Antrodia Cinnamomea ameliorates neointimal formation by inhibiting infl ammatory cell infi ltration through downregulation of adhesion molecule expression in vitro and in vivo
2021-05-24YnZhngAijinHoXiNingChenRongWngChenhuiYngJinngChenPinFupingZhengWenyiKng
Yn Zhng, Aijin M, Ho Xi, Ning Chen, Rong Wng, Chenhui Yng, Jinng Chen, Pin Lü,,*, Fuping Zheng,*, Wenyi Kng*
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing 100048, China
b Cardiovascular Medical Science Center, Department of Cell Biology, Hebei Medical University, Shijiazhuang 050091, China
c Hebei Food Safety Key Laboratory, Hebei Food Inspection and Research Institute, Shijiazhuang 050091, China
d National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, Henan 475004, China
Keywords:Antrodia cinnamomea Vascular smooth muscle cells Infl ammation Adhesion molecule Neointimal hyperplasia
ABSTRACT The increased vascular infl ammation is a key event in the development of atherosclerotic lesions.Antrodia cinnamomea has been shown to promote anticancerogenic activity through decreasing infl ammation.However, the potential role of A.cinnamomea in cardiovascular diseases remains unexplored.Herein, using carotid arterial ligation models, we found that ethanol extract from A.cinnamomea (EEAC) signifi cantly inhibited neointimal hyperplasia in a dose-dependent manner, accompanied with the reduced expression of activated p65 and infl ammatory cytokines.We also show that EEAC ameliorated TNF-α-induced phosphorylation of p65 and pro-infl ammatory cytokine expression in both vascular smooth muscle cells (VSMCs) and macrophages in vitro.Mechanistically, EEAC suppressed expression levels of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) in VSMCs, which attenuates the ability of monocytes/macrophages adhesion to VSMCs.Furthermore, the expression level of these adhesion molecules and infi ltration of monocytes/macrophages were also decreased in neointimal VSMCs of arteries pretreated with EEAC.Altogether, our results reveal a novel function of A.cinnamomea in suppressing vascular infl ammation upon ligation injury during neointimal formation, likely through inhibition of infl ammatory cell infi ltration via downregulating the adhesion molecules in VSMCs.Thus, A.cinnamomea may offer a pharmacological therapy to slow down disease progression in patients with vascular injury.
1.Introduction
Atherosclerosis, restenosis after angioplasty, and graft atherosclerosis after coronary artery bypass surgery are pathologic process occurring within the artery, in which many cell types, such as macrophages, endothelial cells (ECs), and vascular smooth muscle cells (VSMCs), cause chronic infl ammation through their interaction, in response to various inner- or outer-cellular stimuli [1,2].In animal models, monocytes/macrophages infi ltration and production of cytokines in the arterial wall are considered as the primary players regulating atherosclerotic inflammation, which is associated with intimal growth, and blockade of the leukocyte recruitmentviadownregulating the expression of cell adhesion molecules decreases intimal thickening [3].Therefore, decreasing of inflammatory cell recruitment is a major problem to repair vascular injuries.
Previous studies showed that an initial step of monocytes/macrophages infiltration is driven by ECs.Activated ECs mediate immune cell adhesion to the vascular wall and monocyte infi ltration to the intima where they initiate inflammation.Recent human autopsy studies,in vitromechanistic studies andin vivocorrelative data highlighted the important role for VSMC in retention of monocytes and macrophages, contributing to the initiation and propagation of the vascular inflammatory response [2,4].Like endothelial cells, VSMCs can also produce multiple adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM-1) and fractalkine (CX3CL1), promote monocytes and lymphocytes adhere and migrate into the vessel wall [5-7].Furtherly, VSMCs are also able to protect macrophages from apoptosis [5,8].Taken together, VSMCs are indeed important in vascular inflammation.
Antrodia cinnamomea, also namedAntrodia camphorata,Ganoderma camphoratum, orTaiwanofungus camphoratapreviously, is a unique and precious endemic Taiwanese medicinal mushroom.The ingredients ofA.cinnamomeaencompass triterpenoids, polysaccharides, superoxide dismutase (SOD), adenosine, succinic and maleic acid derivatives, proteins (including immune protein), vitamins, nucleic acid, lectin, ergosterol, and lignin [9,10].Among them, triterpenoids and polysaccharides are considered as the main health-care function sources ofA.cinnamomea.A.cinnamomeais widely used for a traditional medicine for diverse health conditions such as diarrhea, detoxification, liver protection, abdominal pain, hypertension, itchy skin and anti-cancer [9,11-13], which are associated with its anti-inflammatory, anti-oxidant, anti-virus, antiallergy, anti-microorganism, immunomodulation, inhibition of platelet aggregation, hepatoprotection, anti-fatigue, and so on [9,14-17].However, little is known about the role ofA.cinnamomeain prevention of vascular injury and regulating vascular inflammation.In this study, we show that ethanol extract fromA.cinnamomea(EEAC) prevents neointimal hyperplasia and nuclear factor kappa-B (NF-κB) activation induced by ligation injury, and has an inhibitory effect on expression of inflammatory cytokines in VSMCs and monocytes/macrophages.Additionally, EEAC treatment significantly downregulates the expression of adhesion molecules in VSMCs and subsequently reduces the ability of macrophages adhesion to VSMCsin vivoandin vitro.
2.Materials and methods
2.1 Preparation of ethanolic extract of fruiting bodies of A.cinnamomea
One gram fruiting body powder ofA.cinnamomeawas weighted and soaked in 10 mL of 95% ethanol extractive solvent for 72 h at room temperature.The solid residue of the above soaked herbs was centrifuged at 13000 r/min for 20 min followed by being filtered and discarded through a Buchner funnel lined with 0.8 μm Whatman filter paper, and the filtrate was vacuumed dry by rotary vacuum distillation for 24 h.The powder form of purifiedA.cinnamomeawas redissolved in DMSO with a concentration of 100 μg/μL for subsequent experiments.
2.2 Carotid artery ligation models
Ten to 12-week-old male mice underwent a complete carotid artery ligation.Briefly, male C57BL/6J mice were anesthetized by inhalation of isoflurane-N2O.The left common carotid artery was completely ligated just near its bifurcation using a 6-0 silk ligature [18,19].Ethanol extract fromA.cinnamomea(EEAC) was orally administered at doses of 50 and 100 mg/kg·day by gastric gavage from 7 days before ligation injury to 14 days after the injury (n= 8 per group).All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee of Hebei Medical University.
2.3 Haematoxylin and eosin (H&E) staining and immunohistochemistry
Carotid arteries were harvested at 14 days after injury.The animals were euthanized by an intraperitoneal injection of ketamine (80 mg/kg)/xylazine (5 mg/kg).The left ventricle was cannulated and perfused with phosphate buffered saline (PBS) containing heparin, and then perfused and fixed with 4% paraformaldehyde in PBS under physiological pressure.The left carotid artery was then removed, further fixed for 16 h, and paraffin-embedded without further dissection.Serial sections (5-μm thick) were obtained at 500 μm.The cross-sectional areas of the intima and media were measured in H&E stained sections in a blinded manner by a single observer using Image Pro Plus 6.0 software (Media Cybernetics).A mean value was determined from at least 4 sections for each animal.Neointima formation was determined as the ratio of the intimal area to the medial area (I/M).
The immunohistochemical analyses were processed according to standard procedures.Immunostained sections were preincubated with 5% normal goat serum and then incubated with anti-proliferating cell nuclear antigen (PCNA) (1:1000, ab92552, Abcam), anti-NF-κB p65 (phospho S536) (1:500, ab28856, Abcam), anti-intercellular cell adhesion molecule (ICAM)-1 (1:200, sc-8439, Santa Cruz), antivascular cell adhesion molecule (VCAM)-1 (1:200, sc-8304, Santa Cruz),anti-CD14 (1:500 dilution, ab182032, Abcam), and anti-CD68 (1:500 dilution, ab955, Abcam) antibody.The sections were incubated with the horseradish peroxidase streptavidin biotinylated secondary antibody (Dako) followed by diaminobenzidine (DAB kit, Vector Laboratories) and counter stained with Gill’s haematoxylin (Fisher Scientific).For the negative controls, the primary antibody was replaced with non-immune rabbit or mouse serum.Staining intensities were determined by measurement of the integrated optical density (IOD) with light microscopy using a computer-based Image-Pro Morphometric System by two independent observers in a doubleblind manner.IOD of objects expressing the protein (brown coloured) was compared with the overall number of cells counted on the basis of IOD blue-related nuclei.The results are expressed as the mean value of at least 3 randomly chosen slides in each group.
2.4 Cell culture and treatment
The mouse VSMC line MOVAS was purchased from ATCC (CRL-2797), and cultured in high-glucose DMEM containing 10% FBS and 0.2 mg/mL G-418.Before treated by different stimulations, all of the VSMCs were incubated in serum free medium for 24 h.The RAW264.7 cells were purchased from ATCC (TIB-71™), and cultured in low-glucose DMEM containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.Before isolating, VSMCs and RAW264.7 were pretreated by different concentrations of EEAC for 24 h and then stimulated with TNF-α (10 ng/mL, PeproTech) for indicated time periods.
2.5 RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) and cDNAs were synthesized by reverse transcriptase SuperScript II (Invitrogen).The qRT-PCR was performed using SYBR Green RT-PCR Master Mix (Invitrogen) and 7300 Real Time PCR System (Applied Biosystems, Carlsbad, CA, USA).For RT-PCR analysis, the following specific primers were used:PCNAforward, 5’-TTTGAGGCACGCCTGATCC-3’and reverse, 5’-GGAGACGTGAGACGAGTCCAT-3’;p21forward, 5’-GTGTGCCGTTGTCTCTTCG-3’ and reverse, 5’-GGTCTGCCTCCGTTTTCG-3’;iNOSfor ward, 5’-CCTTGGTGAAGGGACTGAGC-3’and reverse 5’-CAACGTTCTCCGTTCTCTTGC-3’;MCP-1forward, 5’-CTTCTGGGCCTGCTGTTCA-3’ and reverse, 5’-CCAGCCTACTCATTGGGATCA-3’';IL-6forward, 5’-TAGTCCTTCCTACCCCAATTTCC-3’ and reverse, 5’-TTGGTCCTTAGCCACTCCTTC-3’;IL-1βforward, 5’-GCAACTGTTCCTGAACTCAACT-3’ and reverse, 5’-ATCTTTTGGGGTCCGTCAACT-3’;VCAM-1for ward, 5’-AGTTGGGGATTCGGTTGTTCT-3’and reverse, 5’-CCCCTCATTCCTTACCACCC-3’;ICAM-1forward, 5’-CTCATCCTGCGCTGTCTGGT-3’ and reverse, 5’-CCGGAGCTGCCTGACCTCGG-3’;GAPDHforward, 5’-AAGCCCATCACCATCTTCCA-3’and reverse, 5’-CCTGCCTCACCACCTTCTTG-3’.For the vascular tissues, the samples were pooled to analyse by the qRT-PCR in triplicate, and averaged by experiment, in 3 times repeatedly (n= 3).
2.6 Western blot analysis
Cells were prepared with lysis buffer (1% Triton X-100,150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 1 mmol/L EGTA, pH 8.0, 0.2 mmol/L Na3VO4, 0.2 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 0.5% Nonidet P (NP)-40).Equal amounts of protein (60-100 μg) were separated by 10% SDS-PAGE and electro-transferred on to a polyvinylidenefluoride (PVDF) membrane.Membranes were blocked with 5% non-fat milk in tris-buffered saline-Tween (TBST) for 2 h at room temperature and incubated with specific primary antibodies against PCNA (1:1000, ab92552, Abcam), p21 (1:500, ab188224, Abcam), NF-κB p65 (phospho S536) (1:500, ab28856, Abcam), NF-κB p65 (1:500, ab16502, Abcam), ICAM-1 (1:500, sc-8439, SantaCruz), VCAM-1(1:500, sc-8304, Santa Cruz), or GAPDH (1:1000, ab181602, Abcam) at 4 °C overnight.The membranes were then incubated with IRDye800®conjugated secondary antibody (1:20000, Rockland Biochemicals) for 1 h at room temperature, following scanning with the Odyssey Infrared Imaging System.The protein bands of interest were quantified using Quantity One software (Bio-Rad).
2.7 Cell viability assay
The detail MTT-based cell viability assay has been described in previous publication [20,21].VSMCs or RAW264.7 cells were seeded onto 96-well plates (1 × 104cells/well) and pretreated with various concentrations of EEAC for 24 h before stimulation with or without TNF-α (10 ng/mL).Cell viability was measured using MTT assay.
2.8 Adhesion assay
VSMCs plated in 96-well culture plates were pretreated with EEAC for 24 h followed by stimulated with TNF-α (10 ng/mL) for 24 hor not, and then RAW264.7 cells (labeled with Calcein-AM, Life Technologies) were added to each well.After 30-min incubation at 37 °C, non-adherent cells were removed carefully by washing with cold PBS.Six fields were randomly chosen, and adherent cells were photographed by fluorescence microscopy.The number of adherent cells was counted using ImageJ.
2.9 Statistical analysis
Data analysis was perfo rmed using SPSS version 16.0 or Graphpad Prism 6 software.Data are presented as means ± standard deviation (SD) from at least 3 independent experiments, and each independent experiment was repeated 3 times to obtain the mean.Normally distributed datasets were analyzed with the unpaired Student’st-test for 2 independent groups or pairedt-test for 2 dependent groups, and the one-way analysis of variance (ANOVA) followed by the post Bonferroni’s multiple comparisons test for ≥ 3 groups.For all statistical comparisons, a value ofP< 0.05 was considered statistically significant, and denoted with one, two and three asterisks when lower than 0.05, 0.01 and 0.001, respectively.
3.Results
3.1 EEAC inhibits progression of neointimal hyperplasia
To investigate the role of EEAC in neointima formation, mouse carotid arteries were harvested on day 14 after carotid ligation, and an increased I/M thickness ratio of carotid arteries was observed (Fig.1A).Compared with injured controls, pre-administration with 50 and 100 mg/kg·day EEAC for 1 week significantly reduced I/M thickness ratio by over 55% and 87%, respectively (Fig.1A).Immunohistochemical staining showed that the expression of PCNA was downregulated in neointimal VSMCs of arteries pretreated by EEAC (Fig.1B).Furtherly, qRT-PCR and western blot analysis demonstrated that the expression of PCNA was markedly decreased with an increase of p21 expression in EEAC-pretreated neointimal arteries (Fig.1C, 1D).These data indicate that EEAC exhibits an inhibitory effect on neointimal hyperplasia and VSMC proliferationin vivo.
3.2 EEAC inhibits NF-κB activation during neointimal hyperplasia

Fig.1 EEAC inhibits progression of neointimal hyperplasia. (A) H&E staining (upper) and I/M thickness ratio analysis (below) (n = 8).Bar = 25 μm.The sections of mouse carotid arteries were prepared on day 14 after ligation injury pretreated with EEAC (50 and 100 mg/kg·day) or not.(B) Immunohistochemistry analysis of the expression of PCNA (n = 8).Bar = 25 μm.(C) RT-PCR of PCNA and p21 mRNAs in carotid arteries (n = 3).(D) Western blot and densitometric analysis of expression of PCNA and p21 levels in carotid arteries (n = 3).The mouse carotid arteries were prepared on day 14 after ligation injury pretreated with EEAC (50 mg/kg·day) or not in B, C and D.Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
Inflammation is a key response to neointimal formation during various vascular disorders, including restenosis, atherosclerosis and pulmonary artery hypertension [22-24].Because TNF-α is known to promote VSMC proliferation, and inflammation exerts important effects on cell proliferation [25,26], we sought to determine the relationship between EEAC-suppressed neointimal hyperplasia and TNF-α-induced VSMC inflammation.Upon inflammatory stimulation, transcription factor NF-κB is activated and plays a master regulating role in inflammatory responds [27].Therefore, using p-p65 immunohistochemical staining, we showed that activated p65 was observed in the injury group, mostly in VSMCs, and pretreatment of EEAC nearly eliminated p65 activation (Fig.2A).In addition, we evaluated the mRNA levels of inflammatory molecules, includingiNOS,MCP-1,IL-6andIL-1β, and found that the expression levels of these inflammatory genes were decreased after EEAC administration (Fig.2B-2E), suggesting that EEAC may contribute to anti-inflammatory activity associated with vascular disease.

Fig.2 EEAC attenuates NF-κB activation in carotid arteries.The mouse carotid arteries were prepared on day 14 after ligation injury pretreated with EEAC(50 mg/kg/day) or not.(A) Immunohistochemistry analysis for the expression of activated p65 in carotid arteries (n = 8).Bar = 25 μm.(B-E) RT-PCR of pro-inflammatory molecule mRNAs in carotid arteries (n = 4).Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
3.3 EEAC attenuates expression levels of inflammatory cytokines in VSMCs and macrophages in vitro
To further investigate how EEAC halts vascular inflammation, we turned to culture VSMCs and macrophages, two primary sources of inflammatory cytokines.Firstly, we examined the effect of EEAC on TNF-α-induced cell viability of VSMCs using the MTT assay.VSMCs were pretreated with different concentrations (0-800 μg/mL)of EEAC for 24 h, followed by stimulation with TNF-α (10 ng/mL) for 24 h.As shown by MTT assay, TNF-α promoted VSMC viability, whereas pretreatment of VSMCs with 400 and 800 μg/mL of EEAC for 24 h dose-dependently abrogated the inducing effects of TNF-α on cell viability (Fig.3A).To further provide evidence that EEACinhibited VSMC viability was associated with its inhibition of TNF-α- induced inflammation, we investigated the effect of EEAC on expression levels of inflammatory cytokines.Western blot showed that TNF-α caused an obvious phosphorylation of p65 in VSMCs (Fig.3B).Pretreating VSMCs with EEAC for 24 h greatly attenuated phosphorylation of p65 in TNF-α-stimulated groups (Fig.3B).Next, we examined whether EEAC inhibits the expression levels of of NF-κB target inflammatory cytokine in VSMCs.As shown in Fig.3C-3F, TNF-α increased the mRNA levels ofiNOS,MCP-1,IL-6andIL-1β, and preincubation of EEAC for 24 h decreased the production of these inflammatory cytokines upon TNF-α stimulation, while almost have no effect on their expression in basal groups.
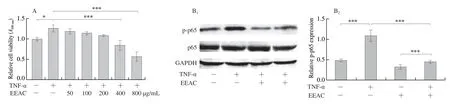
Fig.3 EEAC inhibits NF-κB activation and expression levels of pro-inflammatory molecules in VSMCs stimulated by TNF-α.(A) MTT for the viability of VSMCs, cultured in serum-starved medium containing 50, 100, 200, 400, 800 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 24 h (+) or remained untreated (-).(B) Western blot and densitometric analysis of expression of activated p65 in VSMCs, cultured in serum-starved medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 30 min (+) or remained untreated (-).(C-F) RT-PCR of pro-inflammatory molecule mRNAs in VSMCs, cultured in serum-starved medium containing 400 ug/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 6 h (+) or remained untreated (-).Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
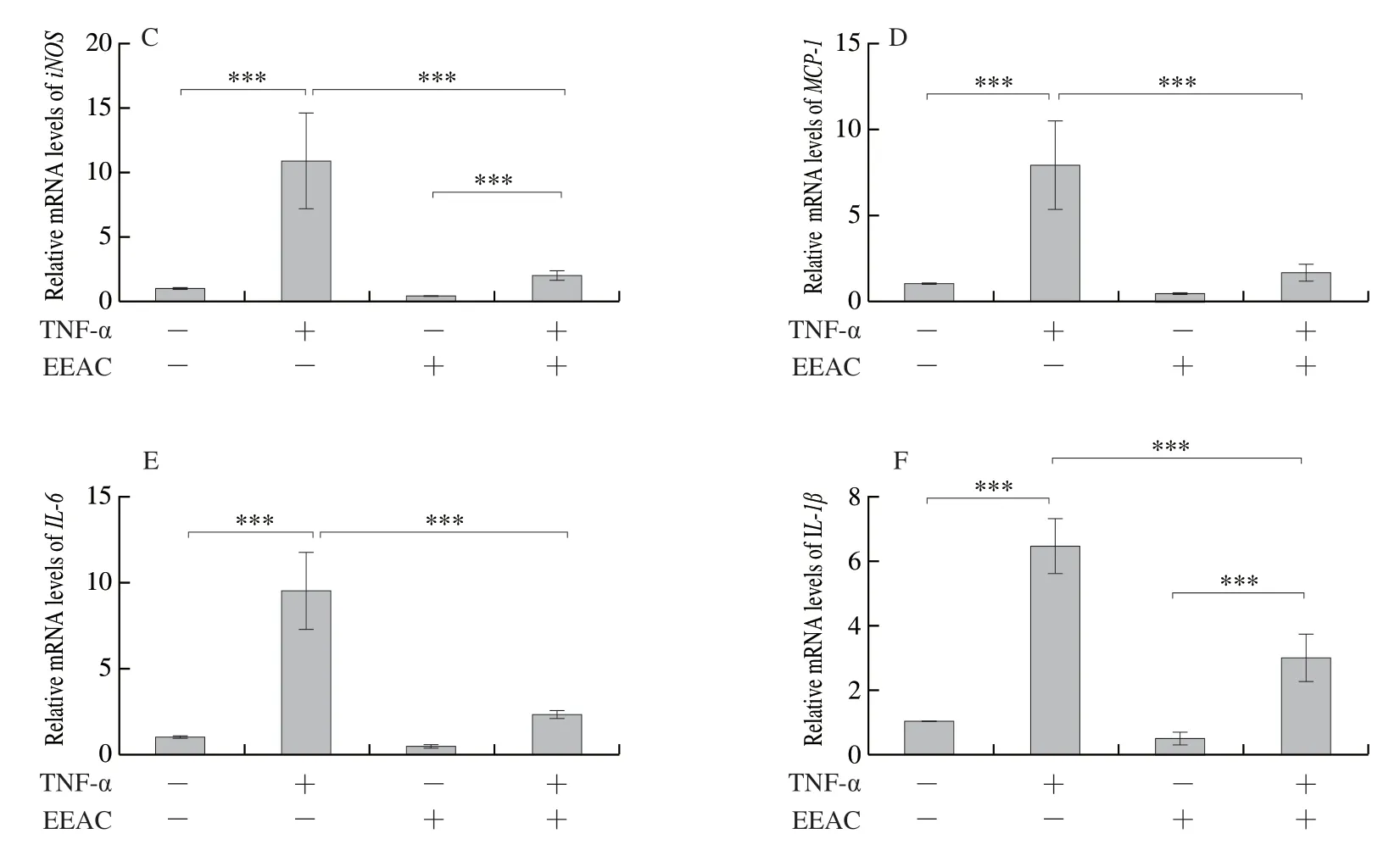
Fig.3 (Continued)
Parallel with the experiments in VSMCs, results from macrophages also showed that EEAC inhibited TNF-α-induced RAW264.7 viability in a concentration dependent manner, and at the EEAC concentration of 400 and 800 μg/mL, the significant decrease of cell viability was observed (Fig.4A).Furtherly, results from Western blot showed that TNF-α upregulated p-p65 expression was reversed by EEAC in both basal and TNF-α stimulated groups (Fig.4B).qRT-PCR showed that EEAC blocked TNF-α-inducediNOS,MCP-1,IL-6andIL-1βmRNA levels (Fig.4C-4F).Altogether, these findings suggest that EEAC inhibits TNF-α-induced inflammation both in VSMCs and macrophages by inhibiting NF-κB signaling pathway.
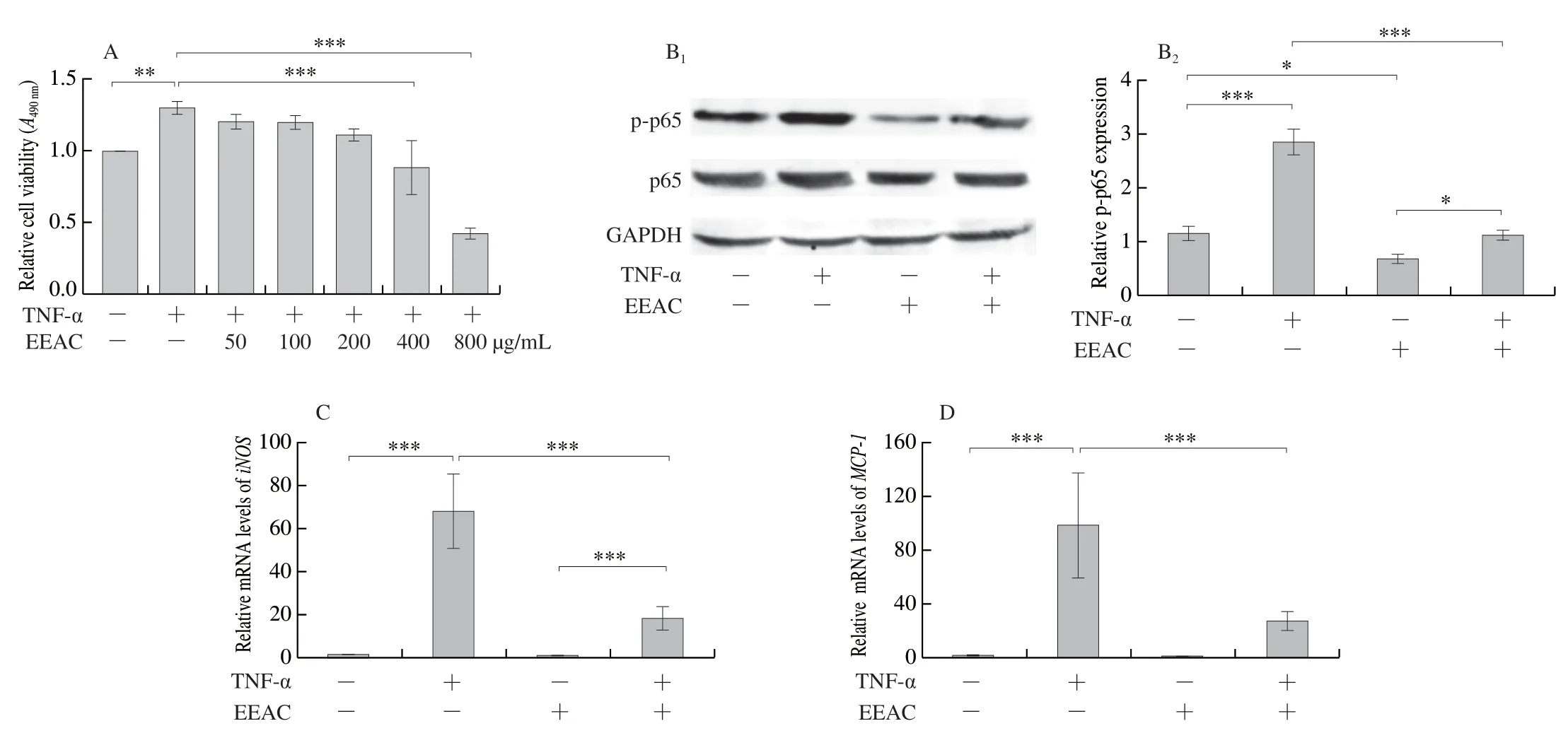
Fig.4 EEAC inhibits NF-κB activation and expression levels of pro-inflammatory molecules in macrophages stimulated by TNF-α.(A) MTT for the viability of RAW264.7 cells, cultured in medium containing 50, 100, 200, 400, 800 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 24 h (+) or remained untreated (-).(B) Western blot and densitometric analysis of expression of activated p65 in RAW264.7 cells, cultured in medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 30 min (+) or remained untreated (-).(C-F) RT-PCR of pro-inflammatory molecule mRNAs in RAW264.7 cells, cultured in medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for (+) or remained untreated (-).Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
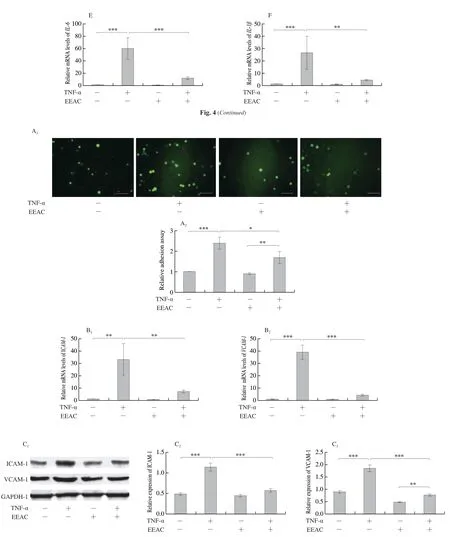
Fig.5 EEAC decreased the adherence of macrophages to VSMCs.(A) Representative images and quantification of calcein-AM-labeled RAW264.7 cell adhesion to VSMCs, cultured with serum-starved medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 24 h (+) or remained untreated (-).(B) RT-PCR assay of the mRNA levels of adhesion molecules in VSMCs, cultured with serum-starved medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 6 h (+) or remained untreated (-).(C) Western blot of the protein expression levels of adhesion molecules in VSMCs, cultured with serum-starved medium containing 400 μg/mL EEAC (+) or DMSO (-) for 24 h, and then stimulated by TNF-α for 24 h (+) or remained untreated (-).Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
3.4 EEAC inhibits macrophages adhesion to VSMCs through decreasing the levels of adhesion molecules released by VSMCs in vitro
The adhesion of monocytes/macrophages to VSMCs is an early event of vascular inflammation [4].We examined the effect of EEAC on TNF-α-induced the interaction between macrophages and VSMCs by anin vitroadhesion assay, in which calcein-AMlabeled RAW264.7 cells were allowed to adhere to a monolayer of VSMCs for 30 min, then the unadhered macrophages were cleared away by vigorously washing, and the adhered RAW264.7 cells were imaged and counted.Treatment with TNF-α for 24 h increased the basal adhesion of macrophages to VSMCs by more than two folds.Compared with TNF-α alone, EEAC pretreatment for 24 h caused a 25% reduction of cell adhesion (Fig.5A).
VCAM-1 and ICAM-1 have been well characterized on VSMCs as adhesion molecules that are induced in retention of monocytes and macrophages [5,28].Therefore, we tested whether EEAC affects their expression in VSMCs.As shown in Fig.5B, treatment with EEAC resulted in 70%-80% decrease in theVCAM-1andICAM-1mRNA levels relative to those of TNF-α alone.Moreover, a significant reduction of VCAM-1 and ICAM-1 protein level was also observed in EEAC-treated groups (Fig.5C).Taken together, the data showed that pretreatment of VSMCs with EEAC decreases the interaction between macrophages and VSMCs through inhibiting the expression levels of VCAM-1 and ICAM-1 secreted by VSMCs.
3.5 EEAC inhibits expression levels of adhesion molecules and inflammatory cell infiltration during neointimal hyperplasia
Next, the effect of EEAC on adhesion molecules expression and inflammatory cell infiltration were verifiedin vivo.Immunohistochemical staining showed that the expression levels of VCAM-1, ICAM-1, CD14 and CD68 (makers of monocyte and macrophage) were markedly decrease in neointimal VSMCs of arteries pretreated with EEAC (Fig.6).These results further suggest that EEAC exhibits an inhibitory effect on vascular inflammationin vivo, and may be an effective agent for prevention of restenosis after angioplasty.
4.Discussion
For decades, chronic inflammation has been recognized as a key feature of early stages during neointimal hyperplasia and vascular remodeling [29-31].Injured VSMCs actively recruit inflammatory cells by producing adhesion molecules [2,4].Thus, prevention of pathological vascular inflammation still remains as a major clinical challenge, which underscores the need for new therapeutic strategies.Herein, firstly, we demonstrate that EEAC suppresses intimal hyperplasia and pro-inflammatory cytokine production induced by carotid artery ligation.Moreover, EEAC attenuates TNF-αinduced pro-inflammatory cytokine expression in both VSMCs and macrophages.In thein vitromacrophage-VSMC interaction study, we found that pretreating VSMC with EEAC inhibits macrophages adhesion to VSMCs, which is associated with decreased expression of VCAM-1 and ICAM-1 in VSMCs.Finally,in vivodata verify that EEAC exerts its inhibitory effect on vascular inflammation by downregulating VCAM-1 and ICAM-1 expression and macrophage infiltration in the intimal hyperplasia site.
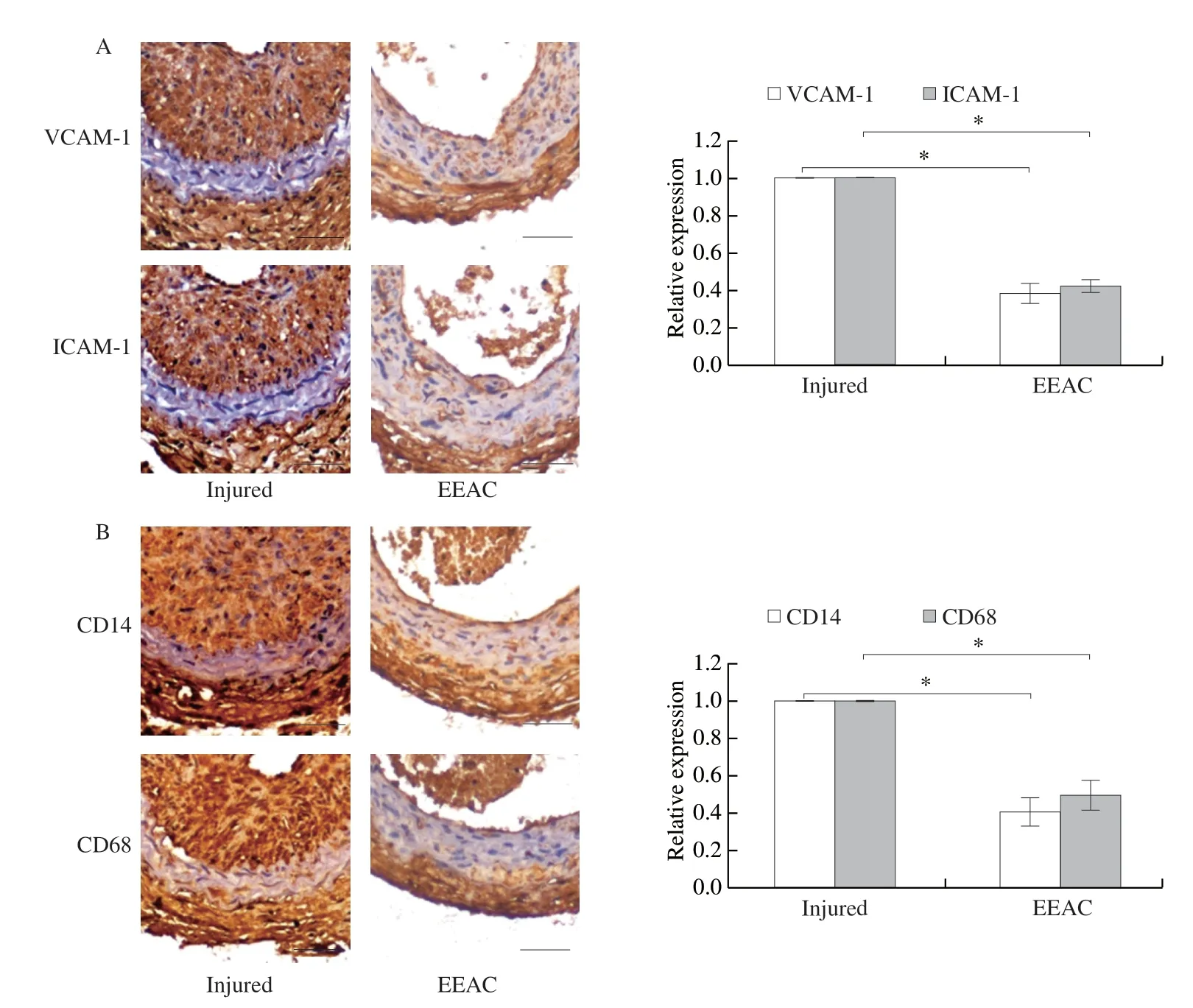
Fig.6 EEAC inhibits expression of adhesion molecules and inflammatory cell infiltration in carotid arteries.The sections of mouse carotid arteries were prepared on day 14 after ligation injury pretreated with EEAC (50 mg/kg·day) or not.Immunohistochemistry analysis for the expression of VCAM-1, ICAM-1 (A), CD14 and CD68 (B) (n = 8).Bar = 25 μm.Data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
The inflammatory responds, oxidative stress and abnormal proliferationare the common pathologic basis of cancer and vascular injury.A.cinnamomeahas been shown to harbor a broad spectrum of antitumor efficacy, such as liver, prostate, bladder, lung and breast through its antiflammatory and antioxidant activity [12,13,32,33].In addition, polysaccharides isolated fromA.cinnamomeainhibit angiogenesis in endothelial cells [34,35].Thus, we speculate thatA.cinnamomeamay play a protective role in vascular remodeling.In thein vivostudy, we found that EEAC suppresses ligation-induced intimal hyperplasia and expression of inflammatory cytokines in carotid arteries, which had not been shown before.
VSMCs are the major cell type in blood vessels.Unlike many other mature cell types in the adult body, VSMCs do not terminally differentiate but retain a remarkable plasticity.VSMCs in the normal arterial media express a range of SMC markers, such as smooth muscle cell myosin heavy chain (MYH11), SM22α, ACTA2, and smoothelin, while VSMCs in culture and in atherosclerosis reduce the expression of these markers, at leastin vitro, acquire increased capacity for cell proliferation, migration, and secretion of various extracellular matrix (ECM) proteins and cytokines [36,37].Although activated ECs mediate immune cell adhesion to the vascular wall and monocyte infiltration to the intima where they initiate inflammation, VSMCs are the soil of vascular inflammation, inflammatory amplifiers and tissue.Evidencein vitropresented here proved that inhibition of expression of inflammatory cytokines by EEAC was replicated to a large extent in cultured aortic SMCs, as well as in a monocyte/macrophage cell line RAW264.7.Of note, inhibition of inflammatory cytokines, such as MCP-1 and IL-6, was more profound in macrophages than in VSMCs, meaning that NF-κB pathway plays a more dominant regulatory role in inflammatory cytokines transcription in monocytes/macrophages than in VSMCs, because EEAC produced a similar reduction in phosphorylation of p65 in these two cell types, which needs to be addressed in future studies.
Aside from the reported colocalization of VSMCs and monocytes observed in the intima [5,8], like endothelial cells, VSMCs can also express multiple adhesion molecules such as VCAM-1 and ICAM-1 to recruit monocytes and macrophages adhesion and migration into the vessel wall [38,39], furtherly suggesting the important role of VSMCs in retaining macrophages within the lesion.The present study demonstrated that EEAC decreased the interaction between VSMCs and RAW264.7in vitro, which may be associated with the downregulation of VCAM-1 and ICAM-1 in VSMCs.Moreover, immunohistochemistry analyses revealed that EEAC prevented the increased expression of VCAM-1, ICAM-1, CD14 and CD68 in the damaged arteries, meaning decreased infiltration of monocytes/macrophages.These results substantiate thein vitrofindings and strongly support the notion thatA.cinnamomeaconfers protection against injury-induced pathological vascular remodeling.
5.Conclusion
In summary,A.cinnamomeaprevents neointimal hyperplasia and vascular inflammation after ligation injury, and exhibits an antiinflammatory effect on VSMCs and monocyte/macrophages upon TNF-α stimulation.This process is associated with the downregulated expression of VCAM-1 and ICAM-1 in VSMCs and the resultant decreased macrophage adhesion to VSMCsin vitroandin vivo.
Conflict of Interest
None.
Acknowledgments
This work was supported by the National Key Research Project of China (2019YFC1606400), Major Public Welfare Projects in Henan Province (201300110200), National Key Research Project of Hebei Province (20375502D), Natural Science Foundation of Hebei Province (H2019206212), High-level Talent Funding Project of Hebei Province (A201905006), Fund of National R & D Center for Edible Fungus Processing Technology, Henan University (20200109), and the Open Fund from Beijing Advanced Innovation Center for Food Nutrition and Human Health (20182025).
杂志排行
食品科学与人类健康(英文)的其它文章
- Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia
- Antioxidant and hepatoprotective effects of Hypsizygus ulmarius polysaccharide on alcoholic liver injury in rats
- Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo
- The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense
- The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice
- Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice
