Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice
2021-05-24KudrtRndhwVrinderSinghSnimrdeepKurRvinderKurSureshKumrRichShri
Kudrt Rndhw, Vrinder Singh*, Snimrdeep Kur, Rvinder Kur, Suresh Kumr, Rich Shri,*
a Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala 147002, India
b Chitkara College of Pharmacy, Chitkara University, Punjab, India
Keywords:Acetylcholinesterase inhibitor Alzheimer’s disease Mushrooms Pleurotus florida Resveratrol
ABSTRACT Our earlier work showed that hydromethanol extract (HME) of Pleurotus fl orida had antioxidant and anticholinesterase potential.This study aimed at isolating the constituent responsible for the activities.HME was subjected to bioactivity guided fractionation using in vitro anti-acetylcholinesterase (Ellman method) and antioxidant (DPPH scavenging assay).The most active constituent was evaluated in vivo employing i.c.v.streptozotocin (STZ) induced dementia in mice.Morris water maze test was used for evaluating memory, followed by biochemical estimations and histopathological studies.Bioactivity guided fractionation resulted in isolation of resveratrol (identifi ed by IR, NMR and MS) and its content in P.fl orida fruiting bodies was 0.0098% (m/m, by a validated TLC densitometric method).It improved STZ induced dementia and neurodegeneration in mice by reducing brain acetylcholinesterase action and oxidative stress.The observed effects might be the presence of resveratrol.Our results further endorse that P.fl orida derived resveratrol can be a promising therapy for Alzheimer’s disease.
1.Introduction
Alzheimer’s disease (AD) is a neurodegenerative condition responsible for immense suffering worldwide especially in aged population.The pathological signs of AD include cholinergic defi cit, oxidative stress,β-amyloid deposition, formation of neurofibrillary tangles of hyper-phosphorylated tau protein and neuroinflammation [1].The available drug therapy is unable to stop the disease progression and is limited by side effects [2].Hence, alternatives are being examined for mitigation of cognitive dysfuctions [3,4].Studies have shown neuroprotective potential of mushrooms mediated by acetylcholinesterase (AChE) inhibition, reducing oxidative stress, increasing endogenous antioxidant levels, stimulation of neuritogenesis, decreasing neurofibrillary tangles andβ-amyloid plaques [5,6].Pleurotus(Jacq.: Fr.) P.Kumm.species, or oyster mushrooms, are the second most frequently grown mushrooms [7].These species are traditionally used in nervous disorders and as memory enhancer [8].Pleurotusgenus contains diverse phytochemical groups, such as polysaccharides, terpenoids, polyphenols, fl avonoids and fatty acids [9].Studies have revealed the beneficial effect of some members ofPleurotusgenus in managing AD [10,11].In our earlier study, thein vitroantioxidant and AChE inhibitory activities of fourPleurotusspecies was examined and hydromethanol extract (HME) ofP.floridaEger (nom.nudum) (P.ostreatusvar.florida) demonstrated antioxidant and AChE inhibitory potential [12].The current investigation aimed to isolate the compound liable for such effects ofP.fl oridaemploying bioactivity guided fractionation (Fig.1) usingin vitroAChE inhibitory and antioxidant assays.Further, isolated bioactive compound was tested for cognitive improvement effects against streptozotocin (STZ) induced dementia in mice.
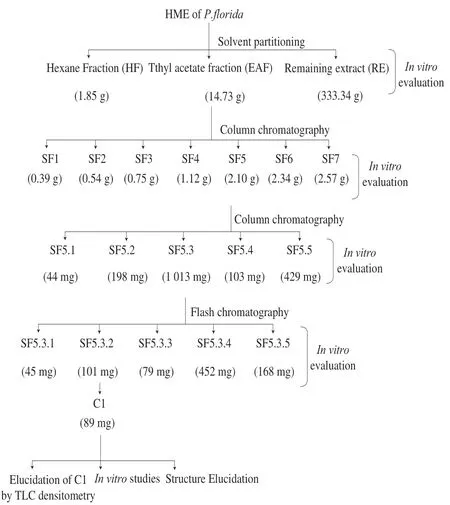
Fig.1 Schematic representation of bioactivity guided isolation.
2.Methods
2.1 Chemicals
AChE, acetylthiocholine iodide, DPPH (2,2-diphenyl-1-picrylhydrazyl), reduced glutathione (GSH), streptozotocin, 1,1,3,3-tetraethoxypropane, 5,5’-dithiobis-(2-nitrobenzoic acid) and donepezil were obtained from Sigma (St.Louis, MO, USA).All other chemicals and reagents were of analytical grade.
2.2 Preparation of HME and its fractions
P.floridafruiting bodies procured from Directorate of Mushroom Research, Solan, Himachal Pradesh, India (voucher no.12249), were shade dried and powdered.The hydromethanol (methanol: water = 70:30,V/V) extract of dried material was prepared by maceration [12].The bioactive HME was suspended in distilled water and sequentially partitioned (10 times) with equal volume of hexane and ethylacetate by refluxing (30 min).The layers were separated, pooled and dried under vacuum to obtain hexane fraction (HF), ethylacetate fraction (EAF) and aqueous fraction (AF).
2.3 Evaluation of in vitro anti-AChE and antioxidant activities of fractions
AChE inhibition by the fractions was determined with the modified Ellman method [13,14].Percentage enzyme inhibition was calculated by using the following formula:
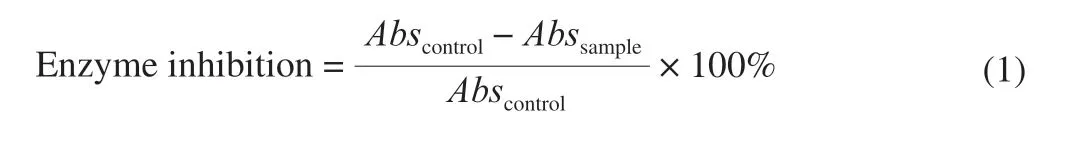
whereAbscontrolrepresents the absorbance of the control andAbssampleis the absorbance of the sample.The control consisted of 10 μL AChE (0.1 unit/mL), 10 μL ATCI (75 mmol/L), 10 μL DTNB (10 mmol/L), and 270 μL 0.1 mol/L phosphate buffer (pH 8.0); this was read against a blank consisting of 10 μL ATCI (75 mmol/L), 10 μL DTNB (10 mmol/L), and 280 μL 0.1 mol/L phosphate buffer (pH 8.0).Analyses were run in triplicate, and half-maximal inhibitory concentrations (IC50) were determined.
Antioxidant potential of the fractions was determined with the use of the DPPH scavenging assay [15].Ascorbic acid was used as the standard.DPPH scavenging activity was calculated using the following equation:
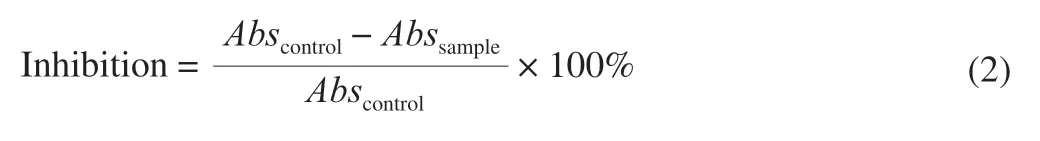
whereAbscontrolrepresents the absorbance of the control (1 mL DPPH solution and 1 mL methanol) andAbssampleis the absorbance of the sample.The control was read against a blank (methanol).Analyses were run in triplicate, and IC50was determined.
2.4 Bioactivity guided isolation of compound from the most active fraction of HME
Silica gel (mesh size 100-200) column chromatography was employed to fractionate the bioactive fraction (EAF, 12 g).Gradient elution with different proportions of hexane (100%), hexane:chloroform (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 0:100,V/V) and chloroform:methanol (99.5:0.5, 99:1, 98:2, 97:03, 96:04, 95:05, 92.5:7.5, 90:10, 85:15, 80:20,V/V) yielded a total of 850 fractions (250 mL each).Each fraction was monitored by thin layer chromatograms (TLC) and those exhibiting similar chromatogram were pooled together to obtain 7 sub-fractions namely, SF1-SF7.These sub-fractions were tested forin vitroAChE and DPPH inhibitory potential as described under section 2.2
The most active sub-fraction (SF 5) was again subjected to silicagel column chromatography using different solvent compositions comprising of mixture of hexane:chloroform (75:25, 50:50, 25:75, 00:100,V/V) and chloroform:methanol (99.9: 0.1, 99.8: 0.2, 99.7:0.3, 99.6:0.4, 99.5:0.5, 99:1, 98:2, 97:3, 96:4, 95:5, 90:10, 80:20, 50:50,V/V) to get total 300 fractions.Fractions with similar TLC profiles were pooled together to yield 5 sub-fractions (SF5.1-SF5.5).These were evaluated for AChE and DPPH scavenging potential as described under section 2.2.
The most active sub-fraction (SF5.3) was further purified using flash chromatography (Biotage Isolera rapid preparation liquid chromatography).A silica gel column (dimensions 20 cm × 3 cm) was loaded with SF5.3 and eluted gradiently with different concentration of hexane, chloroform and methanol at a fixed flow rate of 10 mL/min.The polarity was increased gradually by 5% for every 100 mL of effluent collected.Effluents showing similar purity and TLC chromatogram were pooled together to get fractions (SF5.3.1-SF5.3.5).Their biological activities were tested as described under section 2.2.
A white coloured crystalline compound (C1) was purified from bioactive fraction SF5.3.2 by crystallisation in methanol.The isolated compound was characterised spectroscopically (IR, NMR and MS).
2.5 Estimation of C1 in P.florida fruiting bodies by TLC densitometry
The content of C1 inP.floridafruiting bodies was estimated by TLC densitometric method using Camag HPTLC system (CAMAG Scientific Inc., Wilmington, NC).A 10 μL volume of C1 was applied (Linomat V) at various concentrations (5-30 μg/mL) on silica gel plate (0.2 mm; aluminium backed, E Merck, Mumbai, India) which was developed to 8 cm using the mobile phase-chloroform:methanol (9:1,V/V) in a pre-saturated TLC chamber (10 min).The developed plate was scanned at 306 nm, after drying, using TLC scanner (TLC scanner IV) and area under the curve of the peak of C1 was recorded with WinCATS software 1.4.8.The developed analytical method was validated following ICH guidelines [16,17].The content of C1 was determined from its standard plot and reported as its percent proportion in air dried mushroom material.
2.6 In-vivo studies with C1
2.6.1 Animals
Swiss Albino mice (20-25 g, either sex) purchased from Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, Haryana in May, 2018 were used forin vivoexperiments.Animals were kept in departmental animal house ((25 ± 2) °C; 12 h light-dark cycle) and provided waterad libitumand standard laboratory pellet chow diet.The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC), Punjabi University, Patiala, Punjab (Approval number: 107/GO/ReBi/S/99/CPCSEA/2018-01).Before the start of experiment, mice were adapted to lab conditions for 7 days.All experiments were performed as per the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Ministry of Environment, Forests and Climate Change, Government of India.
2.6.2 Induction of dementia
Cognitiveimpair mentin mice was induced by intracerebroventricular (i.c.v) injections of STZ (3 mg/kg, dissolved in artificial cerebrospinal fluid (ACSF)).STZ was injected bilaterally in two divided doses, on the first and the third day of the protocol [18].The concentration was adjusted so as to deliver 10 μL at a site.Control group mice were given i.c.v.injection of ACSF.
2.6.3 Experimental groups
Mice were divided into following 6 groups (6 animals per group).Treatment and evaluation of memory in animals was done as described in Fig.2.
1)Group I (ACSF group): Mice were administered with ASCF (10 μL, i.c.v.) on day 1 and day 3 of the study period.
2)Group II (STZ + vehicle treated): Mice were injected with STZ (3 mg/kg, 10 μL, i.c.v.) on day 1 and day 3 of study period.
3)Group III (STZ + Donepezil treated group): STZ (3 mg/kg, i.c.v.) treated mice were administered with donepezil (5 mg/kg; p.o.) from day 9 to day 22 of the study period.
4)Group IV, V and VI (STZ + C1 treated groups): STZ (3 mg/kg, i.c.v.) treated mice were administered with C1 at the doses of 5, 10 and 20 mg/kg, p.o., respectively, from day 9 to day 22 of the study period.
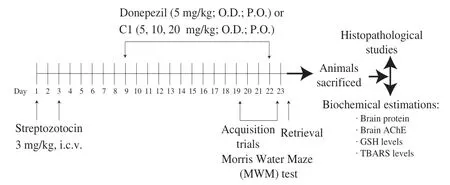
Fig.2 Experimental protocol.
2.6.4 Evaluation of cognitive functions (learning and memory) of animals by MWM
MWM test was employed to evaluate the learning and memory of mice [19,20].Animals were given trainings from day 19 to day 22 (4 times a day) to search the platform hidden under water in one quadrant of the MWM.The time taken by animals to find the hidden platform was measured as escape latency time (ELT).The training session was performed 60 min after treatment with donepezil or C1.On day 23, platform was removed and the same procedure was adopted once to measure the total time spent by mouse in each quadrant.The ability of animals to retain the learnt task was measured as the time spent in target quadrant (TSTQ).The cut off time was 120 s.
2.6.5 Brain biochemical estimations
At the end of behavioural experiment, animals were sacrificed by cervical dislocation (under mild anaesthesia) and their brains were removed.Intact brains were used for histopathological studies (3 per group) while brain homogenates, prepared in buffer (pH 7.4), were used to measure total protein levels [21], AChE activity [13], thiobarbituric acid reactive species (TBARS) levels [22]and reduced GSH levels [23-25].
2.6.6 Histopathological studies
Intact brains were stored in freshly prepared Bouin’s solution.The Hematoxylin and Eosin (which stains nuclei blue and cytoplasm appears pink) was used to stain the brain sections (5 μm thick) and observed under light microscope [26].
2.7 Statistical analysis
Results are expressed as mean ± standard deviation (SD).The results of behavioural study and biochemical parameters were compared by 2-way and one way ANOVA (analysis of variance), respectively, followed by post hoc analysis (Tukey’s test) using GraphPad Prism 7 software.
3.Results
3.1 Bioactivity guided fractionation of HME
HME (yield 32.87%,m/m) was partitioned into HF, EAF and RE.Among the fractions, EAF showed most marked anti-AChE and DPPH scavenging activities (Table 1).Thus, it was fractionated further using column chromatography (Fig.1) and resulted in 7 subfractions (SF1-SF7) which were subjected toin vitrostudies.SF5 was found to have the strongest AChE and DPPH inhibitory activities (indicated by lowest IC50values).The bioactive sub-fraction SF5, on further column chromatography, gave 5 sub-fractions (SF5.1-SF5.5).SF5.3 was found to have lowest IC50values in both the tests (Table 1).Therefore, it was purified by flash chromatography which resulted in 5 sub-fractions (SF5.3.1-SF5.3.5).From SF5.3.2, a white coloured crystalline compound (C1) was obtained by crystallisation.In vitroanalyses revealed that C1 had strongest AChE and DPPH inhibitory activities (Table 1).Therefore, C1 was characterised by spectroscopic techniques and its content in HME was determined by TLC densitometric method.On the basis of spectroscopical (IR, NMR and MS) data, the structure of C1 was found to be resveratrol (Supplementary Fig.1-4).

Table 1 IC50 values of various sub-fractions and compounds in AChE inhibitory and DPPH scavenging activity.
3.2 Estimation of C1 (resveratrol) in P.florida fruiting bodies by TLC densitometry
The content of resveratrol inP.floridafruiting bodies was determined by a validated TLC densitometric method.The developed method was found to be accurate (as depicted by recovery studies), precise and specific (Table 2).Standard plot of resveratrol was prepared between different amounts (50-300 ng) and their peak areas.The chromatogram and spectra overlay of resveratrol and HME are given in Supplementary Fig.5.The content of C1 inP.floridafruiting bodies was estimated as 0.009 8% (m/m).
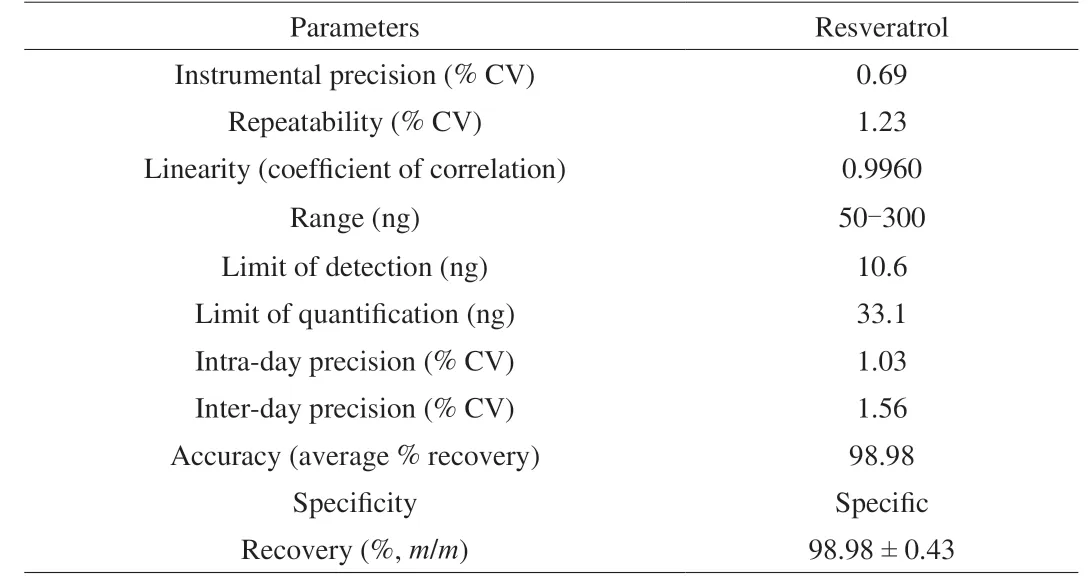
Table 2 Method validation parameters for estimation of C1 (resveratrol) in P.florida fruiting bodies by TLC densitometry.
3.3 Effect of C1 (resveratrol) on learning and memory of animals in MWM
A sharp and significant decline in ELT was observed for control group animals from day 19 to day 22 which reflects their normal learning ability.On day 23, the same group showed significantly higher TSTQ (Q4) when compared to other quadrants which depicted the normal memory function of mice.The STZ-treated group showed marked deterioration of learning and memory capacity evidenced by significantly higher day 22 ELT and lower TSTQ on day 23 in comparison to the ACSF control group.Treatment of STZ injected mice with donepezil (5 mg/kg, p.o.) and resveratrol (5, 10 and 20 mg/kg, p.o.) significantly decreased the ELT on day 22 and increased TSTQ on day 23, as expected, in comparison with STZ group indicating improvement in cognitive functions of animals (Table 3, Fig.3).

Table 3 Effect of various treatments on ELT of mice in MWM test after i.c.v.STZ induced memory deficit.
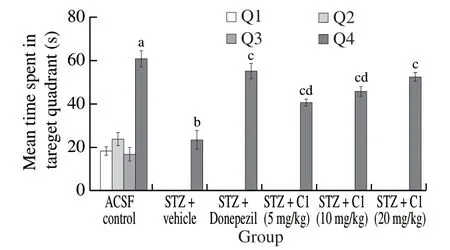
Fig.3 Effect of C1 on TSTQ by mice in MWM test.The data is expressed as mean ± SD; n = 6; a P < 0.05 vs.time spent in Q1, Q2 and Q3 by ACSF control group; bP < 0.05 vs.TSTQ of ACSF control group; c P < 0.05 vs.TSTQ of STZ + vehicle treated group; d P < 0.05 vs.TSTQ of STZ + Donepezil treated group; analysed by two way ANOVA followed by Tukey’s test.
3.4 Effect of C1 (resveratrol) on brain AChE activity and oxidative stress
In comparison to the ACSF control group, the STZ group animals showed significant increase in brain AChE activity and TBARS levels (indicator of MDA levels) and decrease in GSH levels (Fig.4).Donepezil as well as resveratrol treatment significantly reversed the i.c.v.-STZ-induced increase in central AChE activity and TBARS levels and decrease in endogenous antioxidant (GSH levels).
3.5 Histopathological studies
The hematoxylin and eosin stained histological sections (Fig.5) showed well defined neuronal area in the hippocampus and normal pyramidal cells (PC) in cortex of the ACSF control group.STZ group mice showed increased vacuolation, decreased number PC and focal gliosis in comparison to control group.Donepezil and resveratrol treatments showed reversal of STZ induced hippocampal and cortical alterations.
4.Discussion
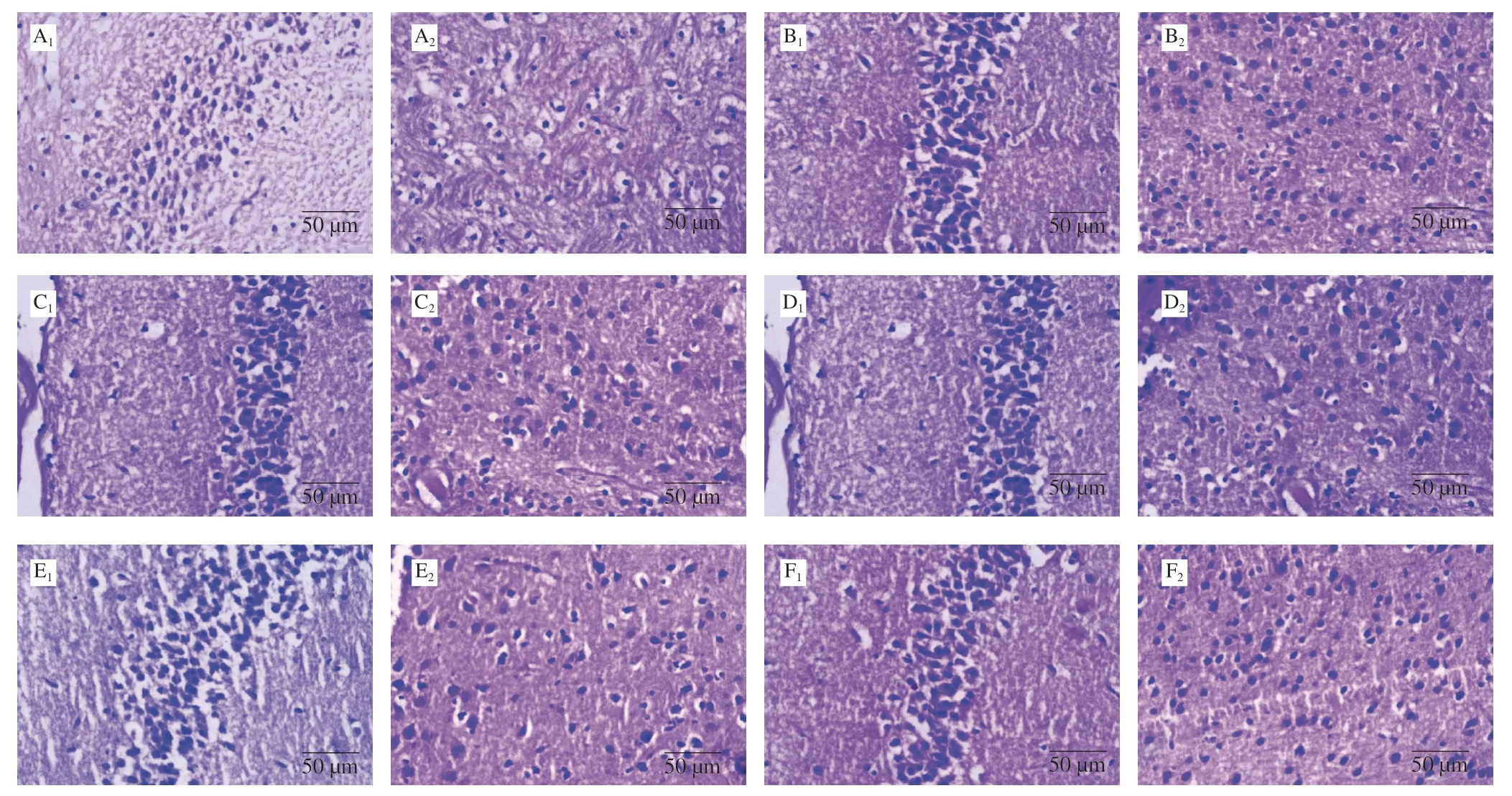
Fig.5 Histological sections of hippocampal (1) and cortical (2) areas of brain (stained with haematoxylin and eosin).A.STZ + vehicle treated; B.ACSF group; C.STZ + Donepezil treated; D.STZ + C1 treated (5 mg/kg) treated; E.STZ + C1 treated (10 mg/kg) treated; F.STZ + C1 treated (20 mg/kg) treated.
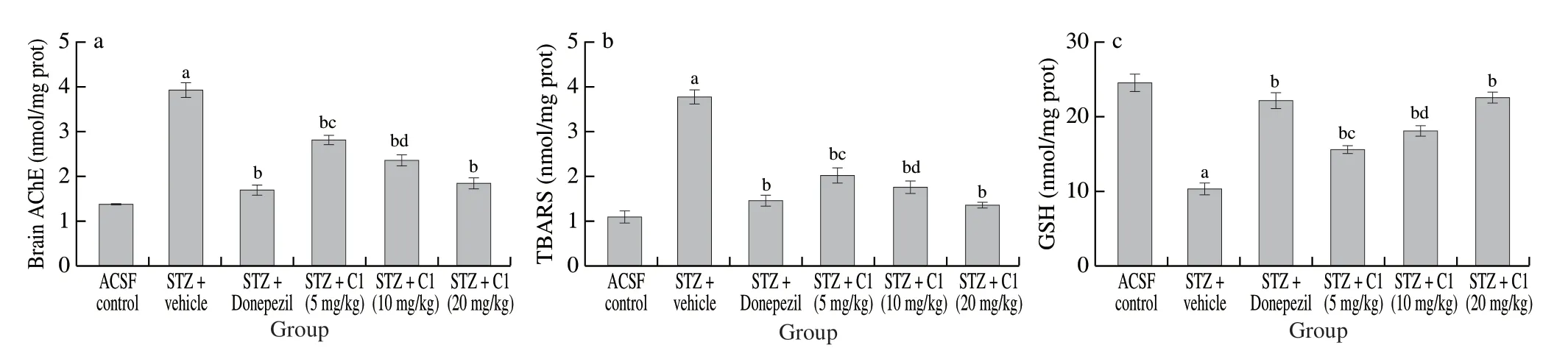
Fig.4 Effect of C1 on (a) brain AChE activity, (b) brain TBARS levels, and (c) brain GSH level of mice.Data is expressed as mean ± SD; n = 6; a P < 0.05 vs.ACSF control group; b P < 0.05 vs.STZ + vehicle treated; c P < 0.05 vs.STZ + Donepezil treated; d P < 0.05 vs.STZ + Donepezil treated.Analysed by one way ANOVA followed by Tukey’s test.
In the present investigation Ellman and DPPH scavenging assays were used for bioactivity guided fractionation of HME.These methods are quick, reliable and produce reproducible results when employed to assess AChE inhibition and antioxidant activities, respectively [13,14].This resulted in isolation of C1 i.e.resveratrol.Its cognitive improvement effects were further establishedin vivoby employing STZ (i.c.v.) induced dementia in mice.Bilateral administration of two sub-diabetogenic doses of STZ, 48 h apart, in cerebroventricles produces behavioural (progressive impairment of learning and memory), molecular, neuropathological and metabolic symptoms in rodents via impairing cerebral energy metabolism, mitochondrial disturbances and generating reactive oxygen species that are reminiscent of the human AD [27,28].The consequent oxidative stress damages the myelin sheath [18], accompanied by decreased acetyl coenzyme-A synthesis in hippocampus causing elevation of AChE activity (cholinergic deficit) leading to neuropathological and cognitive disturbances similar to that in AD [27].Hence, this model is employed widely to study interventions (synthetic and herbal drugs) for AD [29].MWM test is a widely employed and accepted method to measure cognitive parameters of rodents [29].In harmony with earlier studies [18,30], STZ-treated group, in the present investigation, showed marked deterioration of learning and memory capacity which was reversed dose dependently by C1 treatment.
STZ (i.c.v.) administration causes progressive loss of memory and neurodegeneration in mice by elevating the brain AChE activity and free radical generation in the forms of peroxides and superoxides causing oxidative stress [18,30,31].Therefore, the present study revealed the mechanism of action of C1, these cerebral parameters were determined in mice brain homogenates.Oxidative stress due to i.c.v.STZ injection results in generation of malondialdehyde (MDA) by lipid peroxidation and depletion of endogenous antioxidants such as GSH [27,31].Hence, brain MDA and GSH levels that served as biomarkers for brain oxidative stress were measured in mice brain homogenates.In concordance with literature [31,32], i.c.z.STZ injection induced cholinergic deficit and elevation of brain oxidative stress in the present study.Treatment with C1 showed amelioration of these STZ induced effects in mice.
In AD patients, neuronal damage in the brain regions associated with memory, i.e., the cortex and hippocampus is reported.As per literature i.c.v STZ administration causes neuronal degradation in hippocampal and cortical brain areas [18,32].This is also evident on histopathological examination of cortical and hippocampal regions of mice brain after STZ (i.c.v.) administration [28,31].Treatment with C1 reduced the neurodegenerative effects of STZ (Fig.5).
In the present study, a compound named as C1 was isolated as AChE inhibitor from HME which was identified as resveratrol (based on IR, NMR and HRMS studies).Resveratrol, a naturally occurring polyphenol, is commonly found in various food sources.There are reports that describe the presence of this polyphenol in mushrooms includingPleurotusspecies [33,34].Similar to plants, secondary metabolites like stilbenes are biosynthesized from either phenylalanine or tyrosine obtainedviashikimic acid pathway in higher fungi [35].During the biosynthesis of resveratrol, first of all,p-coumaric acid is formed by eitherL-phenylalanine orL-tyrosine by the removal of ammonia under the action of phenylalanine ammonia lyase, producingtrans-cinnamic acid, which is further hydroxylated with the help of cinnamate-4-hydroxylase to givep-coumaric acid [35,36].Then thep-coumaric acid by its esterification with coenzyme A is changed intop-coumaroyl-CoA by the action of 4-coumaroyl-CoA ligase.Finally, with the help of stilbene synthase, three molecules of malonyl-CoA are condensed with 4-coumaroyl-CoA through a repeating decarboxylating reaction to cyclize a tetraketide intermediate to produce resveratrol [35,36].
Resveratrol is effective in preventing STZ-induced cognitive impairment in rodents [37]and has antioxidant mediated neuroprotective effects [38].The findings of our study are in accordance with these earlier reports and further corroborate that resveratrol protects against i.c.v.STZ induced memory deficit in animals.
5.Conclusion
Bioactivity guided isolation of HME ofP.floridalead to the separation of resveratrol.The anti-Alzheimer’s activity ofP.floridais being reported for the first time and is attributed to the presence of resveratrol.Experimental studies as well as clinical trials have shown the effectiveness of resveratrol for management of AD.The findings of our study further endorse that resveratrol can be a promising therapy for management of cognitive dysfunction.Furthermore, resveratrol may be used as the marker for standardization ofP.floridawhen it is developed as a nutraceutical or drug for Alzheimer’s patients.
Declaration of conflict of interest
None.
Acknowledgements
The study was funded by University Grants Commission under UGC BSR fellowship scheme (Award no.F.4.25-1/2013-14(BSR)/7-265/2009(BSR)).The authors are thankful to Prof.Nirmal Singh and Mr.Pankaj Bhatia, Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India for their help in carrying out animal studies.The authors are also thankful to DBTIPLS project (Grant no: BT/PR4548/INF/22/146/2012), Punjabi University, Patiala, Punjab, India for providing HPTLC facility and Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India for providing the necessary laboratory facilities for this work.
Appendix A.Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2021.04.011.
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
- Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo
- The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense
- The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice
- The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics
