落叶松针叶化学成分研究
2020-07-04付美玲张文治白丽明韩松庞婧慧
付美玲 张文治 白丽明 韩松 庞婧慧
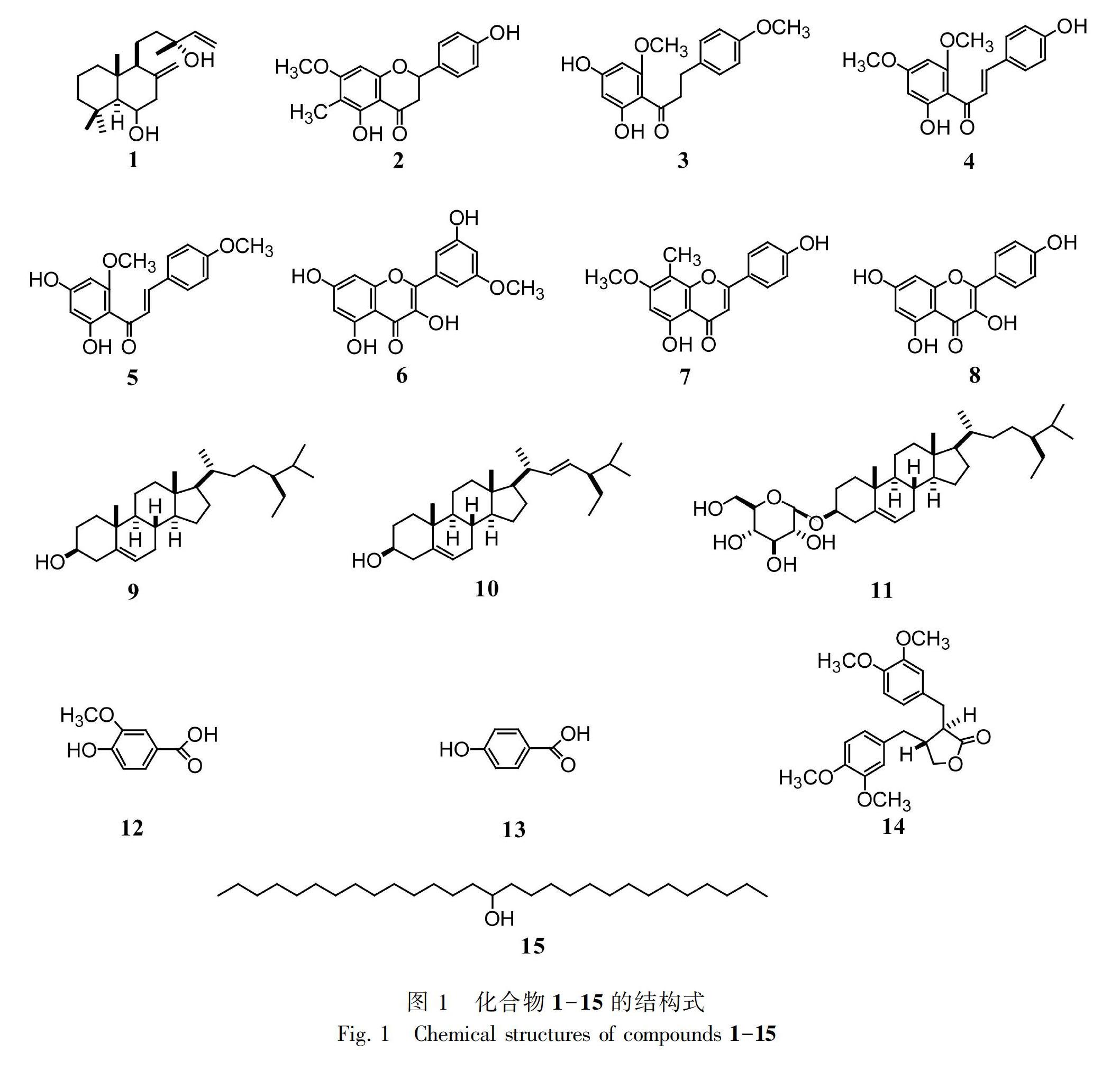
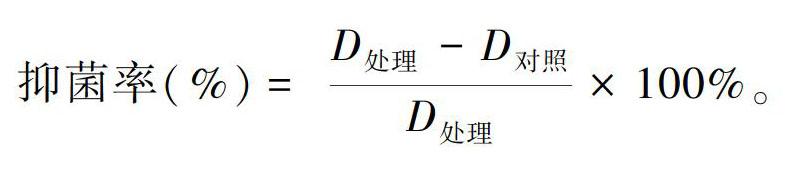

摘 要: 该研究采用硅胶柱色谱、半制备型高效液相色谱和重结晶等方法对落叶松针叶乙酸乙酯萃取物进行分离纯化,利用NMR、MS现代波谱技术结合相关文献报道对分离得到的化合物进行结构鉴定,并对提取浸膏的抑菌活性进行了测试。结果表明:从落叶松针叶乙酸乙酯萃取物中分离得到15个化合物,分别鉴定为Larixol (1)、(2R)-5,4′-二羟基-6-甲基-7-甲氧基-黄酮 (2)、2′,4′-二羟基-4,6′-二甲氧基二氢查尔酮 (3)、2′,4-二羟基-4′,6′-二甲氧基查尔酮 (4)、2′,4′-二羟基-4,6′-二甲氧基查尔酮 (5)、异鼠李素 (6)、4′,5-二羟基-7-甲氧基-8-甲基黄酮 (7)、山奈酚 (8)、β-谷甾醇 (9)、豆甾醇 (10)、胡萝卜苷 (11)、香草酸 (12)、对羟基苯甲酸 (13)、二甲基罗汉松脂素 (14)、15-二十九烷醇 (15)。其中,化合物2,4,5和7为首次从该属植物中分离得到。抑菌活性实验结果显示,乙酸乙酯萃取浸膏在浓度为5~100 mg·mL-1时对大肠杆菌、枯草芽孢杆菌、蜡样芽孢杆菌和金黄色葡萄球菌的抑菌率分别为55%~70%、53%~72%、61%~71%和33%~65%。上述結果为更加深入探究落叶松针叶化学成分和药理活性提供了一定理论依据。
关键词: 落叶松针叶, 化学成分, 黄酮, 香草酸, 抑菌活性
中图分类号: Q946
文献标识码: A
文章编号: 1000-3142(2020)05-0654-09
Chemical constituents of Larix gmelinii needles
FU Meiling, ZHANG Wenzhi*, BAI Liming, HAN Song, PANG Jinghui
( College of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, Heilongjiang, China )
Abstract: To study on chemical constituents and bioactivity of Larix gmelinii needles, separation and purification of ethyl acetate extract from Larix gmelinii needles were carried out by silica gel column chromatography, semi-preparative high performance liquid chromatography, and re-crystallization. The structures of isolated compounds were identified by analysis of spectra of MS, NMR, and compared with the reported conferences. The antibacterial activity of the extract was also tested. The results were as follows: Compounds 1-15 were isolated from the ethyl acetate extract and identified as Larixol (1), (2R)-5,4′-dihydroxy-6-methyl-7-methoxy flavone (2), 2′,4′-dihydroxy-4,6′-dimethoxydihydro-chalcone (3), 2′,4-dihydroxy-4′,6′-dimethoxy-chalcone (4), 2′,4′-dihydroxy-4,6′-dimethoxy-chalcone (5), isorhamnetin (6), 4′,5-dihydroxy-7-methoxy-8-methyl flavone (7), kaempferol (8), β-sitosterol (9), stigmasterol (10), daucosterol (11), vanillic acid (12), hydroxyl-benzoic acid (13), dimethyl-matairesinol (14), and 15-nonacosanol (15). Compounds 2, 4, 5, and 7 were isolated from the genus Pinus for the first time. The result of antibacterial activity indicated that the inhibition rates of ethyl acetate extract to Escherichia coli, Bacillus subtilis, B. cereus, and Staphylococcus aureus were 55%-70%, 53%-72%, 61%-71%, 33%-65%, respectively, at the concentration range of 5-100 mg·mL-1. The results of this study will provide a theoretical basis for further study on the chemical composition and pharmacological activity of Larix gmelinii.
Key words: Larix gmelinii needles, chemical constituents, flavanone, vanillic acid, antibacterial activity
落叶松(Larix gmelinii)是松科落叶松屬的落叶乔木,是松科中的重要成员之一,被誉为“北方杉木”(于震,2014),在我国主要分布于东北大小兴安岭、河北、山西、陕西及四川北部等地(姜贵全,2013)。其针叶部分主要含有黄酮类、单萜和倍半萜类挥发油(韩芬等,2008),药理研究表明落叶松针叶具有抗氧化(Kolhir et al.,1996;Willfor et al.,2003;Wang et al.,2011;Ma et al.,2012)、抗炎(Pferschy-Wenzig et al.,2008)、抗病毒(张卫鹏等,2013)、抗癌(刘妍和王遂,2011)、抗肿瘤(Ohtsu et al.,2001;张泽生等,2012)、抗辐射(杨磊等,2009)、抗突变(周恩宝,2009)、调节免疫(崔晓霞等,2012)、抑菌(马海旭和郭阿君,2017)、杀虫(崔义等,2016)等生物活性。
为了进一步明确落叶松针叶的化学成分及生物活性,本课题组对落叶松针叶乙酸乙酯萃取物的化学成分和抑菌活性进行了研究,共分离得到15个单体化合物,分别鉴定为Larixol (1)、(2R)-5,4′-二羟基-6-甲基-7-甲氧基-黄酮 (2)、2′,4′-二羟基-4,6′-二甲氧基二氢查尔酮 (3)、2′,4-二羟基-4′,6′-二甲氧基查尔酮 (4)、2′,4′-二羟基-4,6′-二甲氧基查尔酮 (5)、异鼠李素 (6)、4′,5-二羟基-7-甲氧基-8-甲基黄酮 (7)、山奈酚 (8)、β-谷甾醇 (9)、豆甾醇 (10)、胡萝卜苷 (11)、香草酸 (12)、对羟基苯甲酸 (13)、二甲基罗汉松脂素 (14)和15-二十九烷醇 (15),化合物1-15结构式见图1所示。此外,不同浓度乙酸乙酯萃取物对大肠杆菌、枯草芽孢杆菌、蜡样芽孢杆菌和金黄色葡萄球菌均表现出不同程度的抑菌活性。
研究结果进一步表明,黄酮类化合物2,4,5和7为首次从落叶松属植物中分离得到,初次发现落叶松针叶乙酸乙酯萃取物在一定浓度范围内对枯草芽孢杆菌和蜡样芽孢杆菌具有抑菌作用,该研究结果为进一步开发利用落叶松针叶的药用价值提供理论依据。
1 材料与方法
1.1 仪器、试剂和材料
1.1.1 仪器 Bruker AV-600核磁共振仪(美国Bruker公司);Yanako熔点仪(北京泰克仪器有限公司);YXQ-LS-50S11型立式压力蒸汽灭菌器(上海博讯实业有限公司医疗设备厂);SW-CJ-LED型超净工作台(上海锦屏仪器仪表有限公司通州分公司);MIR-253型恒温培养箱(上海精宏实验设备有限公司);5988A 型质谱仪(美国安捷伦公司);Waters 2489高效液相色谱系统(Waters科技上海有限公司);薄层色谱板20 cm silicagel GF254(青岛海洋化工厂);C18半制备色谱柱(250 mm×10 mm,5 μm,美国 kromsil 公司);RE-52AA旋转蒸发器(上海亚荣生化仪器厂)。
1.1.2 试剂 琼脂粉(北京奥博星生物技术有限责任公司),氯化钠(天津市凯通化学试剂有限公司),胰蛋白胨(北京奥博星生物技术有限责任公司),酵母浸粉(北京奥博星生物技术有限责任公司),除高效液相色谱使用的溶剂为色谱纯(天津市科密欧化学试剂有限公司)外,其他溶剂均为分析纯。
1.1.3 材料 落叶松针叶,于2017年8月采集于黑龙江省齐齐哈尔市青年林场,经齐齐哈尔大学生命学院杨晓杰教授鉴定为落叶松(Larix gmelinii)针叶。
1.1.4 供试菌种 大肠杆菌(Escherichia coli)、蜡样芽孢杆菌(Bacillus cereus)、枯草芽孢杆菌(B. subtilis)和金黄色葡萄球菌(Staphylococcus aureus)均由齐齐哈尔大学生命学院提供。
1.2 提取和分离
取干燥落叶松针叶15 kg,粉碎,用无水乙醇浸泡提取3次,每次8 d,合并提取液,减压浓缩得浸膏460 g。将浸膏溶于热水中形成混悬溶液,依次以正己烷、乙酸乙酯和正丁醇萃取。分别合并各部位萃取液,减压蒸馏得到正己烷萃取物(124 g),乙酸乙酯萃取物(98 g)和正丁醇萃取物(74 g)。
乙酸乙酯萃取物(98 g)经硅胶柱色谱进行梯度洗脱(正己烷:乙酸乙酯=1:0~0:1),分离得到6个流分(Fr.1-6),Fr.2经硅胶柱色谱分离(正己烷:乙酸乙酯=5:1,7:3),得到10个流分(Fr.2-1~2-10),Fr.2-3经过重结晶(正己烷:乙酸乙酯=5:1),得到化合物1(15.2 mg)。Fr.3经硅胶柱色谱分离(正己烷:乙酸乙酯=6:4),得到8个流分(Fr.3-1~3-8),Fr.3-7经 HPLC [Cosmosil 5C18 AR-II(10 mm × 250 mm),水:甲醇=1:9,流速 2 mL·min-1]进一步纯化,得到化合物2(14.3 mg)。Fr.4经硅胶柱色谱分离(二氯甲烷:乙酸乙酯=9:1,8:2,7:3),得到16个流分(Fr.4-1~4-16),Fr.4-4经过重结晶(二氯甲烷:乙酸乙酯=8:2),得到化合物3(7.1 mg),分别将Fr.4-6和Fr.4-10进行重结晶(二氯甲烷:乙酸乙酯=7:3)处理,得到化合物4(9.4 mg)和化合物5(12 mg)。Fr.4-11经过重结晶(乙酸乙酯),得到化合物6(9.4 mg)。Fr.4-13经硅胶柱色谱,以正己烷-乙酸乙酯(7:3)洗脱,得到2个流分(Fr.4-13-1~4-13-2),Fr.4-13-1重结晶(乙酸乙酯)得到化合物7(5.5 mg)。Fr.4-15重结晶(乙酸乙酯)得到化合物8(6.9 mg)。Fr.1经硅胶柱色谱分离(正己烷:乙酸乙酯=9:1,8:2),得到12个流分(Fr.1-1~1-12),Fr.1-2经过重结晶(乙酸乙酯),得到化合物9(16.3 mg)。Fr.2-6经过重结晶(乙酸乙酯),得到化合物10(5.4 mg)。Fr.5经硅胶柱色谱分离(二氯甲烷:甲醇=9:1),得到9个流分(Fr.5-1~5-9),Fr.5-7经过重结晶(甲醇),得到化合物11(20 mg)。Fr.4-13-2经过重结晶(乙酸乙酯),得到化合物12(8.6 mg)。Fr.4-14经硅胶柱色谱,以正己烷-乙酸乙酯(7:3)洗脱,得到化合物13(7.1 mg)。Fr.4-7经硅胶柱色谱,以正己烷-乙酸乙酯(7:3)洗脱,经过重结晶(乙酸乙酯)得到化合物14(7.2 mg)。Fr.1-5经HPLC [Cosmosil 5C18 AR-II(10 mm × 250 mm),水:甲醇=5:95,流速2 mL·min-1]进一步纯化得到化合物15(20.3 mg)。
1.3 抑菌活性实验
乙酸乙酯萃取物以丙酮作为溶剂,配制浓度分别为100、50、20、10和5 mg·mL-1溶液,用于抑菌活性测定。参照赵赟鑫(2010)的实验方法,首先,选择直径为9 cm培养皿制备PDA平板,灭菌;再用涂布器在平板上均匀涂布大肠杆菌、枯草芽孢杆菌、蜡样芽孢杆菌和金黄色葡萄球菌的菌悬液;最后,采用打孔法,用直径为6 mm的无菌打孔器在已涂布菌悬液的培养基上打孔,每孔加入上述不同浓度萃取物药液50 μL,以等体积的丙酮为空白对照,每个处理重复3次。用十字交叉法(田双娥,2019)测量菌落直径,D处理为处理菌落直径,D对照为对照菌落直径,并通过以下公式计算抑菌率。
2 结果与分析
2.1 结构鉴定
化合物1 白色晶体(正己烷-乙酸乙酯),mp: 101~102 ℃, EI-MS m/z: 306 [M]+, 分子式为C20H34O2, 1H-NMR (600 MHz, CDCl3) δ: 0.69 (3H, s, H-20), 1.01 (3H, s, H-19), 1.16 (3H, s, H-18), 1.26 (3H, s, H-16), 2.05 (1H, t, J=11.8 Hz, H-7), 2.67 (1H, dd, J=4.8, 12.0 Hz, H-7), 3.86 (1H, m, H-6), 4.59 (1H, d, J=1.4 Hz, H-17), 4.89 (1H, d, J=1.4Hz, H-17), 5.06 (1H, dd, J=1.2, 10.6 Hz, H-15), 5.21 (1H, dd, J=1.2, 17.1 Hz, H-15), 5.91 (1H, dd, J=10.9, 17.1 Hz, H-14); 13C-NMR (150 MHz, CDCl3) δ: 39.3 (C-1), 19.2 (C-2), 43.8 (C-3), 33.9 (C-4), 60.6 (C-5), 71.7 (C-6), 49.2 (C-7), 145.6 (C-8), 56.5 (C-9), 39.6 (C-10), 18.1 (C-11), 41.4 (C-12), 73.6 (C-13), 145.3 (C-14), 111.7 (C-15), 27.8 (C-16), 108.5 (C-17), 36.7 (C-18), 22.7 (C-19), 16.1 (C-20)。根據文献报道(杨保华,2004),鉴定化合物1为Larixol。
化合物2 黄色油状物(正己烷-乙酸乙酯),EI-MS m/z: 301 [M]+, 分子式为C17H17O5, 1H-NMR (600 MHz, CDCl3) δ: 2.05 (1H, s, CH3-6), 2.76 (1H, dd, J=2.7, 17.1 Hz, H-3), 3.09 (1H, dd, J=13.1, 17.1 Hz, H-3), 3.83 (3H, s, OCH3-7), 5.35 (1H, dd, J=2.7, 13.1 Hz, H-2), 6.07 (1H, s, H-8), 6.88 (2H, d, J=8.3 Hz, H-3′, H-5′), 7.34 (2H, d, J=8.3 Hz, H-2′, H-6′), 12.06 (OH-5); 13C-NMR (150 MHz, CDCl3) δ: 79.1 (C-2), 43.3 (C-3),196.1 (C-4), 160.4 (C-5), 106.0 (C-6), 165.8 (C-7), 90.7 (C-8), 161.3 (C-9), 102.8 (C-10), 130.6 (C-1′), 128.0 (C-2′, C-6′), 115.7 (C-3′ and C-5′), 156.3 (C-4′), 8.2 (CH3-6), 55.8 (OCH3-7)。根据文献报道(Leyte-Lugo et al.,2017),鉴定化合物2为(2R)-5,4′-dihydroxy-6-C-methyl-7-methoxy-flavanone。
化合物3 黄色结晶(正己烷-乙酸乙酯),mp: 172~173 ℃, EI-MS m/z: 302 [M]+, 分子式为C17H18O5, 1H-NMR (600 MHz, CDCl3) δ: 2.95 (2H, t, J=7.5 Hz, H2-β), 3.30 (2H, t, J=7.5 Hz, H2-α), 3.78 (3H, s, OCH3-4′), 3.80 (3H, s, OCH3-6′), 6.18 (1H, d, J=2.0 Hz, H-5′), 6.88 (2H, d, J=8.5 Hz, H-3, H-5), 7.16 (2H, d, J=8.5 Hz, H-2, H-6), 13.96 (1H, s, OH-2′); 13C-NMR (150 MHz, CDCl3) δ: 133.8(C-1), 129.7 (C-2, C-6 ), 157.9 (C-4), 115.0 (C-3, C-5), 105.0 (C-1′), 166.6 (C-2′), 96.2 (C-3′), 165.3 (C-4′), 91.8 (C-5′), 163.5 (C-6′), 55.4 (OCH3-4′), 56.3 (OCH3-6′), 45.9 (C-α), 29.8 (C-β), 204.3 (C=O)。根据文献报道(Seidel et al.,2000),鉴定化合物3为2′,4′-dihydroxy-4,6′-dimethoxydihydrochalcone。
化合物4 黄色针晶(正己烷-乙酸乙酯),mp: 186~187 ℃, EI-MS m/z: 300 [M]+, 分子式为C17H16O5, 1H-NMR (600 MHz, DMSO-d6) δ: 3.81 (3H, s, OCH3-4′), 3.88 (3H, s, OCH3-6′), 5.92 (1H, d, J=2.1 Hz, H-5′), 6.01 (1H, d, J=2.2 Hz, H-3′), 6.93 (2H, d, J=8.5 Hz, H-3, H-5), 7.54 (2H, d, J=8.5 Hz, H-2, H-6), 7.69 (1H, d, J=15.8 Hz, H2-α), 7.74 (1H, d, J=16.0 Hz, H2-β); 13C-NMR (150 MHz, DMSO-d6) δ: 127.9 (C-1), 130.2 (C-2, C-6), 161.0 (C-4), 115.6 (C-3, C-5), 105.6 (C-1′), 166.8 (C-2′), 95.3 (C-3′), 165.2 (C-4′), 92.1 (C-5′), 163.1 (C-6′), 55.8 (OCH3-4′), 56.5 (OCH3-6′), 125.3 (C-α), 142.7 (C-β), 192.3 (C=O)。根据文献报道(Seeram et al.,1996;Dharmaratne et al.,2002),鉴定化合物4为2′,4-dihydroxy-4′,6′-dimethoxychalcone。
化合物5 黄色针晶(正己烷-乙酸乙酯),mp: 162~163 ℃, EI-MS m/z: 300 [M]+, 分子式为C17H16O5, 1H-NMR (600 MHz, CDCl3) δ: 3.87 (3H, s, OCH3-4), 3.92 (3H, s, OCH3-6′), 5.33 (1H, brs, OH-4′), 5.96 (1H, d, J=2.4 Hz, H-5′), 6.05 (1H, d, J=2.4 Hz, H-3′), 6.94 (2H, d, J=8.8 Hz, H-3, H-5), 7.56(2H, d, J=8.8 Hz, H-2, H-6), 7.75 (1H, d, J=15.6 Hz, H-β), 7.78 (1H, d, J=15.6 Hz, H-α), 14.32 (1H, s, OH-2′); 13C-NMR (150 MHz, CDCl3) δ: 128.3 (C-1), 130.1 (C-2, C-6), 161.4 (C-4), 114.4 (C-3, C-5), 106.5 (C-1′), 167.9 (C-2′), 96.8 (C-3′), 162.7 (C-4′), 91.1 (C-5′), 163.2 (C-6′), 55.9 (OCH3-6′), 55.4 (OCH3-4), 125.1 (C-α), 142.6 (C-β), 192.7 (C=O)。根据文献报道(Seidel et al.,2000),鉴定化合物5为2′,4′-dihydroxy-4,6′-dimethoxychalcone。
化合物6 黄色针晶(正己烷-乙酸乙酯),mp: 216~217 ℃, EI-MS m/z: 316 [M]+, 分子式为C16H12O7, 1H-NMR (600 MHz, DMSO-d6) δ: 3.85 (3H, s, OCH3-3′), 6.25 (1H, d, J=2.2 Hz, H-6), 6.49 (1H, d, J=2.2 Hz, H-8), 6.96 (1H, d, J=2.2 Hz, H-5′), 7.70 (1H, dd, J=2.2, 8.8 Hz, H-6′), 7.77 (1H, d, J=2.2 Hz, H-2′), 9.74 (1H, brs, OH-4′), 9.84 (1H, brs, OH-3), 10.98 (1H, s, OH-7), 12.49 (1H, s, OH-5); 13C-NMR (150 MHz, DMSO-d6) δ: 156.6 (C-2), 132.9 (C-3), 176.3 (C-4), 159.8 (C-5), 98.9 (C-6), 164.3 (C-7), 95.0 (C-8), 161.2 (C-9), 105.2 (C-10), 122.2 (C-1′), 115.5 (C-2′), 149.3 (C-3′), 151.5 (C-4′), 116.0 (C-5′), 122.0 (C-6′), 56.2 (OCH3-3′)。根据文献报道(李胜华,2018),鉴定化合物6为异鼠李素。
化合物7 黄色结晶(正己烷-乙酸乙酯),mp: 280~281 ℃, EI-MS m/z: 297 [M]+, 分子式为C17H13O5, 1H-NMR (600 MHz, DMSO-d6) δ: 2.24 (3H, s, CH3-8), 3.90 (3H, s, OCH3-7), 6.54 (1H, s, H-3), 6.82 (1H, s, H-3), 6.95 (2H, d, J=8.5 Hz, H-3′, H-5′), 7.95 (2H, d, J=8.5 Hz, H-2′, H-6′); 13C-NMR (150 MHz, DMSO-d6) δ: 163.2 (C-2) ,101.2 (C-3), 182.9 (C-4), 160.0 (C-5), 95.5 (C-6), 162.1 (C-7), 103.0 (C-8), 154.2 (C-9), 103.5 (C-10), 121.9 (C-1′), 129.0 (C-2′, C-6′), 162.1 (C-4′), 115.6(C-3′, C-5′), 8.11 (CH3-8), 56.8 (OCH3-7)。根据文献报道(Junio et al.,2011),鉴定化合物7为4′,5-dihydroxy-7-methoxy-8-methylflavone。
化合物8 黃色针晶(正己烷-乙酸乙酯),mp: 271~272 ℃, EI-MS m/z: 286 [M]+, 分子式为C15H10O6, 1H-NMR (600 MHz, DMSO-d6) δ: 6.19 (1H, d, J=2.0 Hz, H-6), 6.40 (1H, d, J=2.0 Hz, H-8), 6.92 (2H, d, J=8.9 Hz, H-3′, H-5′), 8.04 (2H, d, J=8.9 Hz, H-2′, H-6′); 13C-NMR (150 MHz, DMSO-d6) δ: 147.3 (C-2), 131.6 (C-3), 176.4 (C-4), 161.2 (C-5), 98.7 (C-6), 164.4 (C-7), 94.0 (C-8), 156.6 (C-9), 103.5 (C-10), 122.1 (C-1′), 130.0 (C-2′, C-6′), 159.7 (C-4′), 115.9 (C-3′and C-5′)。根据文献报道(Singh et al.,2008),鉴定化合物8为山奈酚。
化合物9 白色羽状结晶(正己烷-乙酸乙酯),mp: 141~142 ℃, EI-MS m/z: 414 [M]+, 分子式为C29H50O, 1H-NMR (600 MHz, CDCl3) δ: 0.70 (3H, s, J=7.3 Hz, CH3-29), 0.83 (3H, m, J=6.6 Hz, CH3-27), 0.86 (3H, d, J=6.6 Hz, CH3-26), 0.91 (3H, d, J=6.4 Hz, CH3-21), 1.08 (3H, s, CH3-18), 1.10 (3H, s, CH3-19), 3.53 (1H, m, H-3), 5.35 (1H, m, H-1); 13C-NMR (150 MHz, CDCl3) δ: 37.3 (C-1), 26.1 (C-2), 71.8 (C-3), 42.4 (C-4), 140.8 (C-5), 121.7 (C-6), 34.0 (C-7), 31.6 (C-8), 50.2 (C-9), 36.5 (C-10), 21.1 (C-11), 39.8 (C-12), 42.3 (C-13), 56.8 (C-14), 24.3 (C-15), 29.2 (C-16), 56.1 (C-17), 19.6 (C-18), 11.9 (C-19), 36.2 (C-20), 19.2 (C-21), 31.9 (C-22), 28.3 (C-23), 45.9 (C-24), 29.7 (C-25), 12.0 (C-26), 20.2 (C-27), 23.1 (C-28), 19.8 (C-29)。根据文献报道(朱海林等,2016),鉴定化合物9为β-谷甾醇。
化合物10 白色结晶(正己烷-乙酸乙酯),mp: 167~168 ℃, EI-MS m/z: 412 [M]+, 分子式为C29H48O, 1H-NMR (600 MHz, CDCl3) δ: 3.53 (1H, m, H-3), 5.04 (1H, dd, J=8.6, 15.0 Hz, H-23), 5.16 (1H, dd, J=8.6, 15.0 Hz, H-22), 5.36 (1H, m, H-6); 13C-NMR (150 MHz, CDCl3) δ: 37.2 (C-1), 31.9 (C-2), 71.9 (C-3), 42.2 (C-4), 140.9 (C-5), 121.7 (C-6), 31.9 (C-7), 31.9 (C-8), 50.1 (C-9), 36.6 (C-10), 21.2 (C-11), 39.8 (C-12), 42.3 (C-13), 56.8 (C-14), 24.4 (C-15), 28.9 (C-16), 56.1 (C-17), 12.2 (C-18), 19.4 (C-19), 40.5 (C-20), 21.3 (C-21), 138.3 (C-22), 129.4 (C-23), 51.4 (C-24), 31.9 (C-25), 19.4 (C-26), 21.2 (C-27), 25.4 (C-28), 12.2 (C-29)。根据文献报道(Ee et al.,2006),鉴定化合物10为豆甾醇。
化合物11 白色粉末(二氯甲烷-甲醇),mp: 291~292 ℃, EI-MS m/z: 576 [M]+, 分子式为C35H60O6, 1H-NMR (600 MHz, DMSO-d6) δ: 0.68 (3H, s, H-18), 0.86 (6H, d, J=7.4 Hz, H-26, H-27), 0.88 (3H, t, J=6.4 Hz, H-29), 0.96 (3H, d, J=7.4 Hz, H-21), 0.99 (3H, s, H-19), 3.26 (3H, m,H-2′, 4′, 5′), 3.63 (1H, t, J=6.1 Hz, H-3′), 3.51 (1H, m,H-3), 4.21 (1H, dd, J=6.1, 11.6 Hz, H-6′a), 4.43 (1H, d, J=11.6 Hz, H-6′b), 4.89 (1H, d, J=7.9 Hz, H-1′), 5.33 (1H, m, H-6); 13C-NMR (600 MHz, DMSO-d6) δ: 37.9 (C-1), 30.7 (C-2), 79.0 (C-3), 39.6 (C-4), 141.1 (C-5), 121.9 (C-6), 32.5 (C-7), 32.6 (C-8), 50.4 (C-9), 37.1 (C-10), 21.5 (C-11), 40.2 (C-12),42.6 (C-13), 57.3 (C-14), 24.8 (C-15), 29.4 (C-16), 56.4 (C-17), 12.4 (C-18), 19.7 (C-19), 36.4 (C-20), 20.0 (C-21), 34.9 (C-22),26.4 (C-23), 46.2 (C-24), 29.5 (C-25), 20.4 (C-26), 19.6 (C-27), 24.1 (C-28), 12.5 (C-29), 101.9 (C-1′), 74.7 (C-2′), 77.8 (C-3′), 71.2 (C-4′), 77.5 (C-5′), 62.3 (C-6′)。根据文献报道(Luo et al.,2009),鉴定化合物11为胡萝卜苷。
化合物12 白色针晶(正己烷-乙酸乙酯),mp: 209~210 ℃, EI-MS m/z: 168 [M]+, 分子式为C8H8O4, 1H-NMR (600 MHz, DMSO-d6) δ: 3.80 (3H, s, OCH3-3), 6.83 (1H, d, J=7.7 Hz, H-5), 7.45 (2H, m, H-2, H-6); 13C-NMR (150 MHz, DMSO-d6) δ: 122.1 (C-1), 113.2 (C-2), 147.7 (C-3), 151.6 (C-4), 115.5 (C-5), 123.9 (C-6), 56.0 (-OCH3), 167.7 (-COOH)。根据文献报道(倪付勇,2017),鉴定化合物12为香草酸。
化合物13 白色粉末(正己烷-乙酸乙酯),mp: 215~216 ℃, EI-MS m/z: 138 [M]+, 分子式为C7H6O3, 1H-NMR (600 MHz, DMSO-d6) δ: 6.84 (2H, d, J=8.7 Hz, H-3, H-5), 7.81 (2H, d, J=8.7 Hz, H-2, H-6); 13C-NMR (150 MHz, DMSO-d6) δ: 121.8 (C-1), 132.0 (C-2, C-6), 162.1 (C-4), 115.6 (C-3, C-5), 167.6 (-COOH)。根据文献报道(谢雪等,2016),鉴定化合物13为对羟基苯甲酸。
化合物14 无色针晶(正己烷-乙酸乙酯),mp: 127~128 ℃, EI-MS m/z: 386 [M]+, 分子式为C22H26O6, 1H-NMR (600 MHz, CDCl3) δ: 2.51 (1H, dd, J=7.9, 13.5 Hz, H-7b), 2.53 (1H, ddddd, J=5.9, 7.2, 8.1, 8.3, 9.0 Hz, H-8), 2.59 (1H, ddd, J=5.4, 6.4, 9.0 Hz, H-8′), 2.64 (1H, dd, J=7.9, 13.5 Hz, H-7a), 2.91 (1H, dd, J=5.4, 13.8 Hz, H-7′b), 2.96 (1H, dd, J=6.2, 13.8 Hz, H-7′a), 3.83 (3H, s, H-12), 3.84 (3H, s, H-11), 3.86 (3H, s, H-13), 3.87 (1H, s, H-10), 3.88 (1H, dd, J=8.1, 8.7 Hz, H-9b), 4.13 (1H, dd, J=7.2, 8.7 Hz, H-9a), 6.49 (1H, d, J=2.2 Hz, H-2), 6.56 (1H, dd, J=2.2, 8.2 Hz, H-6), 6.65 (1H, dd, J=2.0, 8.3 Hz, H-6′), 6.68 (1H, d, J=2.0 Hz, H-2′), 6.77 (1H, d, J=8.4 Hz, H-5′), 6.78 (1H, d, J=8.0 Hz, H-5); 13C-NMR (150 MHz, CDCl3) δ: 130.5 (C-1), 111.8 (C-2), 147.9 (C-3), 149.2 (C-4), 111.1 (C-5), 120.6 (C-6), 38.2 (C-7), 41.1 (C-8), 71.2 (C-9), 56.0 (C-10), 55.9 (C- 11), 55.9 (C-12), 55.9 (C-13), 130.2 (C-1′), 112.4 (C-2′), 147.8 (C-3′), 149.1 (C-4′), 111.4 (C-5′), 121.4 (C-6′), 34.5 (C-7′), 46.6 (C-8′), 178.7 (C-9′)。根據文献报道(Tanabe et al.,2011),鉴定化合物14为二甲基罗汉松脂素。
(Hydrastis canadensis) [J]. J Nat Prod, 74(7):1621-1629.
KOLHIR VK, BYKOV VA, BAGINSKAJA AI, et al., 1996. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii (Rupr.) Rupr. wood [J]. Phytother Res, 10(6): 478-482.
LEYTE-LUGO M, BRITTON ER, FOIL DH, et al., 2017. Secondary metabolites from the leaves of the medicinal plant goldenseal (Hydrastis canadensis) [J]. Phytochem Lett, 20(15): 54-60.
LI SH, 2018. Chemical constituents of Gendarussa vulgaris [J]. Chin Trad Herb Drugs, 49(17): 27-31. [李胜华, 2018. 小驳骨的化学成分研究 [J]. 中草药, 49(17):27-31.]
LIU Y, WANG S, 2011. Extraction and antioxidaion of dihydroquercetin [J]. Chem Res Appl, 23(1): 107-111. [刘妍, 王遂, 2011. 二氢槲皮素的提取及抗氧化性研究 [J]. 化学研究与应用, 23(1):107-111.]
LUO W, ZHAO M, YANG B, et al., 2009. Identification of bioactive compounds in Phyllenthus emblica L. fruit and their free radical scavenging activities [J]. Food Chem, 114(2): 499-504.
MA C, YANG L, WANG W, et al., 2012. Extraction of dihydroquercetin from Larix gmelinii with ultrasound-assisted and microwave-assisted alternant digestion [J]. Int J Mol Sci, 13(12): 8789-8804.
MA HX, GUO AJ, 2017. Study on antibacterial activity of extracts of two gardening plants [J]. For Invest Design, 36(1): 54-56. [馬海旭, 郭阿君, 2017. 两种园林植物提取液抑菌活性的研究 [J]. 林业勘查设计, 36(1):54-56.]
NI FY, WEN JH, LI M, et al., 2017. Chemical constituents from flower buds of Lonicera japonica [J]. Chin Trad Herb Drugs, 48(18): 3689-3692. [倪付勇, 温建辉, 李明, 等, 2017. 金银花化学成分研究 [J]. 中草药, 48(18):3689-3692.]
OHTSU H, TANAKA R, IN Y, et al., 2001. Abietane diterpenoids from the cones of Larix kaempferi and their inhibitory effects on Epstein-Barr virus activation [J]. Planta Med, 67(1): 55-60.
PFERSCHY-WENZIG EM, KUNERT O, PRESSER A, et al., 2008. In vitro anti-inflammatory activity of larch (Larix decidua L.) sawdust [J]. J Agric Food Chem, 56(24): 11688-11693.
PLADIO LP, VILLASENOR I, 2004. Anti-spasmodic constituents from Drimys piperita hook F. leaves [J]. Philipp J Sci, 133(1): 17-21.
SEERAM NP, JACOBS H, MCLEAN S, et al., 1996. Prenylated hydroxybenzoic acid derivatives from Piper murrayanum [J]. Phytochemistry(Oxford), 43(4): 863-865.
SEIDEL V, BAILLEUL F, WATERMAN PG, 2000. (Rel)-1β,2α-di-(2,4-dihydroxy-6-methoxybenzoyl)-3β, 4α-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii [J]. Phytochemistry, 55(5): 439-446.
SINGH R, SINGH B, SINGH S, et al., 2008. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. [J]. Toxicol In Vitro, 22(8): 1965-1970.
TANABE H, FUKUTOMI R, YASUI K, et al., 2011. Identification of dimethylmatairesinol as an immunoglobulin e-suppressing component of the leaves of Cinnamomum camphora [J]. J Health Sci, 57(2): 184-187.
TIAN SE, 2019. Study on three kinds of inhibition zone tests applied in evaluation of antifungal activity of oregano oil [J]. Flav Frag Cosm, 20(1): 37-41. [田双娥, 2019. 三种不同抑菌圈试验法在牛至油抑菌评价中的应用研究 [J]. 香料香精化妆品, 20(1):37-41.]
WANG Y, ZU Y, LONG J, et al., 2011. Enzymatic water extraction of taxifolin from wood sawdust of Larix gmelini (Rupr.) Rupr. and evaluation of its antioxidant activity [J]. Food Chem, 126(3): 1178-1185.
WILLFOR SM, AHOTUPA MO, HEMMING JE, et al., 2003. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species [J]. J Agric Food Chem, 51(26): 7600-7606.
XIE X, ZHANG HD, WEN JH, et al., 2016. Studies on che-mical constituents in Guizhi Fuling Capsule (VI) [J]. Chin Trad Herbal Drugs, 46(21): 3795-3797. [謝雪, 张宏达, 温建辉, 等, 2016. 桂枝茯苓胶囊化学成分研究(VI) [J]. 中草药, 46(21):3795-3797.]
YANG BH, 2004. Studies on the active constituents of Larix olgensis Henry var. koreana Nakai [D]. Shanghai: The Second Millitary Medical University: 49. [杨保华, 2004.长白落叶松树皮活性成分研究 [D]. 上海:第二军医大学:49.]
YANG L, MA CH, HUANG JM, et al., 2009. Comparison of dihyroquercetin extraction methods from Larix gmelini [J]. For Eng, 25(5): 6-11. [杨磊, 马春慧, 黄金明, 等, 2009. 兴安落叶松中二氢槲皮素提取方法的比较 [J]. 森林工程, 25(5):6-11.]
YU Z, 2014. Extraction, purification of polyphenols from larch bark and its effects of anti-oxidation [D]. Harbin: Northeast Forestry University. [于震, 2014. 落叶松树皮多酚的提取、纯化及抗氧化活性研究 [D]. 哈尔滨:东北林业大学.]
ZHANG WP, LIU W, FU JH, et al., 2013. Structural identification and quantitative analysis of taxifolin in Larix olgensis Henry var. koreana Nakai [J]. Food Sci, 4(16) : 293-296. [张卫鹏, 刘伟, 付警辉, 等, 2013. 长白落叶松中花旗松素的结构鉴定与含量测定 [J]. 食品科学, 34(16):293-296.]
ZHANG ZS, WANG LT, CHEN YZ, et al., 2012. Extraction technology of larch arabinogalactan [J]. Food Ind, 33(7): 55-57. [张泽生, 王利婷, 陈玥舟, 等, 2012. 落叶松阿拉伯半乳聚糖的提取工艺研究 [J]. 食品工业, 33(7):55-57.]
ZHAO YX, LIU KH, DENG BW, et al, 2010. Antibacterial activities of metabolites produced by two endophytic bacteria isolates in Taxus chinensis [J]. J Food Sci Biotechnol, 29(4): 617-623. [赵赟鑫, 刘开辉, 邓百万, 等, 2010. 2株中国红豆杉内生细菌代谢产抑菌活性物质的研究 [J]. 食品与生物技术学报, 29(4):617-623.]
ZHOU EB, 2009. Study on chemical constituents of volatile oil from pine leaves of pinaceae plants [J]. Jiamusi: Jiamusi University. [周恩宝, 2009. 松科植物松叶挥发油化学成分的研究 [D]. 佳木斯:佳木斯大学.]
ZHU HL, WANG ZY, ZHENG BZ, et al., 2016. Chemical constituents from berries of Physalis pubescens [J]. Chin Trad Herb Drugs, 47(5): 732-735. [朱海林, 王振洲, 郑炳真, 等, 2016. 毛酸浆果实的化学成分研究 [J]. 中草药, 47(5):732-735.]
(责任编辑 何永艳)
