The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics
2021-05-24JinbinHaoLiyunYeGuoliangMengYanjiaoSongJunshengFuXiaopingWu
Jinbin Hao, Liyun Ye, Guoliang Meng, Yanjiao Song, Junsheng Fu, Xiaoping Wu*
College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, 350002 Fujian, China
Keywords:Trametes lactinea Polysaccharide Hepatoprotective Transcriptome Metabolome
ABSTRACT Trametes lactinea mycelia polysaccharides (TLMPS) have a wide range of bioactivities.The potential mechanisms of action of TLMPS against acute alcohol-induced liver injury in vivo were investigated by analyzing the physical and chemical properties of TLMPS and its protective effects on a mouse model of alcoholic liver injury.TLMPS protected the liver against alcohol-induced injury, as evidenced by the reduced alcohol-induced elevation of the liver index, serum biochemical indices, and maintenance of hepatic morphology.Potential mechanisms were analyzed using transcriptome and metabolome analyses.The transcriptome data revealed the involvement of many differentially expressed genes in chemical carcinogenesis, drug metabolism, and metabolism of xenobiotics by cytochrome P450.The metabolome analysis revealed that TLMPS significantly regulated specific metabolites in the liver, including organic acids, lipids, nucleosides, and organic oxygen compounds.KEGG enrichment analysis revealed the signifi cant involvement of different metabolites in choline metabolism, ATP-binding cassette transporters, and glycerophospholipid metabolism.Assessment of the changes in gene expression and metabolites revealed the significantly different expression of several genes encoding key enzymes and metabolites in choline metabolism pathway.The collective findings confi rmed that choline metabolism plays an important role in the protective effects of TLMPS against acute alcoholic liver injury.
1.Introduction
Trametes lactineais a mushroom belonging to the family Polyporaceae, order Polyporales, class Agaricomycetes, and kingdom Basidiomycota.Current understanding ofT.lactineais limited and most research has focused on culture conditions and bioactive compounds.Yus et al.[1]found that media composition, pH, inoculum volume, incubation temperature, and time affected the growth ofT.lactinea.Zhang et al.[2]reported that Trametenolic acid B can effectively prevent growth of gastric cancer cells.He et al.[3]revealed that polysaccharides isolated fromT.lactineahave excellent antioxidant and anti-proliferative properties and so may be valuable as a natural source of antioxidants.
The liver is a crucial organ responsible for metabolizing over 80% of alcohol that is consumed [4-5].Liver damage directly affects the metabolism of the body.Alcoholic liver disease (ALD), including alcoholic fatty liver, alcoholic hepatitis, alcoholic hepatic fibrosis, alcoholic cirrhosis, and hepatocellular carcinoma, is one of the most important causes of chronic liver disease worldwide.ALD is commonly treated with clinical drugs, such as glucocorticoids.The side effects of taking drugs are common [6].A number of natural products have been reported to protect the liver.For example, the exopolysaccharides ofInonotus hispidusprotect against acute alcoholic liver injury [7], while melanin fromAuricularia auriculais effective in alleviating alcohol-induced liver damage [8], and salvianolic acid B protect against acute liver injury [9].
The ability ofT.lactineamycelia polysaccharides (TLMPS) to protect against liver injuryin vivoinduced by alcohol has not been assessed.The objective of this study was to assess the protective effects of TLMPS against acute alcoholic liver injury in mice based on examinations of the liver index, serum enzymes, and histopathology.The underlying mechanism of the hepatoprotective effects of TLMPS was analyzed using transcriptome and metabolome analyses.
2.Material and methods
2.1 Preparation of fungal materials
T.lactineastrains were obtained from the Edible Fungal Germplasm Resources Management Center in Fujian Province, China.Mycelia were cultured in potato dextrose broth at 28 °C and 160 r/min for 8 days.The resulting convoluted mycelia were collected and washed with distilled water before freeze-drying.
2.2 TLMPS extraction and purification
The mycelia were ground to a powder, mixed with deionized water at a powder-to-liquid ratio of 1:30, and placed in a 90 °C water bath for 4 h.This extraction procedure was performed twice.The two aqueous extracts were combined.After adding three times the volume of anhydrous ethanol and storing in a refrigerator at 4 °C for 8 h, the aqueous extracts were pelleted by centrifugation at 8 000 r/min for 10 min.Each resulting supernatant was discarded.Each pellet of crude polysaccharides was dissolved in deionized water and deproteinized using Sevage reagent (chloroform:n-butanol at a 4:1 ratio).The resulting mixture was centrifuged at 8000 r/min for 10 min.The volume of the supernatant was adjusted to 25 mL with distilled water.The polysaccharide solution was placed in a dialysis bag for 48 h with flowing water to remove any small molecules.Finally, the dialyzed polysaccharide solution was freeze-dried and used to test TLMPS [10-13].
2.3 Physical and chemical analyses of TLMPS
The total content of TLMPS content was assessed using the established phenol-sulfuric acid method with glucose as the standard [14].The glucuronic acid content was determined by the established sulfuric acid-m-hydroxybiphenyl method [15].Soluble glycoprotein content was determined by Coomassie brilliant blue staining.Sulfate content was determined by barium chloride gelatin turbidimetry with bovine serum albumin and sulfate radical as the respective standards [16].
Spectral analyses were used to detect the purified TLMPS.For ultraviolet (UV) absorption spectrometry, a 0.5 mg/mL solution of TLMPS in deionized water was scanned in the range of 190—400 nm using an Ultraviolet visible (UV-Vis) spectrophotometer (Shimadzu Experimental Equipment Co., Ltd, Shanghai, China) with a scan interval of 1 nm.For infrared (IR) absorption spectrometry, TLMPS and dry potassium bromide were mixed in a 1:200 proportion, ground homogenously.The IR absorption spectrum was recorded in the range of 4000 cm-1to 500 cm-1.TLMPS samples (1 mg/mL) were also examined following staining with 1 mL of Congo red solution (80 μmol/L).The NaOH concentration of the sample was adjusted to 0, 0.1, 0.2, 0.3, 0.4 and 0.5mol/L using 1 mol/L NaOH solution.The resulting 6 reaction systems were examined by UV-Vis spectrophotometry in the wavelength range of 400-700nm.The maximum absorption wavelength was recorded.Deionized water was used instead of the polysaccharide solution as the blank control group.
2.4 Hepatoprotective effect in vivo
Sixty male Kunming mice weighing 18-22 g were purchased online (http://www.fzzmsoft.com/xieli/; Fuzhou, China).The mice were randomly divided into 6 groups of 10 animals in each group.The groups comprised a blank group, model group, low-dose TLMPS group (100 mg TLMPS/kg body weight (bw)), medium-dose TLMPS group (200 mg TLMPS/kg bw), high-dose TLMPS group (400 mg TLMPS/kg bw), and a positive control group (300 mg biphenyl diester/kg bw).After one week of adaptive feeding, the TLMPS groups and the positive control group were administered the corresponding dose of gavage, while the blank and model groups were administered the corresponding distilled water gavage for 8 consecutive days.Food was providedad libitumduring this experimental period [7].At 4 h after the last pretreatment, the model, TLMPS, and positive control groups were administered 50% ethanol (12 mL/kg bw) by oral gavage.The blank group was administered the same volume of distilled water [17].
All mice were fasted for 12 h following ethanol treatment and euthanized.The total weight and the liver weight of each mouse were determined and used to calculate the liver index (ratio of liver weight to body weight).Serum was collected by centrifugation and the activities of malondialdehyde (MDA) and superoxide dismutase (SOD) were determined according to the manufacturer’s instructions (Jiancheng Biochemical Reagent Co., Nanjing, China) [18].The liver from each mouse was prepared for further analysis.The middle section of the left lobe of the liver was fixed with 10% neutral formalin.After paraffin embedding, sectioning, and staining with hematoxylin and eosin (Jiancheng Biochemical Reagent Co), the specimens were observed and photographed (200 × magnification).Serum was collected by centrifugation, and the alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), and triglyceride (TG) levels were calculated according to the manufacturer’s instructions (Jiancheng Biochemical Reagent Co.).
2.5 Total RNA isolation and transcriptome analysis
Total RNA was extracted from the liver using a Total RNA Extraction Kit (Omega Bio-Tek, Guangzhou, China) according to the manufacturer’s instructions.The concentration and quality of RNA were detected using a 2100 RNA Nano 6000 Assay Kit (Agilent Technologies, Santa Clara, CA, USA) [19].RNA-seq libraries were prepared and sequenced using an Illumina HiSeq by the Applied Protein Technology Company (Shanghai, China).Clean reads were obtained by removing low-quality sequences and joint contamination using FASTP software, followed by mapping using HISAT2 [20].The gene expression levels for each sample were expressed according to the fragments per kilobase per million (FPKM).P-adjusted (padj) < 0.05 and |log 2 fold-change| > 1 were used as the criteria of significant difference to identify differentially expressed genes (DEGs) [21].Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis for the DEGs were performed using the OmicShare tools, a free online platform for data analysis (http://www.omicshare.com/tools).
2.6 Metabolite measurements and analysis
Adding 200 μL water to approximately 80 mg of liver tissue and mixed for 60 s.Next, added 800 μL methyl alcohol and acetonitrile solution (1:1,V/V).After mixing for 60 s, took low-temperature ultrasound for 30 min twice.The protein was precipitated at -20 °C for 1 h.Then centrifuged at 4 °C for 20 min.The supernatant was freeze-dried.The metabolite sample was prepared and stored at -80 °C.
Metabolites in each freeze-dried sample were analyzed using an ultra-high performance liquid chromatography-quadrupole timeof-flight mass spectrometry (UHPLC-Q-TOF MS) system.Samples were separated using a model 1290 infinity LC UHPLC system (Agilent Technologies) equipped with a hydrophobic interaction chromatography column.The analytical conditions were a column temperature of 25 °C and a flow rate of 0.3 mL/min.Mobile phase A consisted of water, 25 mmol/L ammonium acetate, and 25 mm ammonia water.Mobile phase B comprised acetonitrile.Electrospray ionization (ESI) was used to detect positive and negative ions.Samples were analyzed using a triple TOF 5600 mass spectrometer (AB SCIEX, Framingham, MA, USA).Peak alignment, retention time correction, and peak area extraction were performed using the XCMS program.The structure of metabolites was identified by accurate mass number matching (< 25 ppm) and secondary spectrum matching.After pretreatment by Pareto-scaling, discriminant analysis was performed by orthogonal partial least squares discriminant analysis (OPLS-DA) in R (version 3.1.1).This analysis was modified on the basis of partial least squares discriminant analysis (PLS-DA) to filter out any noise that was irrelevant to the classification information and improve the analytical ability and effectiveness of the model.Variable importance for the projection (VIP) obtained by OPLS-DA was one of the criteria used to screen for differences among groups.Differential metabolites were selected according to VIP > 1 andP< 0.05 based ont-tests performed in Metaboanalyst 4.0 (http://www.metaboanalyst.ca/) [22,23].Metabolic pathways were constructed based on KEGG (http://www.genome.jp/kegg/pathway.html) and OmicShare tools (http://www.omicshare.com/tools) enrichment analyses.Selected pathways withP< 0.05 were considered as important metabolic pathways that might may be affected by TLMPS.
2.7 Statistical analysis
All data are expressed as the mean ± standard deviation of three independent experiments.Significant differences from the respective controls of each experimental group were assessed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).P< 0.05 denoted statistical significance.
3.Results
3.1 TLMPS physicochemical characteristics
The contents of carbohydrate, soluble glycoprotein, glucuronic acid, and sulfate of TLMPS are summarized in Table 1.The carbohydrate content was the highest ((687.71 ± 4.33) mg/g), followed by sulfate and glucuronic acid ((198.89 ± 7.86) and (103.45 ± 0.71) mg/g, respectively), and soluble glycoprotein ((7.012 ± 0.38) mg/g).
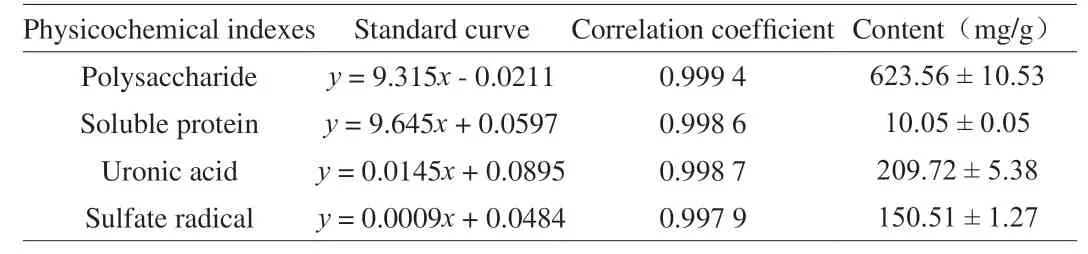
Table 1 Chemical components of TLMPS.
To further characterize TLMPS precisely, UV and IR absorption spectrometry and Congo red analysis were performed.The UV spectrum displayed an obvious absorption peak at 195 nm (Fig.1A), which is the characteristic absorption peak of polysaccharides.No obvious absorption peaks were detected at the wavelengths of 260 and 280 nm, indicating little or no nucleic acids and proteins in the test polysaccharide [24].This result was consistent with the protein content summarized in Table 1.The results indicated that the test polysaccharide was extracted with high purity.
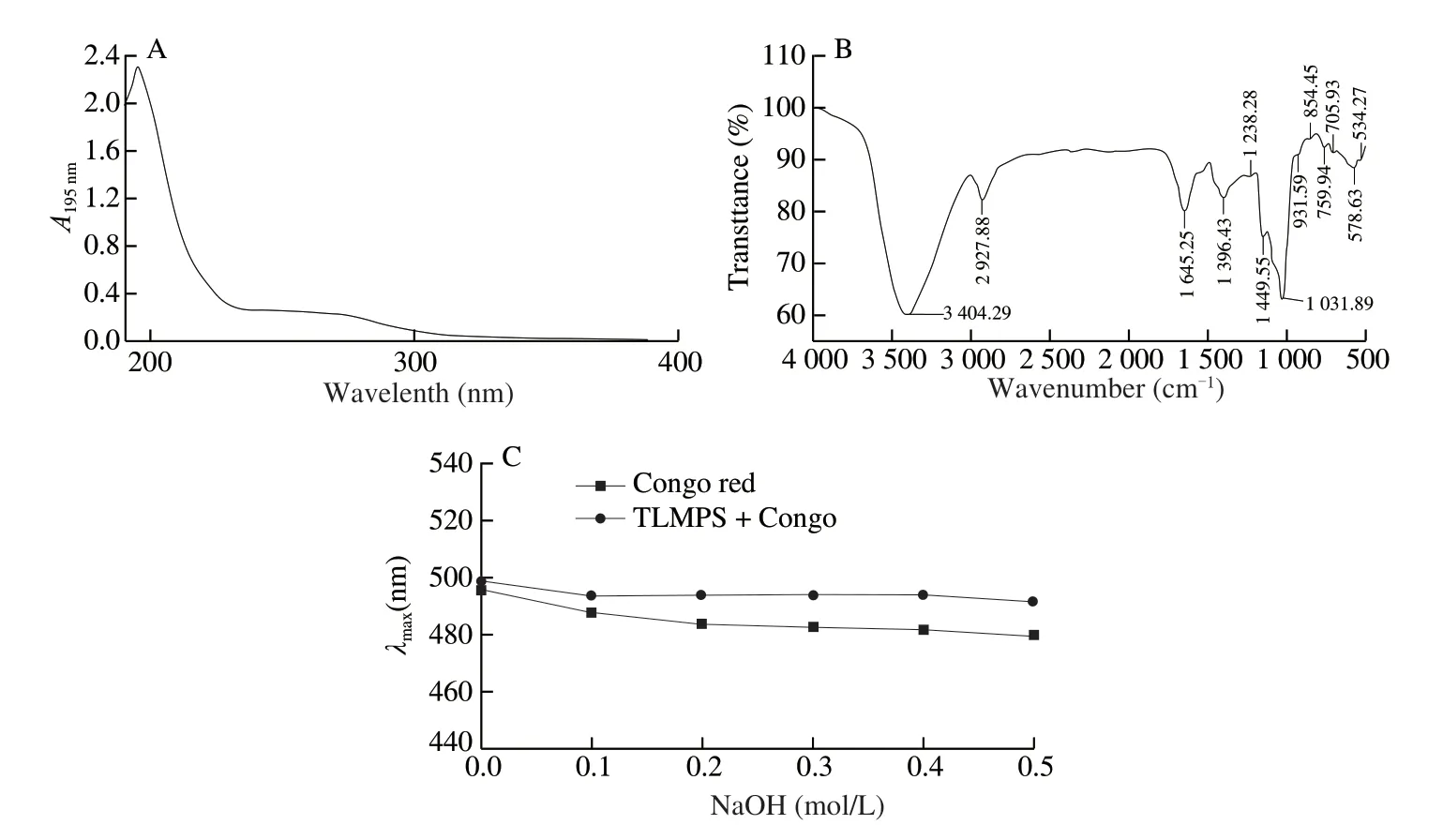
Fig.1 Spectral analyses of the purified TLMPS.(A) UV spectrum of TLMPS.(B) IR spectrum of TLMPS.(C) Congo red detection result of TLMPS.
In the IR spectrum, the intense broad peak at 3 412.00 cm-1was characteristic of hydroxyl groups with stretching vibration, indicating the presence of O—H between or in molecules (Fig.1B).The absorption peak at 2 916.31 cm-1was caused by the asymmetric stretching vibration of C—H [25].The strong absorption peak at 1 639.46 cm-1was caused by the stretching vibration of C=O.The characteristic absorption peak at 1 386.79 cm-1was attributed to the bending vibration of C—H [26].The absorption peak at 1 200 cm-1to 1 000 cm-1was ascribed to the stretching vibrations (C—OH) of the side groups and the glycosidic bond vibration (C—O—C) [27], indicating the presence of a pyranosyl unit.The absorption peak at 896.88 cm-1was due to theβ-type C—H variable angle vibration of pyranose.The collective findings indicated that the TLMPS skeleton may be composed ofβ-pyranosyl units.
Congo red is an acid dye that can complex with polysaccharides to form a triple helix [28].The complex formation produces redshifts in the emission wavelength.A strong alkaline environment will destroy the triple helix structure and the maximum characteristic absorption wavelength change.As the NaOH concentration increased, the maximum absorption wavelength of the polysaccharide-Congo red solution decreased (Fig.1C).Compared with the Congo red solution, TLMPS displayed no red shift, indicating that TLMPS lacked the triple helix structure.Rather, an irregular cluster conformation may have been present.
3.2 Effect of TLMPS on liver and serum
There was no significant difference in body weight betwee n the blank, model, and TLMPS groups (P> 0.05; Table 2), indicating that the appropriate dosage of reagent did not affect the growth and development of the mice, and that TLMPS did not affect the growth of mice.The liver index can reflect pathological changes in the liver.The liver index in the model group was significantly higher than that in the blank group (Fig.2A).The finding indicated that alcohol causes liver tissue damage and edema and thus the success of the model.Compared with the model group, the liver index of the medium-dose, high-dose, and positive control groups decreased significantly (P< 0.05), indicating that adequate amounts of TLMPS can effectively reduce liver edema and acute alcoholic liver injury, with an effect similar to that of biphenyl diester.
ALT, AST, TG, and TC are all important indices of liver injury.The ALT, AST, TG, and TC activities in the mice sera are shown in Fig.2B.Compared with the blank group, the serum levels of ALT, AST, TG, and TC in the model group were significantly increased, indicating that the liver cells of the mice were substantially damaged with consequent disrupted metabolism, evident as the altered serum activities of ALT, AST, TG, and TC.Compared with the model group, the TLMPS and positive groups displayed significantly reduced ALT, AST, TG, and TC activities in the mice with alcoholrelated liver injuries.The reductions were particularly pronounced in the high-dose and positive control groups.The results indicated that TLMPS can alleviate acute ethanol liver injury.
SOD and MDA levels were used as key indices of liver injury.The effects of TLMPS on SOD and MDA activities in the liver are shown in Fig.2C.In comparison with the blank group, the MDA level ((10.63 ± 0.42) nmol/mg protein) was increased and the SOD activity ((78.80 ± 2.5) U/mg protein) decreased significantly in the liver of the model group.These findings indicated that alcohol can cause liver injury and reduce the antioxidant capacity of liver injury in mice.In contrast, the MDA level ((8.40 ± 0.25) nmol/mg protein) was significantly decreased and SOD activity ((94.76 ± 6.28) U/mg protein) improved significantly in the TLMPS group compared with the value in the model group.The findings indicated that TLMPS could alleviate the oxidative stress injury induced by alcohol, and could thus protect against acute alcoholic liver injury in mice.
In addition, changes in the hepatic morphology of mice were observed in the different experimental groups.As shown in Fig.2D, in the blank group, the liver lobule clearly evident, the liver cell cord was arranged in an orderly and radiated manner, and there was no foam change or marked hyperemia in the hepatic sinuses.In contrast, the hepatic lobule was not clearly evident in the model group, the sinusoid was narrowed, and the liver cell cord was disordered.Moreover, the hepatocytes were turbid, foamed, or ballooned, the cytoplasm was pale, the nuclei were unclear, and marked hyperemia was observed in the hepatic sinuses.Compared with the model group, the turbidity, swelling, foam-like changes, and cord disorder of the liver cells were significantly improved in the TLMPS and positive groups.In the low-dose TLMPS group, the morphology of liver tissue was partly improved.However, some hepatocyte lesions and steatosis were present around the central vein.In the middle-dose TLMPS group, the morphology of liver tissue was significantly ameliorated and the arrangement of hepatocyte cords was significantly improved.In the high-dose TLMPS group and positive group, hepatocyte turbidity swelling and foam-like cells were significantly reduced, similar to the blank group.In addition, a small number of necrotic cells were evident.These results indicated that the potential pharmacological activity of TLMPS, especially high-dose TLMPS.Therefore, the high-dose was used to analyze the TLMPS action pathway in the subsequent transcriptome and metabolome analyses.
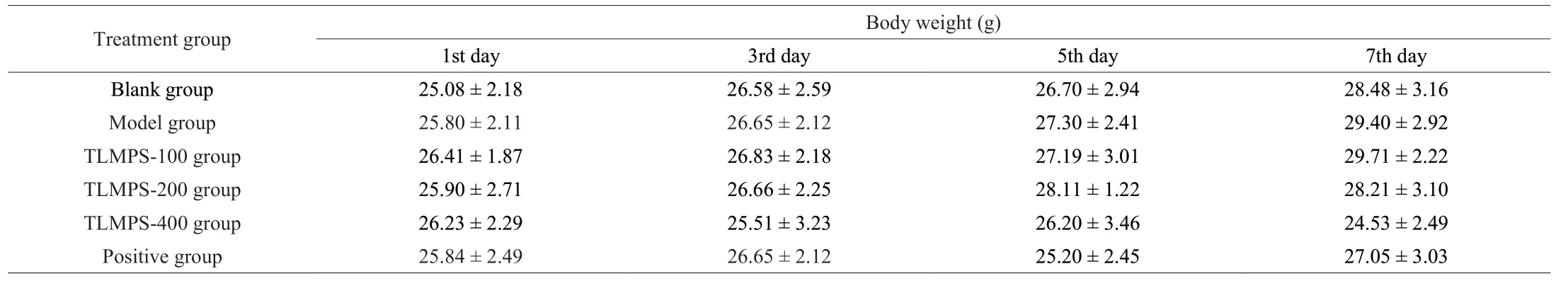
Table 2 Effects of TLMPS on mice body weight.
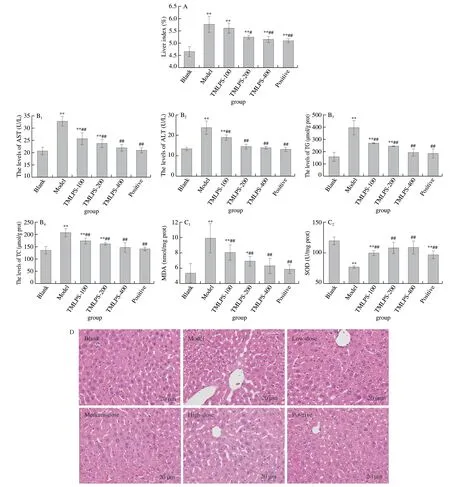
Fig.2 Effects of TLMPS on the liver and serum.(A) Effects of TLMPS on liver index in mice.(B) Effects of TLMPS on AST, ALT, TG, and TC in the serum of acute alcohol liver injury in mice.(C) Effects of TLMPS on SOD and MDA activities in the liver.(D) Effects of TLMPS on hepatic morphological changes in ALD mice.#P < 0.05 and ##P < 0.01 compared with model group; *P < 0.05 and **P < 0.01 compared with blank group.
3.3 Transcriptomic analysis of the hepatoprotective effect of TLMPS
The RNA-Seq data (Table S1) revealed that the transcriptome assembly was robust and reliable.A high Pearson’s correlation between samples (R2> 0.92; Fig.S1) indicated that the biological replicates were reliable.The transcriptome analysis revealed, a total of 520 and 401 DEGs in the model vs.blank, and TLMPS vs.model, respectively (Fig.3A).Comparison of the model and blank group revealed 309 genes were upregulated and 211 genes were downregulated due to alcoholic liver injury.In comparison with the model group, 199 genes were upregulated and 202 genes were downregulated in the TLMPS group.Enrichment analysis was performed for the model vs.blank upregulated and TLMPS vs.model downregulated DEGs, and the model vs.blank downregulated and TLMPS vs.model upregulated DEGs.The DEGs may have contributed to the different protective effects against acute alcoholic liver injury in mice (Fig.3B).GO enrichment analysis of the selected DEGs indicated that a large number were involved in the “cellular of biological process” category, “cell of cellular components” category, and “binding of molecular function” category.(Fig.4A).Gene enrichment analysis of selected DEGs based on the KEGG database revealed that these genes were mainly involved in chemical carcinogenesis, drug metabolism, metabolism of xenobiotics by cytochrome P450, retinol metabolism, and glutathione metabolism (Fig.4B).
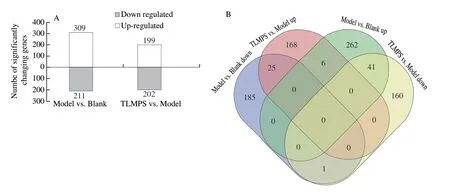
Fig.3 DEGs in different groups of ALD mice.(A) Number of DEGs in different groups.(B) Venn diagram of DEGs in different groups.
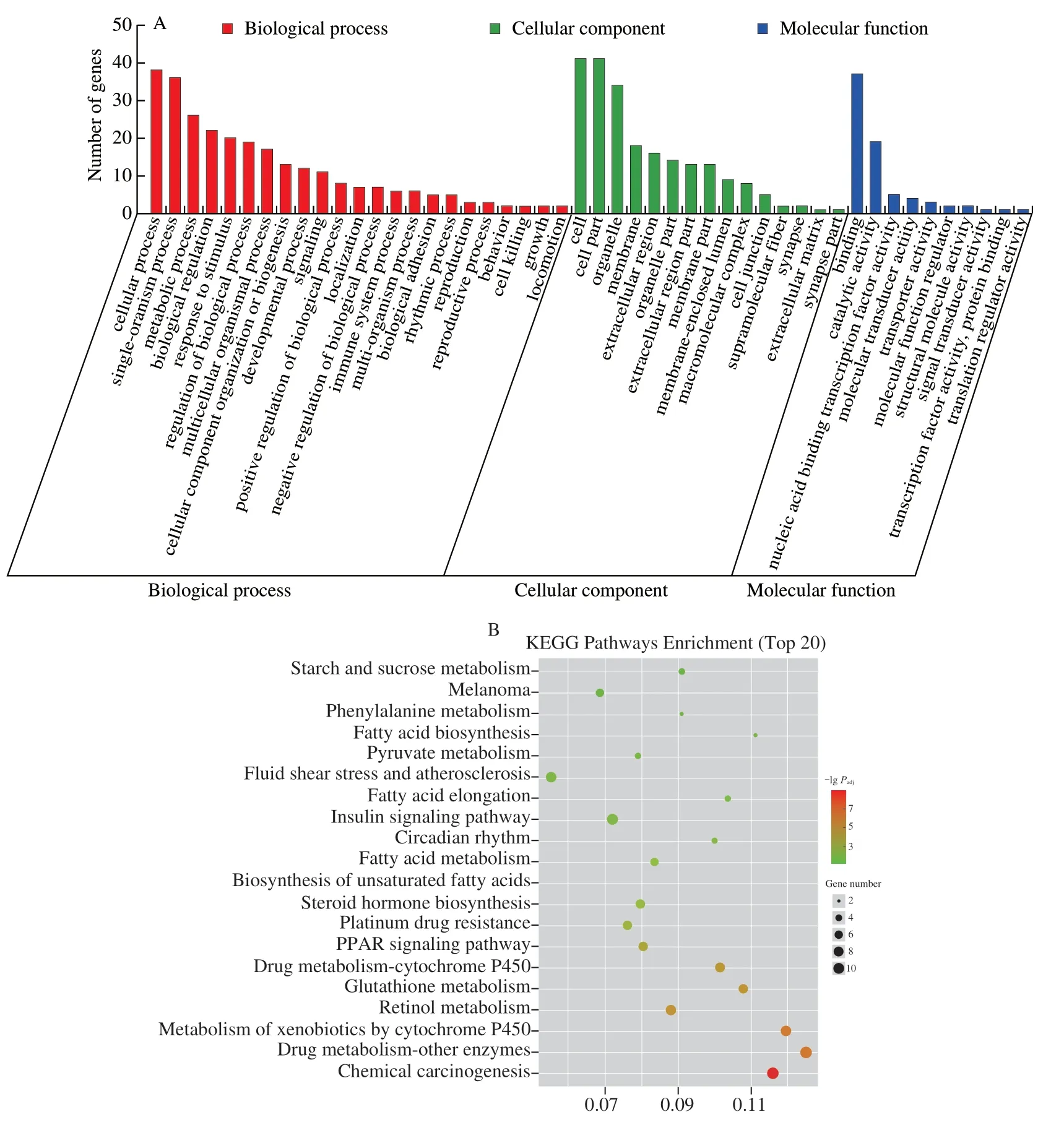
Fig.4 Enrichment analysis of DEGs in ALD mice.(A) GO enrichment of DEGs.(B) Bubble plot of KEGG enrichment of DEGs.
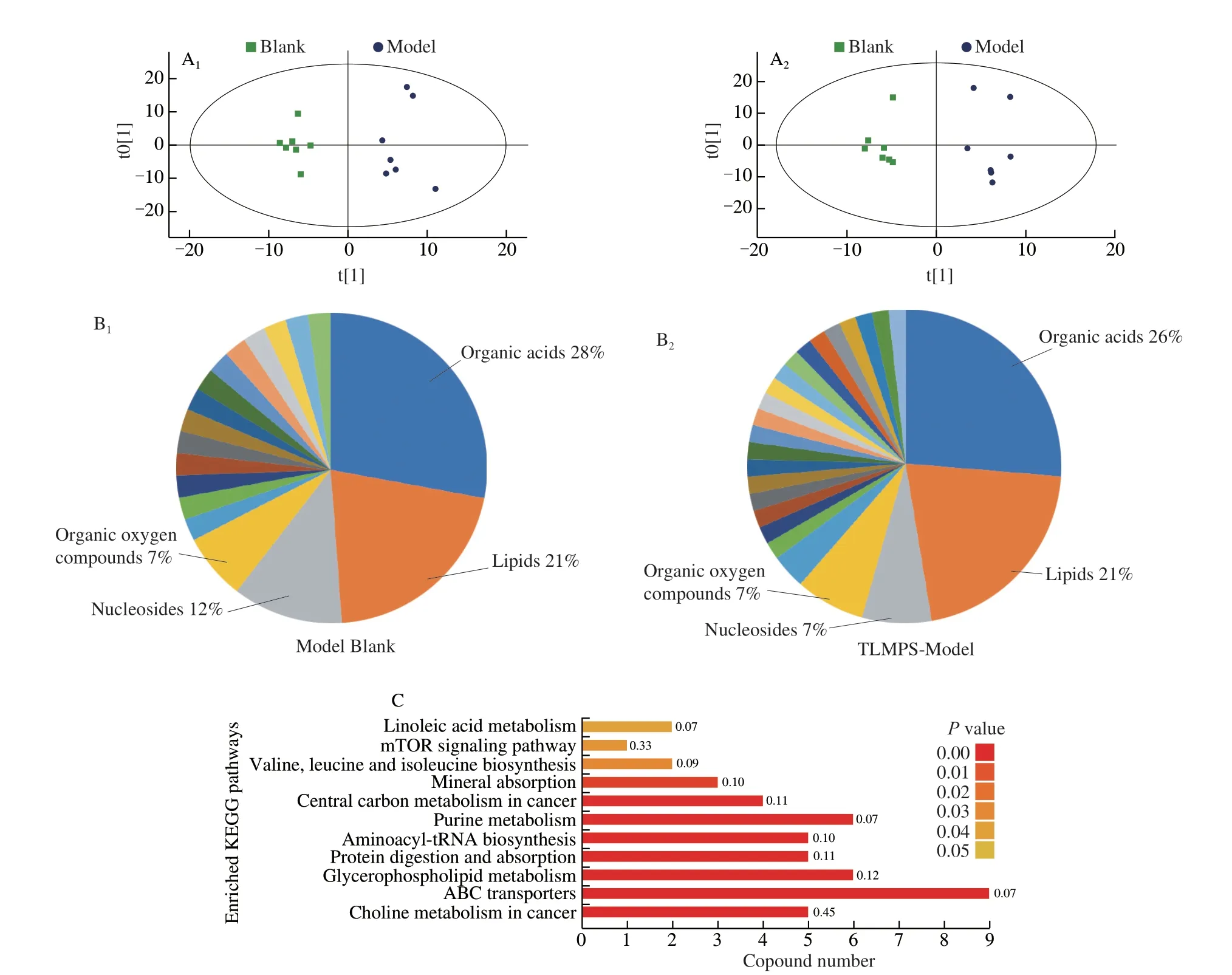
Fig.5 Overall qualitative and quantitative analysis of metabolomics data.(A) OPLS-DA clustering based on the metabolome data.(B) Component analysis of the metabolites differentially expressed between the model vs.blank group, TLMPS vs.model group.(C) KEGG enrichment of different metabolites.
3.4 Metabolic analysis of the hepatoprotective effect of TLMPS
UHPLC-Q-TOF MS detected many metabolites.OPLS-DA was performed to determine whether the metabolomic profiles of the 3 groups differed from each other.The results are depicted in Fig.5A.The blank and model groups were clearly separated.Similarly, the TLMPS and model groups exhibited two distinct categories, indicating a good correlation between the replicates.Compared to the control group, 78 measured metabolites were significantly changed by ethyl alcohol in the model group.These included organic acids, lipids, nucleosides, and organic oxygen compounds (Fig.5B, Fig.S2).Comparison of the TLMPS and model groups revealed significant differences in 57 metabolites.This may have reflected the effect of TLMPS.KEGG enrichment analysis of the significantly different metabolites indicated that they were involved in choline metabolism, ATP-binding cassette transporters, glycerophospholipid metabolism, and protein digestion and absorption.These findings suggested that these metabolic pathways may play important roles in the protective effect against acute alcoholic liver injury in mice of the TLMPS group (Fig.5C).
3.5 Association of transcriptomic and metabolomic changes involved in crucial biological pathways
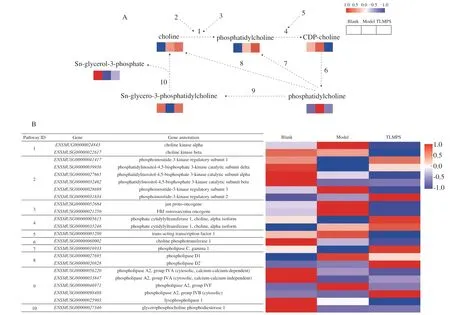
Fig.6 Adaptive changes in the choline metabolism by TLMPS pretreatment in ALD mice.(A) Choline metabolic pathways and metabolite changes associated with the pathways.(B) Changes in expression of genes involved in metabolic pathways.The pathway ID column indicates the gene product in relation to the metabolic pathway.
To obtain more information on the protective effects of TLMPS in acute alcoholic liver injury in mice, the changes in the association between gene expression and metabolite changes among the 3 groups were investigated.Interestingly, after TLMPS pretreatment, several genes encoding key enzymes and metabolites were significantly different in the choline metabolism pathway.The effects of TLMPS on alcoholic liver injury via the choline metabolism pathway and the general pattern of the relative changes of related metabolites are depicted in Fig.6A.TLMPS pretreatment markedly suppressed the content of CDP-choline and phosphatidylcholine, and increased the levels of sn-glycero-3-phosphatidylcholine and sn-glycerol-3-phosphate, which were increased or reduced by alcoholic liver injury.The expression patterns of the genes involved in the choline metabolism pathway are detailed in Fig.6B.In these pathways, the expression of most genes differed in the blank, model, and TLMPS groups.The expression of choline kinase alpha (ENSMUSG00000024843), phosphoinositide-3-kinase regulatory subunit 3 (ENSMUSG00000028698), jun protooncogene (ENSMUSG00000052684), FBJ osteosarcoma oncogene (ENSMUSG00000021250), phospholipase A2, and group IVF (ENSMUSG00000046971) was higher in the model group than in the blank.The expression was relieved in the TLMPS group.Conversely, several genes were downregulated in the blank vs.model group and upregulated in the model vs.TLMPS group.They included phosphoinositide-3-kinase regulatory subunit 1 (ENSMUSG00000041417), phosphate cytidylyltransferase 1, choline, alpha isoform (ENSMUSG00000005615), and phospholipase C, gamma 1 (ENSMUSG00000016933).These results confirmed the important role of the choline metabolism pathway in the preventive effects of TLMPS against alcoholic liver injury.
4.Discussion
The liver has vital functions and important metabolic activities.Serious metabolic disorders can occur as a result of liver damage.Liver injury has become a particularly prominent global health problem, with ALD being one of the most common diseases caused by excessive drinking [29].Alcohol is metabolized into acetaldehyde by alcohol dehydrogenase, the cytochrome P450 system, and the catalase systemin vivo.The many hydroxyl radicals that being produced are responsible for homeostasis of the radicals in the human body.Disruption of this balance occurs with age and can lead to the development of cancer, and a decline in immune system function [30].Several major areas of direct relevance to ALD include oxidative stress, the gut-liver axis and cytokine signaling, malnutrition, fibrin, and stellate cell fibrosis [31].
The liver index is one of the main indicators used to identify liver injury [32].The levels of ALT and AST reflect the degree of liver cell damage in a certain range due to liver cell membrane damage and aminotransferase in the liver cell released into the serum [33-35].The metabolites of TC and TG in the serum are closely related to lipid metabolism.The contents of TC and TG increase when there is excess fat in the liver or when fat metabolism in the liver is impaired [36].In this study, the liver index of the TLMPS group was reduced.Similarly, the serum levels of ALT, AST, TC, and TG confirmed liver injury in the model group and were correspondingly relieved in the TLMPS group, suggesting that TLMPS exerted a preventive effect against acute alcoholic liver injury in mice.Moreover, the histopathological amelioration of liver infiltration and hepatocyte necrosis caused by alcohol in the model group were also remarkably attenuated in the TLMPS group, further confirming the protective effect of TLMPS.In addition, comparison of the different TLMPS dosages revealed that the high-dose group (400 mg/kg bw) showed the most pronounced effects that were most similar to those of the positive control.Thus, thein vivofindings indicated that 400 mg/kg bw has potential as a natural drug for the prevention of acute alcoholic liver injury.
Drug alternatives, such as glucocorticoids andα-interferons, are commonly used for the treatment of ALD.However, they also induce side effects.Therefore, the identification of natural products with few side effects is one of the main goals of ALD treatment research.Aplysin is extracted from the red algaLaurencia tristichawas reported to significantly protect against alcohol-induced liver injury, possibly by alleviating oxidative damage and modulating endogenous apoptosis-related gene expression [37,38].Similarly, betulinic acid, a pentacyclic lupane-type triterpene, was previously found to prevent liver damage induced by alcoholin vivo, possibly due to its increased antioxidant capacity and decreased lipid peroxidation [39].Zhao et al.[40]pointed out that artemisinin could inhibit the activation of nuclear factor-kappa B, induce the expression of nitric oxide synthase, enhance the stability of liver cell membranes, and reduce damage to liver cells and their membranes.In the present study, TLMPS had a preventive effect in acute alcoholic liver injury in mice, based on an assessment of the liver index, aminotransferase levels in the serum, and the examination of pathological changes of tissue sections.Transcriptome and metabolome analyses explored the potential mechanism underlying the ability of TLMPS to prevent liver damage induced by alcohol.The transcriptome analysis showed that TLMPS was mainly involved in chemical carcinogenesis, drug metabolism, and the metabolism of xenobiotics by cytochrome P450, retinol metabolism, and glutathione metabolism.Metabolome analysis clearly indicated that TLMPS changed the metabolism of organic acids, lipids, nucleosides, and organic oxygen compounds in the liver.The combined data revealed the important role of choline metabolism in the protective effect of TLMPS against acute alcoholic liver injury.
Choline is an essential nutrient, and low levels of choline in the blood are often associated with fatty degeneration in the liver and liver dysfunction [41].Over 98% of choline found in the blood and tissues is a component of phosphatidylcholine molecules [42].Phosphatidylcholine, an important component of animal cell membranes, has a wide range of physiological activities, including reducing blood lipids, protecting the liver, and preventing arteriosclerosis.In an early study, Tuma et al.[43]indicated a potential interrelationship between ethanol metabolism and choline oxidation in the liver, where ethanol metabolism would stimulate the oxidative degradation of choline.In the present study, phosphatidylcholine and CDP-choline contents increased markedly, consistent with previous studies [44,45].Interestingly, TLMPS pretreatment suppressed the contents of CDP-choline and phosphatidylcholine but enhanced the levels of sn-glycero-3-phosphatidylcholine and sn-glycerol-3-phosphate, which can be increased or reduced by alcoholic liver injury.Furthermore, the expression of most genes in the choline metabolism pathway was upregulated and downregulated by pretreatment with TLMPS.These results suggest that TLMPS affects acute alcoholic liver injury by regulating choline metabolism, specifically CDP-choline, phosphatidylcholine, and sn-glycero-3-phosphatidylcholine.
The results presented in this study demonstrate that pretreatment with TLMPS mitigates the toxic effects of acute alcohol in the liver.The mechanism of TLMPS may be involved in regulating choline metabolism.Therefore, TLMPS should be regarded as a promising new functional component that could protect the liver against acute alcoholic damage.
5.Conclusions
Analyses of extract and purified TLMPS revealed that the carbohydrate content was the highest, followed by sulfate and glucuronic acid contents.UV and IR absorption spectrometry and Congo red analyses revealed the high purity of the obtained TLMPS.The protective effects of TLMPS against acute alcoholic liver injury were demonstrated by an inhibited alcohol-induced elevation of the liver index, serum biochemical indices, and maintenance of hepatic morphology.Potential mechanisms were analyzed using transcriptome and metabolome analyses showed that TLMPS regulated organic acids, lipids, nucleosides, and organic oxygen compounds in the liver.Choline metabolism was also found to be important role in the TLMPS protection against acute alcoholic liver injury.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant number 2019YFC1710501), the construction of a modern agricultural industrial technology system in Fujian province and an expert workstation of the modern edible mushroom industrial technology systeminvolvingPleurotus pulmonariusandGanoderma lucidum(project number Minnong General [2019]no.144).
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2021.04.010.
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Amelioration of metabolic disorders by a mushroom-derived polyphenols correlates with the reduction of Ruminococcaceae in gut of DIO mice
- Immunomodulatory effects of polysaccharides from edible fungus: a review
- Advances in research on chemical constituents and pharmacological effects of Paecilomyces hepiali
- Healthy function and high valued utilization of edible fungi
- Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
