胆酸二聚体化合物的设计、合成及抑制肝细胞凋亡研究
2020-08-14牛伟萍牛伟晓张国宁朱梅何红伟王菊仙
牛伟萍,牛伟晓,张国宁,朱梅,何红伟,王菊仙
·论著·
胆酸二聚体化合物的设计、合成及抑制肝细胞凋亡研究
牛伟萍,牛伟晓,张国宁,朱梅,何红伟,王菊仙
100050 北京,中国医学科学院北京协和医学院医药生物技术研究所合成室
通过设计合成胆酸二聚体化合物及评价其体外抑制肝细胞凋亡活性,发现新型治疗慢性肝病的候选化合物。
以熊去氧胆酸、鹅去氧胆酸和奥贝胆酸为起始原料,经亲电取代、脱水缩合、氢化等反应制备目标化合物。通过测定半胱氨酸天冬氨酸蛋白酶 3、7的活性水平评价目标化合物体外抑制肝细胞凋亡的活性。
合成胆酸二聚体化合物 24 个,体外活性结果表明部分目标化合物具有较好的抑制肝细胞凋亡活性,12 个化合物(1a、1e、1h、2a、2c、2e、2f、2g、3a、3c、3d、5a)在 20 μmol/L 浓度下抑制肝细胞凋亡的活性优于阳性对照物牛磺熊去氧胆酸(40.94%)。化合物1a、1e、2a和5a的抑制率分别为 75.60%、88.56%、83.25% 和 105.24%,是阳性对照的 2 倍。
熊去氧胆酸和鹅去氧胆酸二聚体具有抑制肝细胞凋亡的活性,奥贝胆酸二聚体无抑制肝细胞凋亡的活性;连接胆酸骨架的连接链长度对活性有明显的影响,其中具有较短连接链(N,N'-二甲基乙二胺、四甲基乙二胺、乙二醇二甲醚)的化合物(1a、1e、2a)的活性优于具有较长连接链(N,N'-二甲基-1,8-辛二胺、2,5,8,11,14,17-六氧十八烷)的化合物(1g、3e)的活性。
合成; 抑制肝细胞凋亡; 熊去氧胆酸; 鹅去氧胆酸; 奥贝胆酸
慢性肝病是多种复杂疾病的总称,包括非酒精性脂肪肝(NAFLD)/非酒精性脂肪性肝炎(NASH)、肝纤维化和肝硬化等,这些慢性肝病的共同特征是肝细胞的凋亡和(或)坏死的激活[1-3]。尽管肝细胞具有强大的再生能力[4-8],然而,当细胞凋亡或坏死程度超过一定的阈值时,肝脏的再生能力不足以抵消肝细胞的大量凋亡,肝细胞被纤维化的瘢痕取代,肝功能受到损害,从而逐渐导致肝纤维化、肝硬化甚至是肝功能衰竭等病情严重、治愈率低的慢性肝病[9]。因此,通过抑制肝细胞的凋亡,减轻肝损伤,可以起到保护肝脏,防治慢性肝病的效果。
胆汁酸是一类具有特殊理化和生物学特性的酸性类固醇分子[10],如熊去氧胆酸(UDCA)、鹅去氧胆酸(CDCA)和 CDCA 的衍生物奥贝胆酸(OCA)等,大部分胆汁酸及其衍生物可抑制肝细胞凋亡,保护肝细胞[10-13]。本课题组在前期工作中,设计合成了 UDCA 与生物素相连生物探针分子,活性结果显示此生物探针具有抑制肝细胞凋亡的作用。结合文献调研结果[14-15],推测胆酸二聚体也可能会具有抑制肝细胞凋亡活性。基于此,我们设计合成了化合物1d及2a,对其进行了初步的活性测定。活性结果显示,化合物2a显示出较好的抑制肝细胞凋亡活性,在 25 μmol/L 浓度下可以显著抑制由三氯乙酸(TCA)诱导的小鼠肝细胞炎症趋化因子水平的上升,在 10 μmol/L 浓度下即可以显著抑制由甘氨鹅去氧胆酸(GCDCA,鹅去氧胆酸的甘氨酸偶联形式)诱导的原代人肝细胞的凋亡。
通过头对头策略合成的胆汁酸二聚体已被证实具有抗革兰氏阳性菌、抗真菌和抗恶性细胞增殖的活性[16-18],而胆汁酸二聚体对抑制肝细胞凋亡方面的研究尚未见报道。综上所述,此文以 UDCA、CDCA 和OCA为先导化合物设计合成了一系列胆汁酸二聚体类化合物,并评价了其抑制肝细胞凋亡的活性,以期获得具有更强抑制肝细胞凋亡作用的化合物,为慢性肝病药物的研发提供思路。
1 材料与方法
1.1 材料
1.1.1 主要仪器 400 MHz、500 MHz 和 600 MHz核磁共振仪购自德国Bruker 公司;MP-70 熔点仪购自瑞士梅特勒公司,未校正;1100 型四级杆液质联用仪购自美国Agilent 公司;自检式 Ultima-TOF 质谱仪购自美国赛默飞科技公司。
1.1.2 主要试剂 UDCA、CDCA、OCA、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDCI)、N-羟基琥珀酰亚胺等试剂均为市售分析纯或化学纯。
1.1.3 实验动物 10 周龄雄性 Balb/C 小鼠,体重 20 ~ 30 g,购自北京维通利华实验动物中心。维持在 12 h 的昼夜节律下,自由摄取标准饮食和水直至实验当天。
1.2 方法
1.2.1 目标化合物的合成 化合物1a~1h的合成如图1 所示。以 UDCA 为起始原料,在无水 N,N-二甲基甲酰胺(DMF)中,以EDCI活化羧酸以获得活性酯中间体1-1,中间体1-1通过与不同的连接链缩合反应得到目标化合物1a~1h[17]。
化合物2a~2g、3a~3e和4a~4b的合成路线如图2 所示。以化合物2a为例,采用 UDCA 为原料,以 3,4-二氢-2H-吡喃保护羟基,得到中间体2-1,在 EDCI/1-羟基苯并三唑(HOBT)/1,8-二氮杂二环十一碳-7-烯(DBU)介导下与连接链 2a 缩合反应获得中间体2a-2,随后在对甲苯磺酸吡啶鎓盐的催化下除去保护基得到目标化合物2a[19]。化合物2b~2g、3a~3e和4a~4b均使用与上述相同的方法合成。
化合物5a~5b的合成路线如图3 所示。分别将 UDCA、CDCA 和OCA 与三氟甲磺酸叔丁基二甲基硅烷酯反应得到中间体5-1、6-1和7-1。在 EDCI/HOBT/DBU 介导下,中间体5-1与等摩尔比的 2-[2-(苄氧基)乙氧基]乙醇缩合得到中间体5-2。5-2在 Pd/C 催化下氢解,除去苄基保护基,得到中间体5-3。5-3在 EDCI/HOBt/DBU 介导下,分别与中间体6-1或7-1缩合,分别得到5a-4和5b-4,以氟化氢吡啶除去叔丁基二甲基硅烷保护基得到目标化合物5a~5b。
1.2.2 抑制肝细胞凋亡活性测试 以(GCDCA)诱导小鼠的原代肝细胞凋亡[13-15, 20-21],通过测定半胱氨酸天冬氨酸蛋白酶 3/7(caspase 3/7)的活性水平评价目标化合物体外抑制肝细胞凋亡的活性。
通过胶原酶灌注技术分离肝细胞。将新鲜分离的肝细胞以 1 × 106个/ml 的密度悬浮在 WilliamE培养基的混合物中(WilliamE 培养基中添加了10% FBS、100 IU/ml 青霉素 G 钠和 100 IU/ml硫酸链霉素)。将肝细胞在 37.0 ℃、5% CO2的培养箱中培养。细胞接种后 4 h 更换培养基,以尽量减少死细胞的污染。将分离的肝细胞在不同的 William E 培养基中孵育 4 h。William E 培养基分为 4 组,分别为加入 GCDCA(200 μmol/L)组、空白对照组(不加任何化合物)、实验组(加入目标化合物1a~5b 20 μmol/L和 GCDCA 200 μmol/L的混合物)及阳性对照组(分别加入 UDCA、CDCA、TUDCA 或 OCA 20 μmol/L和 GCDCA 200 μmol/L的混合物)。
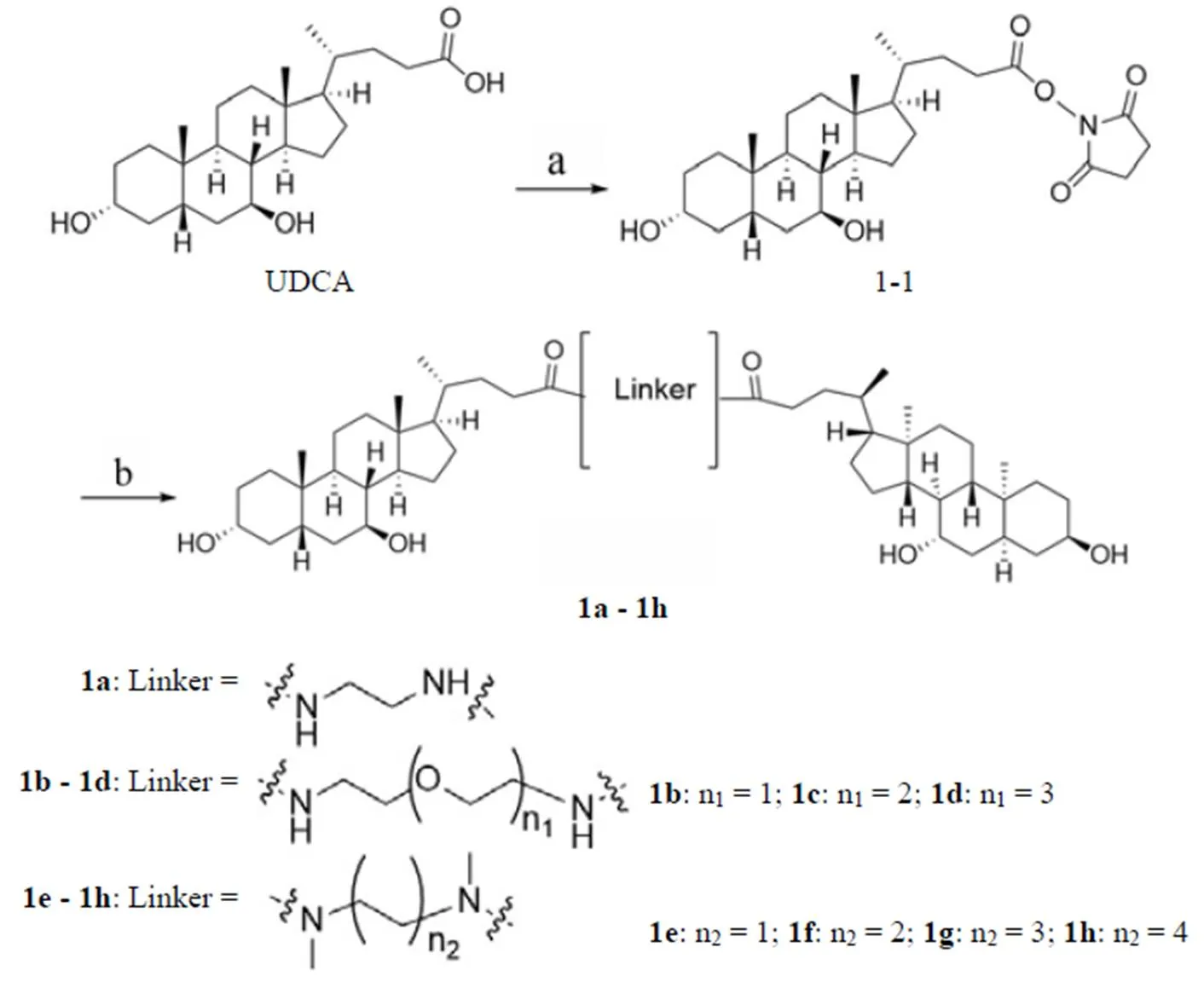
a:1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐、N-羟基琥珀酰亚胺,无水 N,N-二甲基甲酰胺,氩气保护,35℃,12 h;b:连接链 1a ~ 1h,无水 DMF,氩气保护,35 ℃,3 h
a: N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide, anhydrous N,N-Dimethylformamide, Ar, 35 ℃, 12 h; b: linker 1a - 1h, anhydrous DMF, Ar, 35 ℃, 3 h
图1 化合物1a~1h的合成路线
Figure 1 Syntheses of compounds 1a - 1h

a:3-4-二氢-2H-吡喃,对甲苯磺酸一水合物,1-4-二氧六环,rt,3 h,60%;b:1-羟基苯并三唑,1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐,1,8-二氮杂二环十一碳-7-烯,无水 DMF,30 ℃,12 h;c:对甲苯磺酸吡啶鎓盐,甲醇,60 ℃,12 h
a: 3,4-Dihydro-2H-pyran, p-Toluenesulfonic acid monohydrate, 1,4-Dioxane, rt, 3 h, 60%; b: 1-Hydroxybenzotriazole (HOBT), EDCI, 1,8-Diazabicyclo [5.4.0]undec-7-ene, anhydrous DMF, 30 ℃, 12 h; c: Pyridinium p-toluenesulfonate, methanol, 60 ℃, 12 h
图 2 化合物2a~4b的合成路线
Figure 2 Syntheses of compounds 2a - 4b
1.2.3 Caspase3/7 活性测定 根据 caspase3/7 分析试剂盒说明书测量 caspase3/7 活性。
抑制率(%)=[1 –(化合物 caspase – 空白组caspase)/(GCDCA 组 caspase – 空白组 caspase)] × 100%。
2 结果
2.1 目标化合物的合成
共合成 24 个胆酸类二聚体,化合物结构均经1H-NMR、13C-NMR和 MS 确证。部分代表性目标化合物的1H-NMR、13C-NMR 和 MS 数据如下。
2.1.1 (4R,4'R)-N,N'-(乙烷-1,2-二基)双(4-((3R,5S, 7S,8R,9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酰胺)(1a) 白色固体,收率 61%;mp:149 ~ 150 ℃;1H-NMR(600 MHz,CD3OD-4)3.53-3.44(m,4H),3.27(s,4H),2.28-2.20(m,2H),2.12-2.00(m,4H),1.95-1.87(m,4H),1.86-1.75(m,6H),1.66-1.00(m,38H),0.99-0.95(m,12H),0.72(s,6H)。13C-NMR(151 MHz,CD3OD-4)δ 177.19,72.13,71.96,57.55,56.56,44.82,44.51,44.05,41.61,40.73,40.09,38.63,38.03,36.94,36.10,35.19,34.24,33.35,31.06,29.73,27.99,23.95,22.40,19.06,12.69。HRMS-ESI():831.6274(C50H84N2O6Na+;[M+Na]+,Calc. 831.6227)。
2.1.2 (4R,4'R)-N,N'-(氧双(乙烷-2,1-二基))双(4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酰胺)(1b) 白色固体,收率 65%;mp:143 ~ 144 ℃;1H-NMR(600 MHz,CD3OD-4)3.53-3.45(m,8H),3.36-3.33(m,4H),2.30-2.23(m,2H),2.17-2.09(m,2H),2.08-2.02(m,2H),1.95-1.76(m,10H),1.67-1.39(m,21H),1.38-1.00(m,15H),0.98(s,4H),0.72(s,6H)。13C-NMR(151 MHz,CD3OD-4)176.92,72.12,71.93,70.52,57.56,56.58,44.83,44.51,44.05,41.63,40.75,40.31,38.63,38.05,36.93,36.11,35.19,34.16,33.36,31.08,29.75,27.99,23.97,22.42,19.12,12.72。HRMS-ESI():875.6452(C52H88N2O7Na+;[M+Na]+,Calc. 875.6490)。
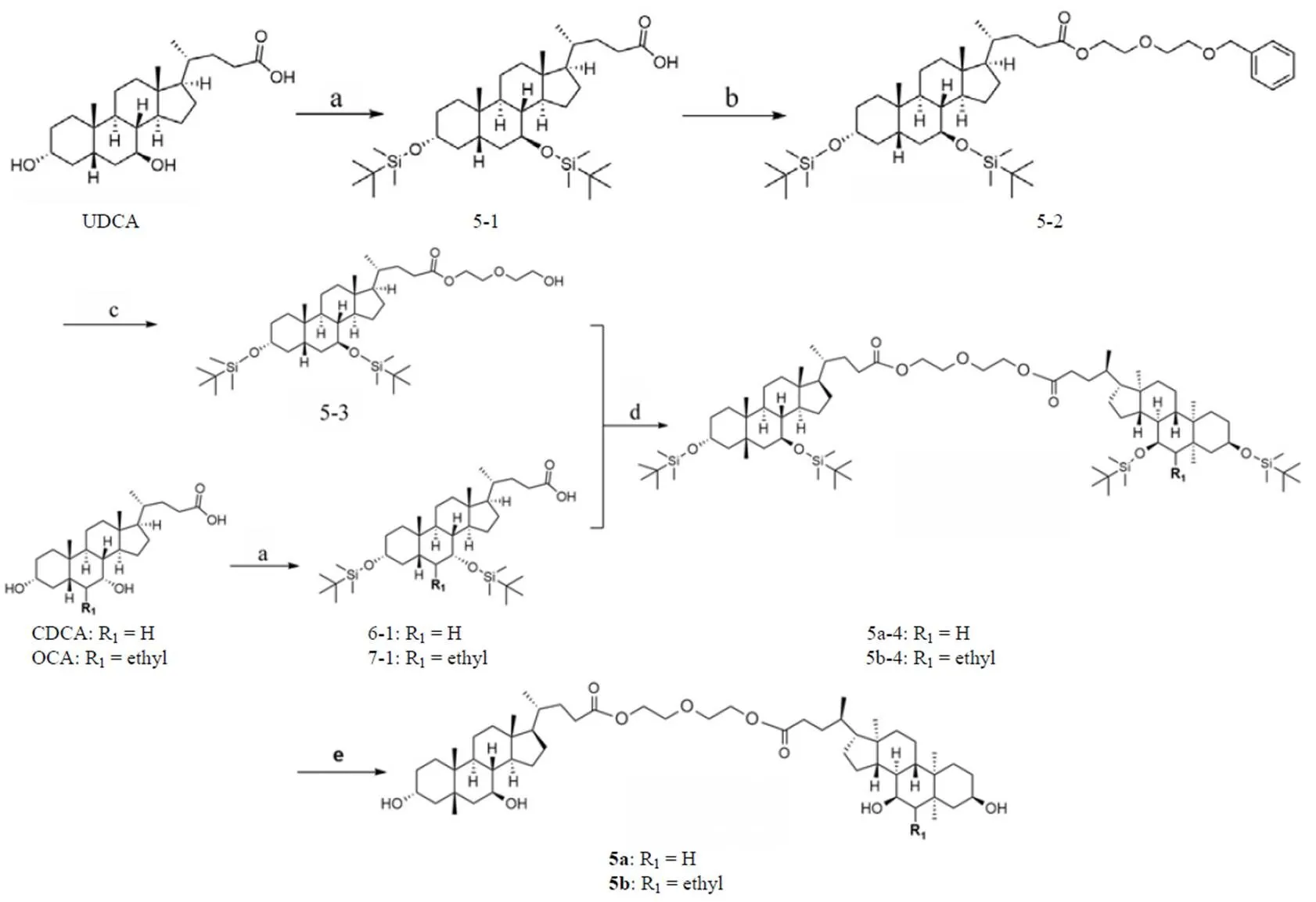
a:三氟甲磺酸叔丁基二甲基硅烷酯,N,N-二异丙基乙胺,无水二氯甲烷,室温,2 h,40%;b:HOBT,EDCI,DBU,2-[2-(苄氧基)乙氧基]乙醇,无水 DMF,35 ℃,12 h,51%;c:Pd/C,H2,乙醇,77%;d:6-1 或 7-1,HOBT,EDCI,DBU,DMF;e:氟化氢吡啶盐,四氢呋喃,室温,2 h
a: Trifluoromethanesulfonic acid tert-butyldimethylsilyl ester, N,N-Diisopropylethylamine, anhydrous DCM, rt, 2h, 40%; b: HOBT, EDCI, DBU, 2-[2-(Benzyloxy)ethoxy]ethanol, anhydrous DMF, 35 ℃, 12 h, 51%; c: Pd/C, H2, ethanol, 77%; d: 6-1 or 7-1, HOBT, EDCI, DBU, DMF; e: Pyridine hydrofluoride, Tetrahydrofuran, rt, 2 h
图 3 化合物5a~ 5b的合成路线
Figure 3 Syntheses of compounds 5a - 5b
2.1.3 乙烷-1,2-二基(4R,4'R)-双(4-((3R,5S,7S,8R, 9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酸酯)(2a) 白色固体,收率61%;mp:99 ~ 100 ℃;1H-NMR(600 MHz,Chloroform-)4.27(s,4H),3.77-3.38(m,5H),2.42-2.33(m,2H),2.30-2.20(m,2H),2.06-1.95(m,3H),1.95-0.98(m,60H),0.94(d,= 9.3 Hz,13H),0.68(s,6H)。13C-NMR(151 MHz,Chloroform-)174.11,71.58,71.47,62.18,55.95,55.07,43.92,43.89,42.59,40.31,39.38,37.46,37.05,35.48,35.08,34.22,31.37,31.12,30.52,28.76,27.08,23.54,21.33,18.55,12.30。HRMS-ESI():833.5912(C50H82O8Na+;[M+Na]+,Calc. 833.5908)。
2.1.4 氧双(乙烷-2,1-二基)(4R,4'R)-双(4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酸酯)(2b) 白色固体,收率53%;mp:73 ~ 75 ℃;1H-NMR(600 MHz,Chloroform-)4.25-4.18(m,4H),3.71-3.65(m,4H),3.61-3.53(m,4H),2.42-2.34(m,2H),2.29-2.21(m,2H),2.11(s,4H),1.99(d,= 12.5 Hz,2H),1.93-1.85(m,2H),1.85-1.74(m,8H),1.69-1.62(m,4H),1.61-1.53(m,4H),1.52-1.19(m,25H),1.17-0.97(m,8H),0.94-0.91(m,12H),0.67(s,6H)。13C-NMR(151 MHz,Chloroform-)174.32,71.53,71.43,69.24,63.44,55.93,55.07,43.89,43.85,42.59,40.30,39.38,37.43,37.04,35.42,35.07,34.20,31.70,31.31,31.08,30.44,28.76,27.04,23.53,22.76,21.32,18.55,14.23,12.28。HRMS-ESI():877.6203(C52H86O9Na+;[M+Na]+,Calc. 877.6170)。
2.1.5 乙烷-1,2-二基(4R,4'R)-双(4-((3R,7R,8R,9S, 10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六烷基-1H-环戊[a]菲基-17-基)戊酸酯)(3a) 白色固体,收率52%;mp:103 ~ 105 ℃;1H-NMR(400 MHz,Chloroform-)4.32(s,3H),3.90(s,2H),3.61-3.43(m,2H),2.49-2.37(m,2H),2.34-2.19(m,4H),2.11-1.01(m,22H),1.01-0.88(m,11H),0.71(s,6H)。13C-NMR(101 MHz,Chloroform-)174.01,72.08,68.57,62.08,55.79,50.45,42.72,41.50,39.88,39.66,39.43,35.39,35.37,35.07,34.62,32.89,31.17,31.01,30.65,28.21,23.79,22.82,20.62,18.30,11.81。HRMS-ESI():833.5909(C50H82O8Na+;[M+Na]+,Calc. 833.5908)。
2.1.6 氧双(乙烷-2,1-二基)(4R,4'R)-双(4-((3R,7R, 8R,9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六烷基-1H-环戊[a]菲基-17-基)戊酸酯)(3b) 白色固体,收率44%;mp:94 ~ 95 ℃;1H-NMR(400 MHz,Chloroform-)4.27(t,= 4.6 Hz,4H),3.89(s,2H),3.78-3.70(m,4H),3.56-3.45(m,2H),2.49-2.37(m,2H),2.35-2.17(m,4H),2.10-1.08(m,37H),1.08-0.99(m,1H),0.99-0.88(m,12H),0.70(s,6H)。13C-NMR(101 MHz,Chloroform-)174.24,72.02,69.16,68.51,63.35,55.81,50.46,42.70,41.51,39.86,39.67,39.44,35.37,35.07,34.64,32.86,31.13,30.95,30.67,28.21,23.74,22.82,20.62,18.32,11.80。HRMS-ESI():877.6232(C52H86O9Na+;[M+Na]+;Calc. 877.6170)。
2.1.7 乙烷-1,2-二基(4R,4'R)-双(4-((3R,7R,8S,9S, 10S,13R,14S,17R)-6-乙基-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酸酯)(4a) 白色油状物,产率 35%;1H-NMR(600 MHz,Chloroform-)3.71-3.68(m,2H),3.65(s,6H),2.38-2.31(m,2H),2.25-2.18(m,2H),1.95(m,2H),1.93-1.86(m,2H),1.85-1.73(m,10H),1.71-1.55(m,4H),1.54-1.08(m,25H),0.99(td,= 14.3,3.5 Hz,2H),0.94-0.86(m,18H),0.65(s,6H)。13C-NMR(101 MHz,Chloroform-)174.79,72.36,70.92,55.77,51.52,50.54,45.22,42.77,41.20,40.05,39.62,35.56,35.53,35.39,34.02,33.26,31.03,30.66,28.19,23.73,23.18,22.26,20.77,18.29,11.81,11.69。HRMS-ESI():867.6741(C54H91O8+;[M+H]+,Calc. 867.6715)。
2.1.8 2-(2-(((R)-4-((3R,5S,7R,8R,9S,10S,13R,14S, 17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酰基)氧基)乙氧基)乙基(R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-二羟基-10,13-二甲基十六氢-1H-环戊[a]菲基-17-基)戊酸酯(5a) 白色固体,收率61%;mp:78 ~ 79 ℃;1H-NMR(600 MHz,Chloroform-)4.26-4.19(m,4H),3.87-3.82(m,1H),3.69(t,= 4.8 Hz,4H),3.63-3.54(m,2H),3.50-3.42(m,1H),2.43-2.34(m,2H),2.30-2.16(m,3H),2.03-0.96(m,29H),0.95-0.90(m,12H),0.67(d,= 10.7 Hz,6H)。13C-NMR(101 MHz,Chloroform-)174.25,174.22,72.03,71.45,71.37,69.15,68.51,65.60,63.34,55.81,55.75,54.90,50.48,43.77,42.71,42.46,41.50,40.16,39.88,39.67,39.44,39.21,37.31,36.89,35.38,35.35,35.29,35.07,34.96,34.63,34.10,32.86,31.15,31.14,30.96,30.94,30.67,30.59,30.34,28.66,28.20,26.92,23.74,23.42,22.80,21.20,20.60,19.21,18.42,18.32,13.76,12.17,11.81。HRMS-ESI():853.6152(C52H85O9-;[M-H]-,Calc. 853.6193)。
2.2 体外抑制肝细胞凋亡活性结果
目标化合物体外抑制肝细胞凋亡活性的结果见表 1,部分目标化合物显示较好的抑制肝细胞凋亡活性,12 个化合物(1a、1e、1h、2a、2c、2e、2f、2g、3a、3c、3d、5a)在 20 μmol/L浓度下抑制肝细胞凋亡的活性高于阳性对照物牛磺熊去氧胆酸(40.94%),化合物1a、1e、2a和5a的抑制率分别为 75.60%、88.56%、83.25% 和 105.24%。
表 1 化合物1a~5b的化学结构、物理性质及抑制肝细胞凋亡活性
Table 1 Structures, physical data and inhibitory activity against GCDCA-induced hepatocyte apoptosis of compounds 1a - 5b
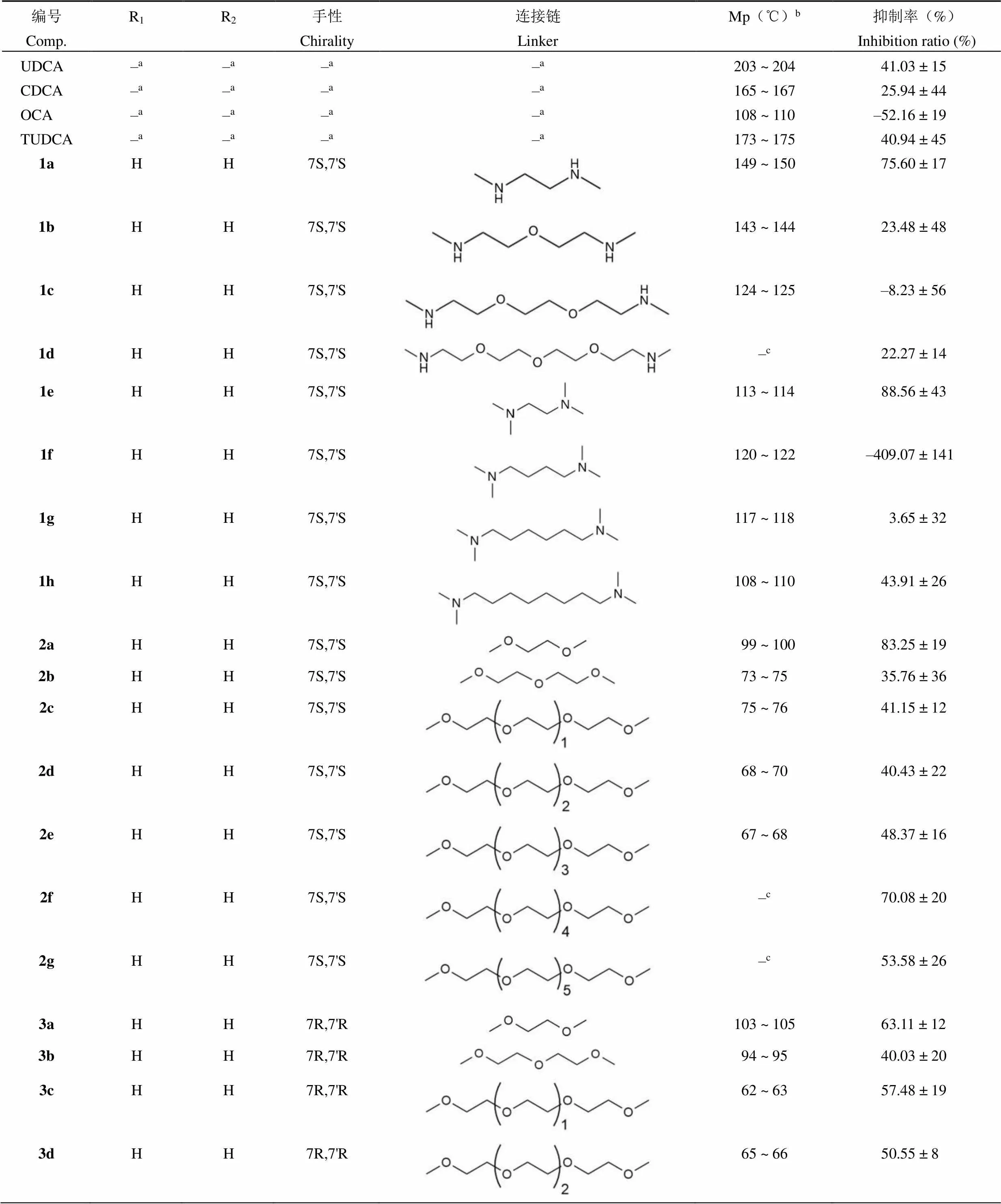
编号Comp.R1R2手性Chirality连接链LinkerMp(℃)b抑制率(%)Inhibition ratio (%) UDCA–a–a–a–a203 ~ 20441.03 ± 15 CDCA–a–a–a–a165 ~ 16725.94 ± 44 OCA–a–a–a–a108 ~ 110–52.16 ± 19 TUDCA–a–a–a–a173 ~ 17540.94 ± 45 1aHH7S,7'S149 ~ 15075.60 ± 17 1bHH7S,7'S143 ~ 14423.48 ± 48 1cHH7S,7'S124 ~ 125–8.23 ± 56 1dHH7S,7'S–c22.27 ± 14 1eHH7S,7'S113 ~ 11488.56 ± 43 1fHH7S,7'S120 ~ 122–409.07 ± 141 1gHH7S,7'S117 ~ 1183.65 ± 32 1hHH7S,7'S108 ~ 11043.91 ± 26 2aHH7S,7'S99 ~ 10083.25 ± 19 2bHH7S,7'S73 ~ 7535.76 ± 36 2cHH7S,7'S75 ~ 7641.15 ± 12 2dHH7S,7'S68 ~ 7040.43 ± 22 2eHH7S,7'S67 ~ 6848.37 ± 16 2fHH7S,7'S–c70.08 ± 20 2gHH7S,7'S–c53.58 ± 26 3aHH7R,7'R103 ~ 10563.11 ± 12 3bHH7R,7'R94 ~ 9540.03 ± 20 3cHH7R,7'R62 ~ 6357.48 ± 19 3dHH7R,7'R65 ~ 6650.55 ± 8
续表 1
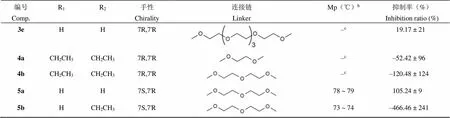
编号Comp.R1R2手性Chirality连接链LinkerMp(℃)b抑制率(%)Inhibition ratio (%) 3eHH7R,7'R–c19.17 ± 21 4aCH2CH3CH2CH37R,7'R–c–52.42 ± 96 4bCH2CH3CH2CH37R,7'R–c–120.48 ± 124 5aHH7S,7'R78 ~ 79105.24 ± 9 5bHCH2CH37S,7'R73 ~ 74–466.46 ± 241
注:a不适用;b熔点仪未经校正;c油状物。
Notes:aNot applicable;bMelting point apparatus uncorrected;cOily substance.
3 讨论
目标化合物体外抑制肝细胞凋亡活性结果表明,胆汁酸二聚体的胆汁酸骨架和连接链都对其抑制肝细胞凋亡的活性产生影响。UDCA 二聚体(1a~2g)和 CDCA 二聚体(3a~3e)显示出抑制肝细胞凋亡的活性,而 OCA 及其二聚体(4a,4b)均不显示抑制肝细胞凋亡的活性。美国食品药品监督管理局(FDA)最近发布的安全警告指出,OCA 可能与增加的肝损伤和死亡风险相关[22],实验结果证实了这一声明。
在 UDCA 二聚体中,连接链的种类对活性的相对贡献如下:N,N,N',N'-四甲基乙二胺(1e,88.56%)> 1,2-二甲氧基乙烷(2a,83.25%)> N,N'-二甲基-1,2-乙二胺(1a,75.60%)> 二甘醇二甲醚,三甘醇二甲醚等(2b-2e)。CDCA 二聚体也显示出类似情况,连接链对活性的相对贡献如下:1,2-二甲氧基乙烷(3a,63.11%)>二甘醇二甲醚(3b,40.03%)> 2,5,8,11,14,17-六氧十八烷(3e,19.17%)。化合物抑制肝细胞凋亡的活性随连接链长度的增加而降低,这表明具有较短连接链的化合物可能比具有较长连接链的化合物具有更明显的抑制肝细胞凋亡的活性。本文推测产生这种活性规律的原因是长链柔性连接链可能导致胆酸片段的疏水面互相重叠形成夹层,从而影响化合物与靶点的结合。综上所述,该研究结果表明部分胆汁酸二聚体具有抑制肝细胞凋亡活性,值得进一步深入研究,从而为慢性肝病药物的研发提供可行的方案。
[1] Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol, 2013, 3(2):567-598.
[2] Campana L, Iredale JP. Regression of liver fibrosis. Semin Liver Dis, 2017, 37(1):1-10.
[3] Guicciardi ME, Malhi H, Mott JL, et al. Apoptosis and necrosis in the liver. Compr Physiol, 2013, 3(2):977-1010.
[4] Huang J, Rudnick DA. Elucidating the metabolic regulation of liver regeneration. Am J Pathol, 2014, 184(2):309-321.
[5] Fan M, Wang X, Xu G, et al. Bile acid signaling and liver regeneration. Biochim Biophys Acta, 2015, 1849(2):196-200.
[6] Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology, 2006, 43(2 Suppl 1):S45-S53.
[7] Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol, 2004, 5(10):836-847.
[8] Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol, 2010, 176(1): 2-13.
[9] Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology, 2006, 43(2 Suppl 1):S31-S44.
[10] Amaral JD, Viana RJ, Ramalho RM, et al. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res, 2009, 50(9):1721- 1734.
[11] Castro RE, Solá S, Steer CJ, et al. Bile acids as modulators of apoptosis.//Sahu SC. Hepatotoxicity: from genomics to in vitro and in vivo models. New York: John Wiley & Sons, Ltd, 2008:391-419.
[12] Solá S, Castro RE, Kren BT, et al. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry, 2004, 43(26):8429-8438.
[13] Rust C, Karnitz LM, Paya CV, et al. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem, 2000, 275(26):20210-20216.
[14] Takikawa Y, Miyoshi H, Rust C, et al. The bile acid-activated phosphatidylinositol 3-kinase pathway inhibits Fas apoptosis upstream of bid in rodent hepatocytes. Gastroenterology, 2001, 120(7):1810- 1817.
[15] Schoemaker MN, Conde de La Rosa L, Buist-Homan M, et al. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology, 2004, 39(6):1563-1573.
[16] Pandey PS, Rai R, Singh RB. Synthesis of cholic acid-based molecular receptors: head-to-head cholaphanes. J Chem Soc Perkin Trans 1, 2002, 1(7):918-923.
[17] Singla P, Dalal P, Kaur M, et al. Bile acid oligomers and their combination with antibiotics to combat bacterial infections. J Med Chem, 2018, 61(22):10265-10275.
[18] Salunke DB, Hazra BG, Pore VS, et al. New steroidal dimers with antifungal and antiproliferative activity. J Med Chem, 2004, 47(6): 1591-1594.
[19] Miyashita M, Yoshikoshi A, Grieco PA. Pyridinium p-toluenesulfonate. A mild and efficient catalyst for the tetrahydropyranylation of alcohols. J Org Chem, 1977, 42(23):3772-3774.
[20] Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis, 2002, 34(6):387-392.
[21] Faubion WA, Guicciardi ME, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest, 1999, 103(1):137-145.
[22] Jiang Z, Liu X, Yuan Z, et al. Discovery of a novel selective dual peroxisome proliferator-activated receptor α/δ agonist for the treatment of primary biliary cirrhosis. ACS Med Chem Lett, 2019, 10(7):1068-1073.
Design, synthesis and efficacy of bile acid oligomers in inhibiting glycochenodeoxycholic acid-induced hepatocyte apoptosis
NIU Wei-ping, NIU Wei-xiao, ZHANG Guo-ning, ZHU Mei, HE Hong-wei, WANG Ju-xian
Organic Synthesis Chamber, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100050, China
To design, synthesize and evaluate the efficacy of bile acid oligomers in inhibiting glycochenodeoxycholic acid-induced hepatocyte apoptosisto provide candidate compounds for the development of drugs for chronic liver disease.
Oligomers of bile acids (ursodeoxycholic acid, chenodeoxycholic acid, and obeticholic acid) were synthesized by electrophilic substitution, condensation reaction, hydrogenation, etc. The inhibitory effects of target compounds were evaluated on glycochenodeoxycholic acid-induced hepatocyte apoptosis by measuring caspase3/7 level.
Most of the synthesized bile acid oligomers displayed inhibitory activity against hepatocyte apoptosis. Among synthesized compounds, 12 target compounds (1a, 1e, 1h, 2a, 2c, 2e, 2f, 2g, 3a, 3c, 3d, 5a) were more potent than positive control tauroursodeoxycholic (40.94%). Especially, compounds 1a, 1e, 2a, and 5a exhibited substantial antiapoptotic activity, with inhibition rates of 75.60%, 88.56%, 83.25% and 105.24%, respectively.
Ursodeoxycholic acid oligomers and chenodeoxycholic acid oligomers, instead of obeticholic acid oligomers, exhibit potent inhibitory effect. Compounds with shorter linker (1a, 1e, 2a) exhibit more obvious activity than that of compounds with longer linker (1g, 3e).
Synthetic; Hepatocyte apoptosis; Ursodeoxycholic acid; Chenodeoxycholic acid; Obeticholic acid
WANG Ju-xian, Email: imbjxwang@163.com
国家自然科学基金(81703366、81673497);中国医学科学院医学科学创新基金(2016-I2M-3-014);“重大新药创制”国家科技重大专项(2019ZX09201001-003)
王菊仙:imbjxwang@163.com
10.3969/j.issn.1673-713X.2020.04.007
2020-02-26
