The First Affi liated Hospital of Nanchang University, Nanchang 330006, China
d Engineering Research Center of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University, Changchun 130118, China
Keywords:An trodia cinnamomea mycelia Triterpenoids Chronic alcohol-induced liver injury Lipid accumulation
ABSTRACT The major pathologic hallmark of the alcoholic liver disease (ALD) is the representation of chronic alcohol-induced hepatocyte lipid accumulation.This study aims to investigate the hepatoprotective role of triterpenoids-enriched extracts from Antrodia cinnamomea mycelia (ACT) in chronic alcohol-induced liver injury mice, establishing in C57BL/6 mice through gradient alcohol feeding for 24 weeks.In longterm alcohol consumption mice, the significantly lost body weight, increased organ indexes, hepatic alanine aminotransferase and aspartate aminotransferase levels were all remissed after 6-week ACT orally administration, showing its hepatoprotective property.ACT suppressed the triglyceride, total cholesterol and low-density lipoprotein levels, and enhanced high-density lipoprotein levels in serum or/and liver of chronic alcohol damaged mice.Combining with the pathological observations, ACT displayed an anti-steatosis effects to restrain the progress of ALD.Based on proteomic analysis and enzyme-linked immunosorbent assay, ACT had been confi rmed to regulate the levels of lipid biogeneration-related factors and depressed the over-accumulation of hepatic reactive oxygen species.According to further data, ACT prevented alcoholic liver injury may be associated with mediating lipid metabolism-related to PGC-1α and NF-κB signaling.In summary, ACT protected the body against chronic alcohol ingest induced liver injury through its regulation lipid on metabolism.
1.Introduction
According to the World Health Organization (WHO) report, the alcohol abuse kills more than 3 million people each year worldwide.Chronic and excessive alcohol consumption causes alcoholic liver disease (ALD) [1], which ranges from steatosis, steatohepatitis to fibrosis, cirrhosis and even hepatocellular carcinoma [2].The prevalence of hepatic steatosis increased to 46% in subjects with a daily intake of > 60 g alcohol, and steatosis was found in even 95% of obese individuals [3].
Alcohol is mainly metabolized in hepatocytes, where alcohol can be oxidized into acetaldehyde, which counteracts fatty acid oxidation promoting fatty acid and glycerolipid synthesis, thus resulting in fat accumulation of hepatocytes [4,5].According to the “two-hit” hypothesis of the pathogenesis of ALD, steatotic liver is the first hit, which is susceptible to the secondary insults including vulnerability to adipocytokines, reactive oxygen species (ROS) and other cytokines [6].
Fatty degeneration promotes the development of ALD, and during this process, the maintaining mitochondrial function plays an important role.Cytochrome P450 2E1 (CYP2E1) has been reported to regulate the oxidization of alcohol to acetaldehyde.Accordingly, mitochondrion-targeted CYP2E1 markedly augmented due to alcohol toxicity; however, the microsome-targeted CYP2E1 had a marginal influence on mediating alcohol toxicity [7].CYP2E1 contributes to suppress oxidation of fatty acids through inhibiting the up-regulated peroxisome proliferator-activated receptor by alcohol, leading to fatty liver [8].The enhanced levels of free fatty acids (FFA) due to the blockage on fatty acids oxidation initiates the activation of nuclear factor-kappa B (NF-κB), which can be inhibited by the activated AMP-activated protein kinase (AMPK) [9].Previous work supported that restraining on the activation of NF-κB would protect against alcohol-induced liver injury [10].The regulation on lipid metabolism is considered as an effective way for treatment on alcoholic liver disease.
Multiple fungi and their natural products, with various biological activities, have been consider as medicines for centuries.Except for polysaccharides, triterpenoids are important active ingredients inAntrodia cinnamomea.A.cinnamomea, also known asniuchang chih, is a precious medicinal fungus in Taiwan, China [11].Some previous studies have reported the hepatoprotective effects of triterpenoid compounds extracted fromA.cinnamomeain alcoholic liver injured mice, such as antrosterol [12]and antroquinonol [13], mainlyviarestraining inflammatory response.In our group, the triterpenoids extracted fromA.cinnamomeamycelia obtained by submerged fermentation contained at least 25 types of triterpenoid compounds, which could attenuate acute alcohol-induced liver injury in mice through the modulation of inflammatory response [14].However, the hepatoprotection ofA.cinnamomeatriterpenoids has not been systematically reported in the chronic alcohol consumption induced liver injured mice, which is different from the acute alcohol liver damaged mice on pathological characteristics and pathogenesis, such as the degree of alcoholic steatohepatitis and inflammatory responses.
In this paper, we aimed to determine the alleviation of triterpenoids-enriched extracts fromA.cinnamomeaon long-term alcohol consumption induced alcoholic liver injury in C57BL/6 mouseviaregulation the lipid metabolism.
2.Materials and methods
2.1 A.cinnamomea culture and sample preparation
The submerged fermentation ofA.cinnamomeaand the extraction of triterpenoids from theA.cinnamomeamycelia were the same as our previous study [14].The strain used in this paper was bred based on nitrosoguanidine (NTG) mutagenesis fromA.cinnamomea(ATCC200183, American Type Culture Collection, MD, USA), identified and conserved in China General Microbiological Culture Collection Center (CGMCC) and numbered AC09.Accordingly, triterpenoids were extracted in 80% ethanol and purified by AB-8 type amberlyst column.The fractions from 80% and 100% ethanol elutions were collected and then ethanol was removed.The concentration of the triterpenoids-enriched extracts fromA.cinnamomeamycelia (ACT) was assessed using vanillin-glacial acetic acid and perchloric acid colorimetric spectrophotometry [15].
2.2 Animal experiment design
The study protocol was approved by the Institutional Animal Ethics Committee of School of Life Sciences, Jilin University (NO.SY0502).Similar to our previous study [16], the chronic alcoholinduced liver injury mice were established as follows briefly.Forty eight C57BL/6 male mice (8-week-old; (18-22) g; SCXK (Liao)-2015-0001, Liaoning Changsheng Biotechnology Co., Ltd, Liaoning, China) were housed in a controlled room (relative humidity: (50 ± 5)%; temperature: (23 ± 1) °C; illumination: 12 h light/dark cycle).
After 7 days acclimatization, to induce alcoholic liver disease, all mice but the control mice (n= 8) were administered a drinking water containing 5% alcohol in sterilized tap water.The alcohol content was gradually increased from 5% to 30%, with a 5% increment per week for adaptation, and then 30% alcohol was used for daily drinking for the next 18 weeks.From the 18th week, alcohol-induced mice were randomized separated for 5 groups (n= 8 per group), and orally received with normal saline, 63 mg/kg of silymarin (Sil) (Madaus AG, Cologne, Germany), 5, 15 and 45 mg/kg of ACT for 6 weeks.The control mice were orally received with normal saline for 24 weeks.The body weights of all group mice were monitored at the 1st, 5th, 10th, 19th, 20th, 21st, 22nd, 23rd, and 24th week (Fig.1).
2.3 Sample collection and organ index measure
After the last treatment, all mice were fasted for 8 h and weighed.Blood samples were drawn from the caudal vein.Then, mice were anesthetized with isoflurane inhalation and sacrificed.The liver, kidney, spleen and heart were collected and weighed, one part of those organ were fixed with 4% paraformaldehyde and the remainder were stored immediately at -80 °C.
The organ index was calculated by the following formula:
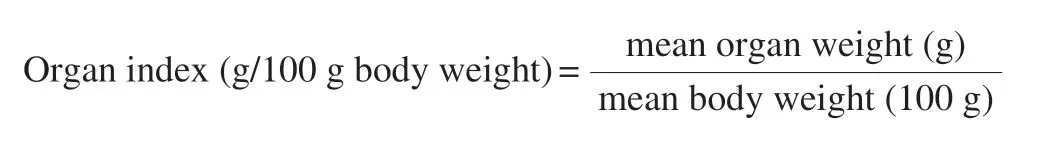
2.4 Pathological examination and TUNEL assay
The fixed liver, kidney, spleen and heart tissues were dehydrated with alcohol and xylol, then were embedded in paraffin and sliced into 5 μm continuous sections.All tissue specimens were processed for haematoxylin and eosin (H&E) and the renal sections were stained with periodic acid-Schiff (PAS) stain.Images were collected with an inverted microscope (400 ×) (IX73, Olympus, Tokyo, Japan).
Cell apoptosis in liver were quantified using the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) kit (C10617, Life Technologies, California, USA) according to manufacturer’s protocol.Fluorescence images were taken using a Nikon Eclipse TE 2000-S fluorescence microscope (200 ×; CCD camera, IX73, Olympus, Tokyo, Japan).
2.5 Proteomics experiments
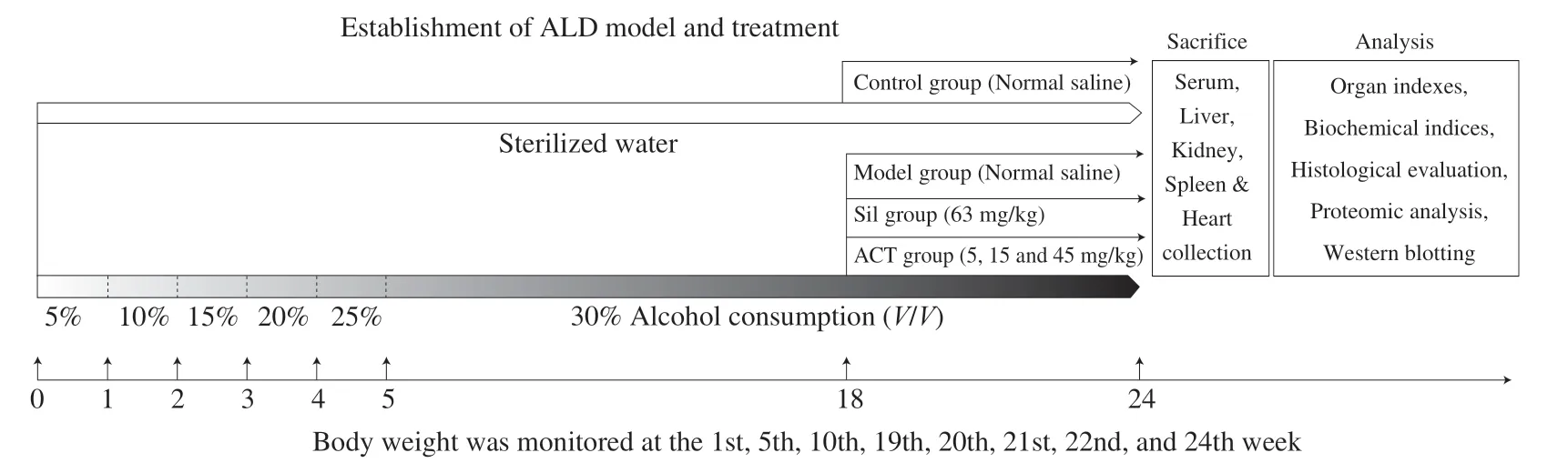
Fig.1 The experimental protocol and drug administration.
A portion of the liver from normal control, model, positive control and 15 mg/kg of ACT-treated group mice were sonicated in ice-cold cell lysis buffer and centrifuged at 12 000 r/min for 15 min.The clear supernatant liquid was separated for total protein content by the bicinchoninic acid (BCA) protein assay kit (Merck Millipore, Burlington, Massachusetts, USA).100 mg of total protein from each sample was diluted with cell lysis buffer to a final 1 mg/mL protein concentration.Proteins were precipitated by adding acetone on ice, and the precipitate was collected by centrifugation at 12 000 r/min for 10 min.Precipitated proteins were dissolved in 100 mmol/L ammonium bicarbonate buffer containing 1% sodium desoxycholate.The disulfide bond of proteins was reduced by adding tris (2-carboxyethyl) phosphine, alkylated with iodoacetamide, and were then incubated with trypsin overnight at 37 °C.The samples were added in trifluoroacetic acid to precipitate sodium deoxycholate (pH < 2), and then centrifuged at 10000 r/min for 10 min.The polypeptides were extracted from the supernatant with 2% trifluoroacetic acid by centrifuging at 10 000 r/min for 20 min.
The final supernatant was loaded onto a C18type column to remove the salt.2 mg of peptides were separated and analyzed with a nano-Ultra Performance Liquid Chromatography (EASY-nLC1200) with Q-Exactive mass spectrometry (Thermo Finnigan).Separation was performed using a reversed-phase column (100 μm, ID × 15 cm, Reprosil-Pur 120C18-AQ, 1.9 μm, Dr.Math) and the conditions were as follows: Mobile phase A: water + 0.1% formic acid + 2% acetonitrile ; Mobile phase B: water + 80% acetonitrile + 0.1% formic acid; Flow rate: 300 nL/min; Gradient time: Mobile phase B: 8%-30% for 92 min, 30%-40% for 20 min, 40%-100% for 2 min, 100% for 2 min,100%-2% for 2 min and 2% for another 2 min.
Data dependent acquisition and positive mode with Orbitrap analyzer was used in mass spectrometry analyses.A resolution of 70000 (at 200m/z), automatic gain control (AGC) of 3.0E+6 with max IT 50 ms apply to MS1; A resolution of 17500, AGC of 1E+5 with max IT 45 ms apply to MS2; normalized collision energy was 27% and isolation window was 2m/z.
The results were performed using MaxQuant (1.5.6.0) and the protein database was derived from UNIPROT database (Uniprot_mouse_2016_09) with the protein sequence and its inverted decoy sequence used in MaxQuant search.Quantitative data are presented as the label free quantitation (LFQ) with match between run.The quantitative results after standardization were analyzed to obtain the corresponding differentially expressed proteins.The proteins with different expression multiples (ratio A/B > 1.5 or ratio A/B < 0.67) were defined as significant proteins, and then subsequent gene ontology (GO), kyoto encyclopedia of genes and genomes (KEGG) pathway were performed and displayed.
2.6 Biochemical assays
A portion of the livers of the mice in each group were weighed and homogenized in saline solutions on ice.After centrifugation (10 000 r/min; 10 min; 4 °C), supernatants were collected and used for determining the levels of alanine aminotransferase (ALT) (CKE90314M), aspartate aminotransferase (AST) (CK-E91386M), triglyceride (TG) (CK-E91733M), total cholesterol (TCHO) (CKE91839M), fibrinogen-like protein 1 (FGL1) (CK-E20558M), fibrinogen γ chain (FGG) (CK-E20559M), fibrinogen β chain (FGB) (CK-E20560M), carnitine acetyltransferase (CrAT) (CK-E20556M), cytochrome P450 8B1 (CYP8B1) (CK-E20569M), stearoyl-CoA desaturase 1 (SCD1) (CK-E20568M)), cytochrome P450 4A10 (CYP4A10) (CK-E20555M), acetoacetyl-CoA synthetase (AACS) (CK-E20547M) and ROS (CK-E91516M) in liver and the levels of high-density lipoprotein (HDL) (CK-E91912M) and low-density lipoprotein (LDL) (CK-E91911M) in the serum and liver of mice in each group according to the manufacturer’s instructions.
2.7 Western blotting
The livers of the mice in each group were weighed and homogenized on ice in cell lysis buffer containing 97% RIPA (Sigma-Aldrich, St.Louis, Missouri, USA), 2% phenylmethanesulfonyl fluoride (Sigma-Aldrich, St.Louis, Missouri, USA) and 1% protease inhibitor cocktail (Sigma-Aldrich, St.Louis, Missouri, USA).Protein concentration of samples were analyzedviaa bicinchoninic acid protein assay kit (Merck Millipore, Massachusetts, USA).Equal amounts of protein in each group were loaded in 10%-12% sodium dodecyl sulphate-polyacrylamide gel electro phoresis (SDS-PAGE), separated electrically, and transferred onto polyvinylidene fluoride (PVDF).Membranes were blocked in 5% bovine serum albumin (BSA) in PBS, and then incubated in primary antibodies at 4 °C for 12 h including phosphorylated (P)-protein kinase B (Akt) (ab131443) (dilution at 1:1000), total (T)-Akt (ab200195) (dilution at 1:2000), P-AMPKα1+α2 (ab23875) (dilution at 1:2000), T-AMPKα1+α2 (ab207442) (dilution at 1:1000), peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (ab54481) (dilution at 1:2000), CYP2E1 (ab28146) (dilution at 1:5000), P-NF-κB p65 (ab194726) (dilution at 1:2000), T-NF-κB p65 (ab16502) (dilution at 1:2000), P-ikappaB kinase α and β (IKKα+β) (ab194528) (dilution at 1:1000), T-IKKα+β (ab178870) (dilution at 1:1000) obtained from Abcam (Cambridgeshire, UK) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (dilution at 1:2000) (ABS16, Merck Millipore, Massachusetts, Germany).The membranes were further exposed to horseradish peroxidase-conjugated secondary antibodies (dilution at 1:2000) (NBP2-30347H and NBP2-30348H) (Novus Biologicals, Littleton, Colorado, USA) for 4 h at 4 °C.An enhanced chemiluminescence detection kit (Merck Millipore, Massachusetts, Germany) combining with an imaging system (BioSpectrum600) were applied to visualize the protein bands.The Image J software (Version 1.8.0) (National Institutes of Health, Bethesda, MD) was used to analyze the pixel density of the band.
2.8 Statistical analysis
All analyses were conducted using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA).One-way analysis of variance followed by post-hoc multiple comparisons (least significance difference and Tamhane’s T2) was used to determine the differences.Data were expressed as mean ± the standard deviation (SD).Statistical significance was declared forP< 0.05.
3.Results
3.1 Protection by ACT against chronic alcohol-induced liver injury
Weight loss and abnormal organ indexes were noted following the chronic alcohol-induced liver injury in mice.Compared with the normal control (CTRL) mice, the body weight of chronic alcohol consumed mice appeared to markedly decrease from 5th to 24th weeks (P< 0.01), which was significantly improved after 3 weeks administration of ACT at doses of 15 and 45 mg/kg (P< 0.05) (Table 1).Compared with chronic alcohol-damaged mice, ACT administration, especially at 45 mg/kg strongly decreased the indexes of liver, kidney and heart (P< 0.05) (Table 1).Sil only reduced liver and heart indexes in mice with chronic alcohol-induced damage (P< 0.05) (Table 1).There was no significant difference in the spleen index among all groups (P> 0.05) (Table 1).
In the alcohol-damaged mice, after 24 weeks, the serum levels of ALT and AST were significantly higher than those of in the healthy mice (P< 0.05), which was served as biochemical markers of liver injury [17].Both Sil and ACT strongly suppressed the levels of ALT and AST (P< 0.05) (Fig.2A, 2B).
Abnormal lipid metabolism is a feature of mice with alcoholic disease [18].Compared with CTRL mice, in chronic alcohol-treated mice, the hepatic levels of TG and TCHO were increased by 14.6% and 14.3% (P< 0.01, Fig.2C, 2D), along with > 9.5% increment onLDL levels and > 10% reduction on HDL levels in serum and liver (P< 0.05, Fig.2E, 2F), respectively.Notably, ACT and Sil ameliorated the increase of TG (P< 0.05, Fig.2C), TCHO (P< 0.01, Fig.2D), LDL (P< 0.05, Fig.2E) and enhanced the levels of HDL (P< 0.05, Fig.2F) in chronic alcohol-treated mice.
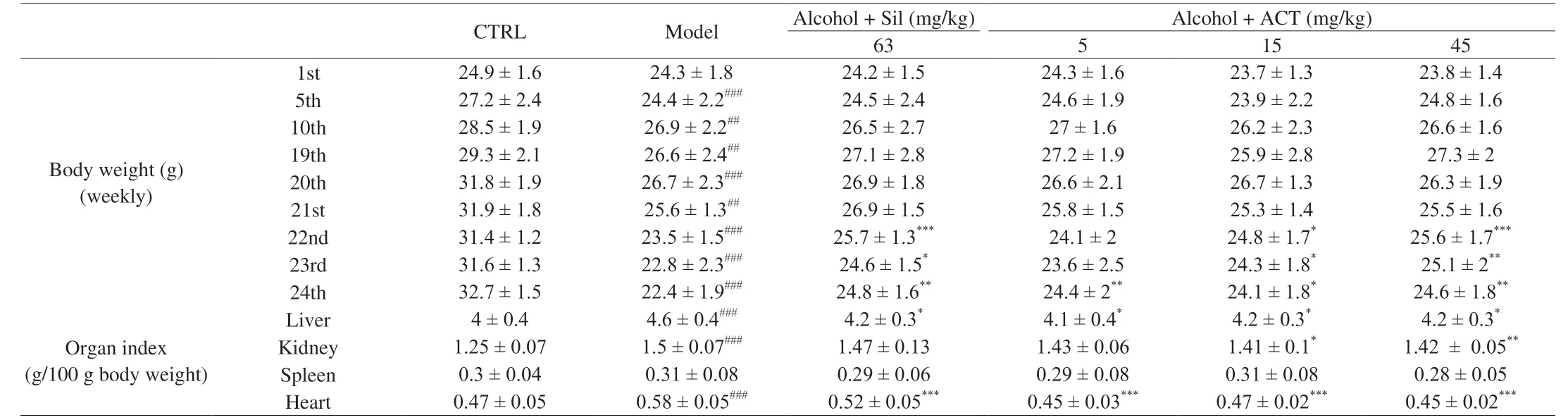
Table 1 The effect of ACT treatment on body weights and organ index of mice with chronic alcohol-induced liver injury.
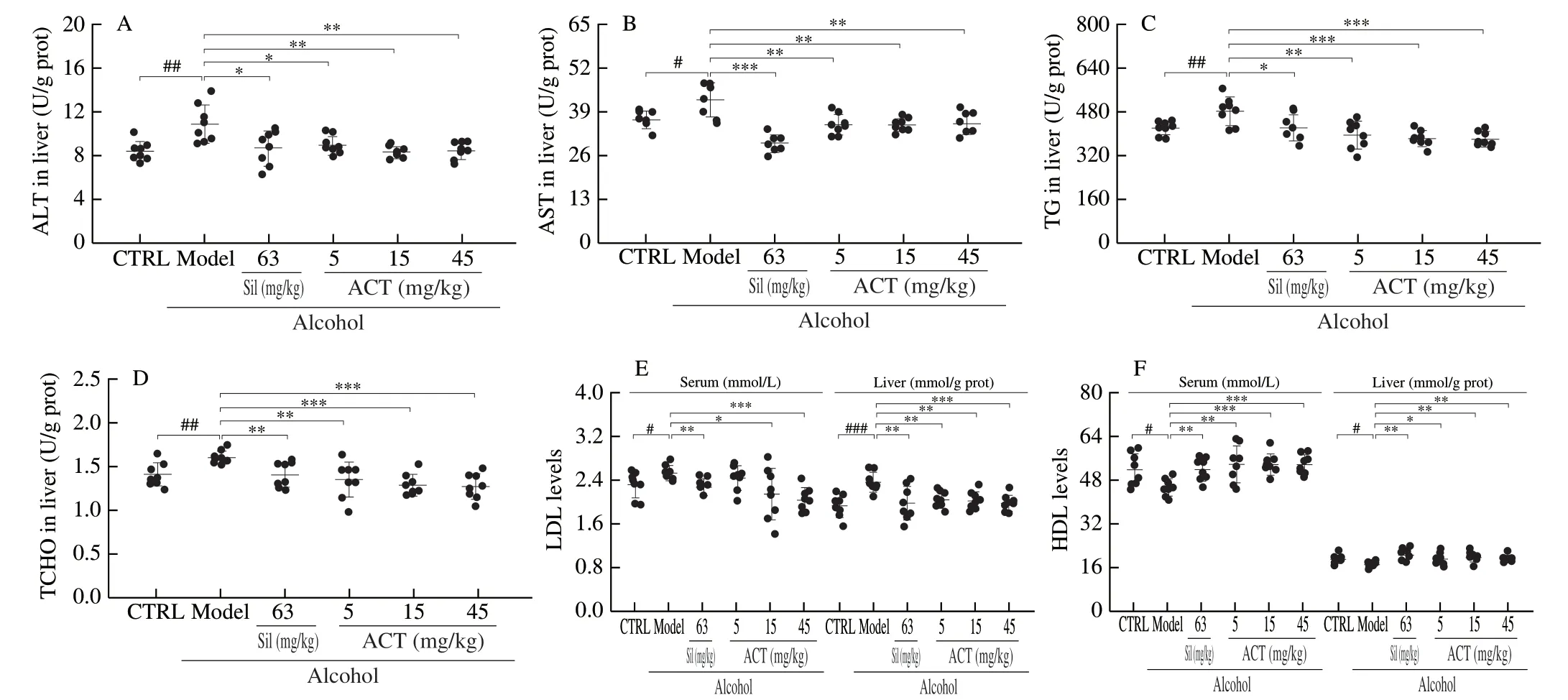
Fig.2 ACT relieves lipid abnormalities of mice with chronic alcohol damage.ACT reduced the levels of (A) ALT, (B) AST, (C) TG and (D) TCHO in liver, (E) suppressed the levels of LDL in the serum and liver, and (F) increased the levels of HDL in the serum and liver of mice with chronic alcohol-induced liver injury, respectively.The data were analyzed with a one-way ANOVA and expressed as means ± SD (n = 8).#P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with alcohol-only treated model group.
The accumulation of large lipid droplets in the vesicles of hepatocytes and eventually fatty degeneration apoptosis of hepatocytes were noted in the mice with chronic alcohol consumption.ACT or Sil effectively alleviated the formation of fat vacuoles (Fig.3A) and apoptosis (Fig.3B).In alcohol-induced HepG2 apoptotic cell model, ACT suppressed the cell apoptosis (Fig.S1A) and relived the loss of mitochondrial membrane potential (MMP) (Fig.S1B).ACT and Sil improved the alcohol-induced kidney damage including the thicken of the basement membrane, glomerular hypertrophy and narrow of the capsular space detected by H&E staining (Fig.3C), and prevented the kidney inflammation indicating by a decrease in PASpositive areas (Fig.3D).Additionally, ACT and Sil suppressed the alcohol-caused inflammatory infiltration of spleen (Fig.3E) and heart (Fig.3F) of mice.
3.2 The anti-steatosis was involved in ACT-mediated hepatoprotective effects
In liver tissue, liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to the isolation and screening of protein among CTRL group, model group, Sil-treated group and ACT-treated group.The MaxQuant and LFQ were used to analyze and quantify the results and proteins with different expression multiples (ratio A/B > 1.5 or ratio A/B < 0.67) were defined as significant proteins.Statistical studies demonstrated that the total number of peptides was 18 336, the total number of proteins was 2 594 groups, the number of quantifiable proteins was 2 467.Moreover, the number of significant changed proteins of model group vs.CTRL group was 177 groups, vs.Sil-treated group was 144 groups, and vs.ACTtreated group was 233 groups (Fig.4A), respectively.STRINGdb analysis was used to reveal the interaction among proteins between different experimental groups (Fig.4B, S2A and S2B), and 62 expected interactions were noted between model and ACT-treated group (Fig.4B).
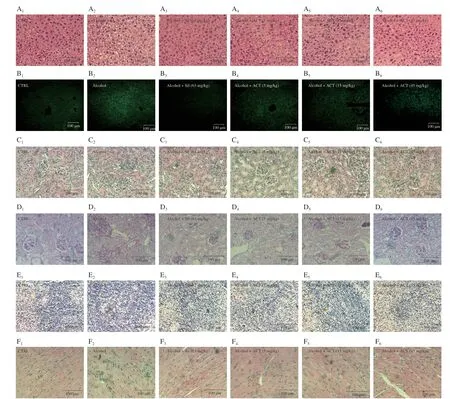
Fig.3 ACT alleviates pathological alternations and hepatocyte apoptosis.Histopathological analyses of the (A) liver, (C) kidney, (E) spleen and (F) heart by H&E staining and of the (D) kidney by PAS staining (Scale bar: 100 μm; Magnification: 400 ×).(B) The apoptosis rated of liver cells were detected via TUNEL staining (Scale bar: 100 μm; Magnification: 200 ×).1.CTRL; 2.Alcohol; 3.Alcohol + Sil (63 mg/kg); 4.Alcohol + ACT (5 mg/kg); 5.Alcohol + ACT (15 mg/kg); 6.Alcohol + ACT (45 mg/kg).
The details of 110 types of proteins were subsequently displayed in Table S1, which simultaneously recorded for two criteria as follows: 1) the ratio of CTRL group vs.model group > 1.5 or < 0.67; 2) the ratio of model groupvs.ACT-treated group > 1.5 or < 0.67.Compared with CTRL group, 60 types of proteins were suppressed and 50 types of proteins were enhanced in chronic alcohol-damaged mice; meanwhile, compared with model mice, ACT treatment resulted in 43 types of proteins enhancement, and 67 types of proteins reduction (Table S1).
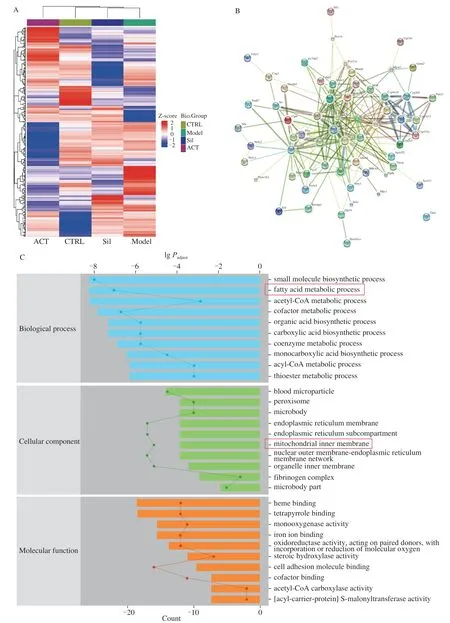
Fig.4 Proteomic analysis of ACT’s significantly regulated factor levels in a chronic alcohol-induced liver injury mouse model.(A) Hierarchical clustering analysis of obvious change factors among control group, model group and Sil-treated group and ACT-treated groups (n = 3).(B) The relationship among proteins processed through STRINGdb (n = 3).(C) GO enrichment analysis, and (D) KEGG analysis.
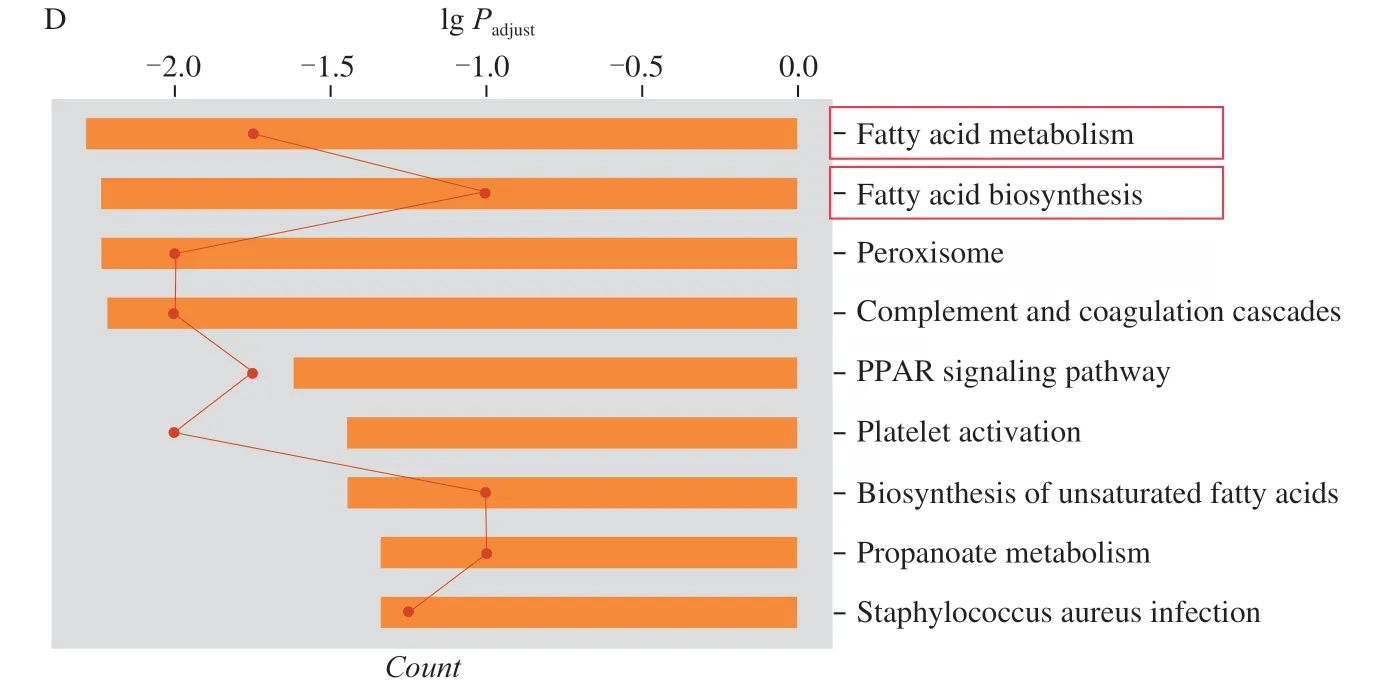
Fig.4 (Continued)
According to GO enrichment analysis, the significant changes proteins mainly related to small molecule biosynthetic process, fatty acid metabolic process of biological process and mitochondrial membrane of cellular component (Fig.4C, S2C and S2D).KEGG pathways analysis displayed that ACT mainly regulated the pathways relevant to fatty acid metabolism and fatty acid biosynthesis in the mouse liver (Fig.4D, S2E and S2F).Accordingly, ACT relieved the chronic alcohol injury in mice may be related to modulation on lipid metabolism.
Consequently, 9 types of cytokines related lipid metabolism were analyzed using ELISA assay.Among all detected cytokines, compared with model mice, ACT treatment resulted in > 13.8%, > 17.4%, > 13.1%, > 12.4%, >15.9%, > 15.4%, > 18.4% and > 12.6% reductions on the levels of AACS (P< 0.05, Fig.5A), SCD1 (P< 0.01, Fig.5B), FGB (P< 0.05, Fig.5C), FGG (P< 0.01, Fig.5D), FGL1 (P< 0.05, Fig.5E), ROS (P< 0.05, Fig.5F), CYP8B1 (P< 0.01, Fig.5G) and CYP4A10 (P< 0.01, Fig.5H), and > 22.3% increment on levels of CrAT (P< 0.01, Fig.5I) in liver tissues.Except for CrAT levels, Sil exhibited the similar regulatory effects as ACT (P< 0.05) (Fig.5).
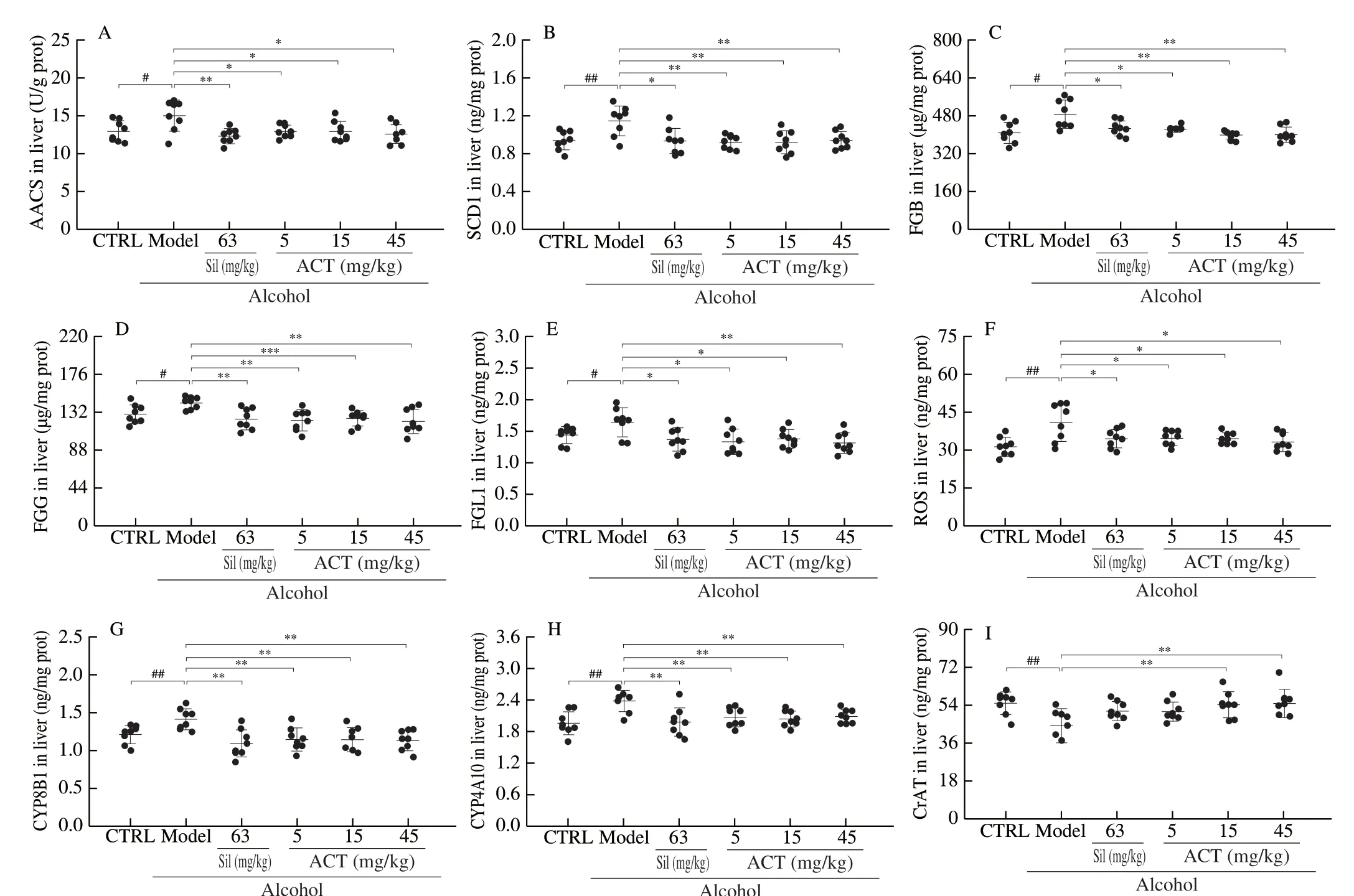
Fig.5 ACT regulated the lipid metabolism related factors in liver of chronic alcohol-induced liver injury mice.ACT treatment decreased the levels of (A) AACS, (B) SCD1, (C) FGB, (D) FGG, (E) FGL1, (F) ROS, (G) CYP8B1, (H) CYP4A10 and (I) CrAT in liver.Data are presented as the mean ± S.D (n = 8) and analyzed via a one-way ANOVA test followed by post-hoc Tukey’s multiple comparison tests.#P < 0.05 and ##P < 0.01 vs.CRTL; *P < 0.05, **P < 0.01 and ***P < 0.001 vs.alcohol-only treated model group.
3.3 PGC-1α and CYP2E1 are involved in ACT-mediated hepatoprotective effects
PGC-1α and CYP2E1 were served as a key regulator of lipid metabolism [19,20].Compared with chronic alcohol-injured mice, ACT significantly lowered the phosphorylation levels of Akt (P< 0.01) and enhanced the phosphorylation levels of AMPK α1+α2 (P< 0.05) and the expression of PGC-1α (P< 0.05) in liver (Fig.6A).ACT prevented the overexpression of CYP2E1 (P< 0.001) and the phosphorylation of IKK α+β (P< 0.05) and NF-κB p65 (P< 0.01) in liver tissues (Fig.6B).However, Sil failed to impact in the overexpression PGC-1α (Fig.6A).
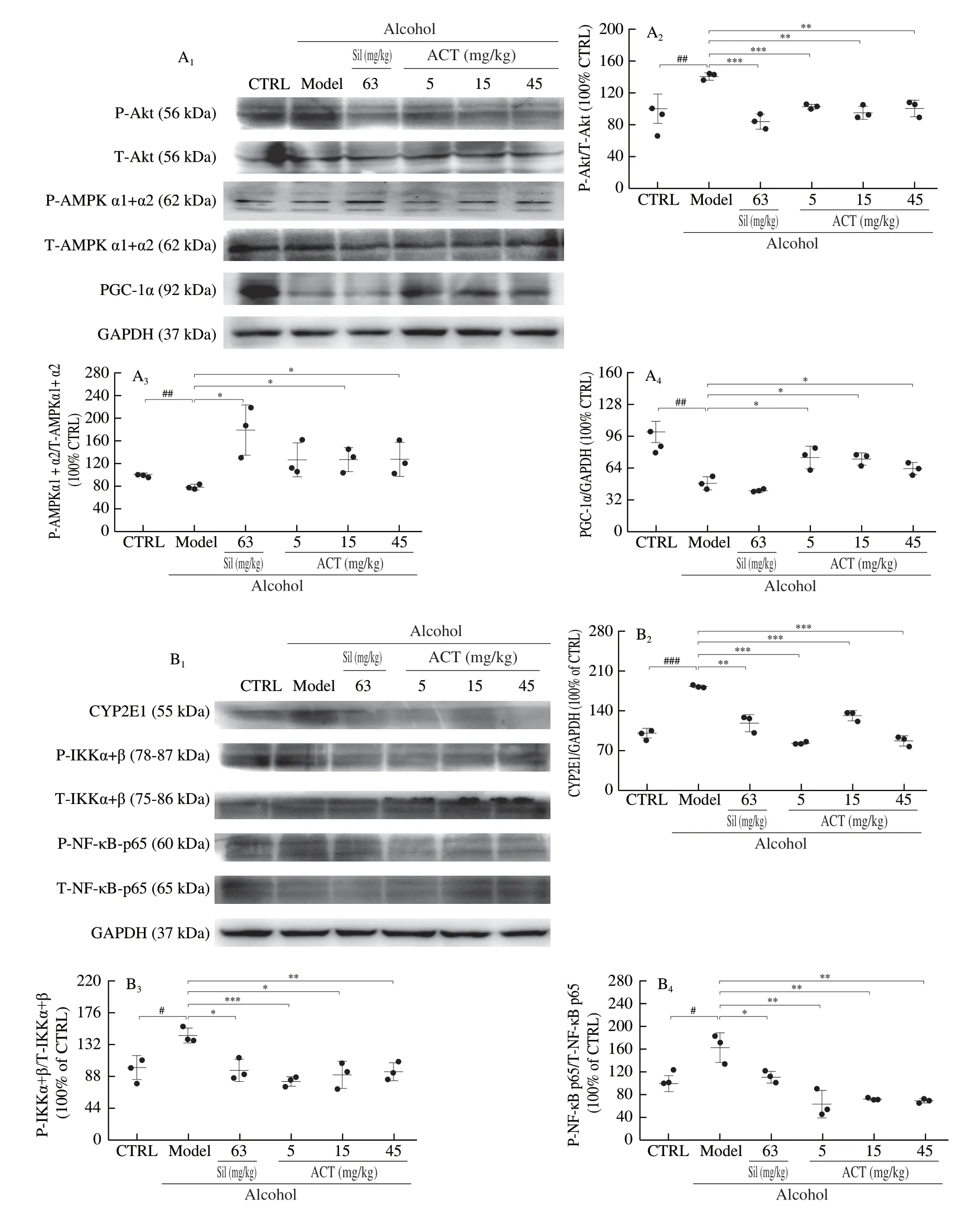
Fig.6 ACT regulated the PGC-1α and NF-κB signaling in liver of chronic alcohol-induced liver injury mice.(A) ACT suppressed the phosphorylation of Akt, and enhanced the phosphorylation of AMPKα1+α2 and expression levels of PGC-1α of liver.(B) ACT suppressed the phosphorylation of IKKα+β and NF-κB, and the expression levels of CYP2E1 of liver.The quantitative expression of each protein was normalized using GAPDH and total proteins.Data are presented as the mean ± S.D (n = 3) and analyzed via a one-way ANOVA test followed by post-hoc Tukey’s multiple comparison tests.#P < 0.05, ##P < 0.01 and ###P < 0.001 vs.CRTL; *P < 0.05, **P < 0.01 and ***P < 0.001 vs.alcohol-only treated model group.
4.Discussion
In this study, the hepatoprotective effects of ACT were confirmed in the mice with chronic alcohol-induced liver injury.In previous work, we analyzed the possible chemical constituents by LC-MS and LC-MS/MS and confirmed the presence of 25 types of triterpenoid compounds in ACT, such as ganodermanondiol, ganoderol, physalin D, ganoderol A, momoridcin, ganolucidic acid B, etc [14].Some of them have been confirmed to show protective effects on hepatocellular injury [21,22], which suggested that the ACT may have a potential hepatoprotective capability.Long-term alcohol consumption can cause mitochondrial damage to trigger the abnormal increases in ALT and AST activities serving as the clinical marker of liver damage [23].ACT effectively reduced ALT and AST activities and alleviated the loss of body weight and the swollen of organs, especially the liver, confirming its hepatoprotection.Excess ingestion of alcohol leads to the accumulation of acetaldehyde in hepatocytes, promoting the synthesis of fatty acids [24], which is responsible for hepatomegaly [25].ACT not only reduced the number of lipid droplets and apoptosis cells in liver, but also relived the abnormal levels of TG, TCHO, HDL and LDL of chronic alcohol-damaged mice, reflecting its modulation of lipid metabolism.
According to the proteomic analysis, ACT influenced the expression and/or activation of proteins related to fatty acid metabolic process.As the lipid metabolism factor, AACS contributes to synthesize fatty acids and cholesterol, and SCD1 distributing throughout the liver, is the rate-limiting enzyme of monounsaturated fatty acids synthesis and markers of TCHO synthesis, which strongly associated with fat metabolism dysfunction [26].CrAT contributes to the intracellular trafficking of fatty acids [27], and its mRNA levels were strongly decreased in type 2 diabetes mellitus patients [28].Chronic alcohol exposure impairs insulin secretion resulting in the increment on free fatty acid mobilization [29], and the withdrawal of insulin raises fibrinogen synthesis rates in type 1 diabetic patients [30].Thus, chronic alcohol exposure reduce insulin levels may also contribute to ALD by raising fibrinogen synthesis.The hyper-levels of fibrinogens (e.g., FGG, FGG and FGL1) have been founded in most patients with severe liver disease [31].Fatty degeneration provides a foundation to the development of ALD, and the membranous structures of mitochondrion rich in the polyunsaturated fatty acid can be attracted by oxyradical causing impairment, such as the dissipation of MMP and even apoptosis [32].CYP8B1 stimulates mitochondria to produce excess hepatic oxyradicalviapromoting the synthesis of cholic acid [33,34].Increasing CYP1A10 is shown to be highly capable of peroxidizing lipids and aggravating steatohepatitisviamodulating peroxisome proliferator-activated receptor [35].The lipid metabolism dysfunction is responsible for the mitochondrial injury, and aggravates the development of ALD.ACT not only regulated on the levels of lipid biogeneration correlative factors in chronic alcoholdamaged mice, but also prevent the over-accumulation of intracellular ROS and the dissipation of MMP in hepatocyte showing its property on controlling the lipid metabolism.
As the potent metabolic regulator, the activated AMPK promotes the lipid catabolism and restrains lipid biosynthesisviaregulating key enzyme expression [36]and enhancing the expression of PGC-1α [37].Akt, a serine/threonine-protein kinase, regulates nutrient metabolismviamediating the AMPK, which can be activated by the inhibitor of Akt [38,39].The high level of hepatic PGC-1α increases lipid metabolism and long-chain fatty acid oxidation through upregulating the tricarboxylic acid cycle genes and the mitochondrial fatty acid oxidation, and decreases triacylglycerol storage and secretion [20,40].In the consistent with the previous investigations, we found that chronic alcohol intake caused the reduction of the phosphorylated AMPK as well as the expression of PGC-1α, and the enhancement of phosphorylated Akt.Based on our results, ACT relieved the abnormal lipid metabolism caused by alcohol, at least partiallyviaenhancing the levels of PGC-1α.
Effects of alcohol on cellular lipid accumulation are dependent on CYP2E1 activity.The inhibition of CYP2E1 can prevent the induction of hepatic steatosis and ROS generation in models of alcoholic steatohepatitis [19,41].The phosphorylated IKK complex (consisting IKKα and IKKβ) induces the phosphorylation of IκB, which helps to active NF-κB to target the related genes [42], promoting inflammation response and aggravating ALD [10].Akt stimulates phosphorylation of p65 through IKK, which is another important mechanism to regulate the transactivation potential of p65 [43].As reported, the activated AMPK and high expression levels of PGC-1ɑ can suppress the activation of NF-κB p65 unequivocally corresponds to the reduction of IKK induction [44,45].The accumulation of FFA caused by alcohol exposure initiates NF-κB activation [46], which further evokes inflammatory responses and oxidative stress [16].In this study, ACT suppressed the phosphorylation of NF-κB to inhibit the accumulation of lipid for alleviating alcoholic damage.
There is still limitation in this study.According to previous study, lipid metabolism is a complex process with multiple factors involved in.On the one hand, PGC-1α and NF-κB signaling plays an important role in regulating the lipid biogeneration and oxidation; on the other hand, these signaling are closely related to alcohol-induced inflammatory responses and oxidative stress.Their exact interactions and relationships are not well understood, which needs further investigation.
5.Conclusions
In conclusion, our study suggest that 6-week ACT treatment could inhibit the development of alcoholic liver injury induced by 24-week alcohol feeding in C57BL/6 mice, its hepatoprotective effects may be related to the regulation lipid metabolism.
Conflicts of Interest Statement
The authors declare no conflict of interest.
Acknowledgements:
This research was funded by the National Key Research & Development Program of China (grant number: 2018YFE0107800), the Special Projects of the Cooperation between Jilin University and Jilin Province (grant number: SXGJXX2017-1), the Science and Technology Develop Project in Jilin Province of China under grant (No.20191102027YY, 20200708091YY and 20200708068YY), and Research and Cultivation Project for Young Teachers of Jiangxi Medical College, Nanchang University (No.PY201901).
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2021.04.012.
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
- Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo
- The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense
- Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice
- The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics
