瓦山锥化学成分研究(1)
2020-07-04李思雯王亚凤何瑞杰戴天歌李典鹏黄永林
李思雯 王亚凤 何瑞杰 戴天歌 李典鹏 黄永林
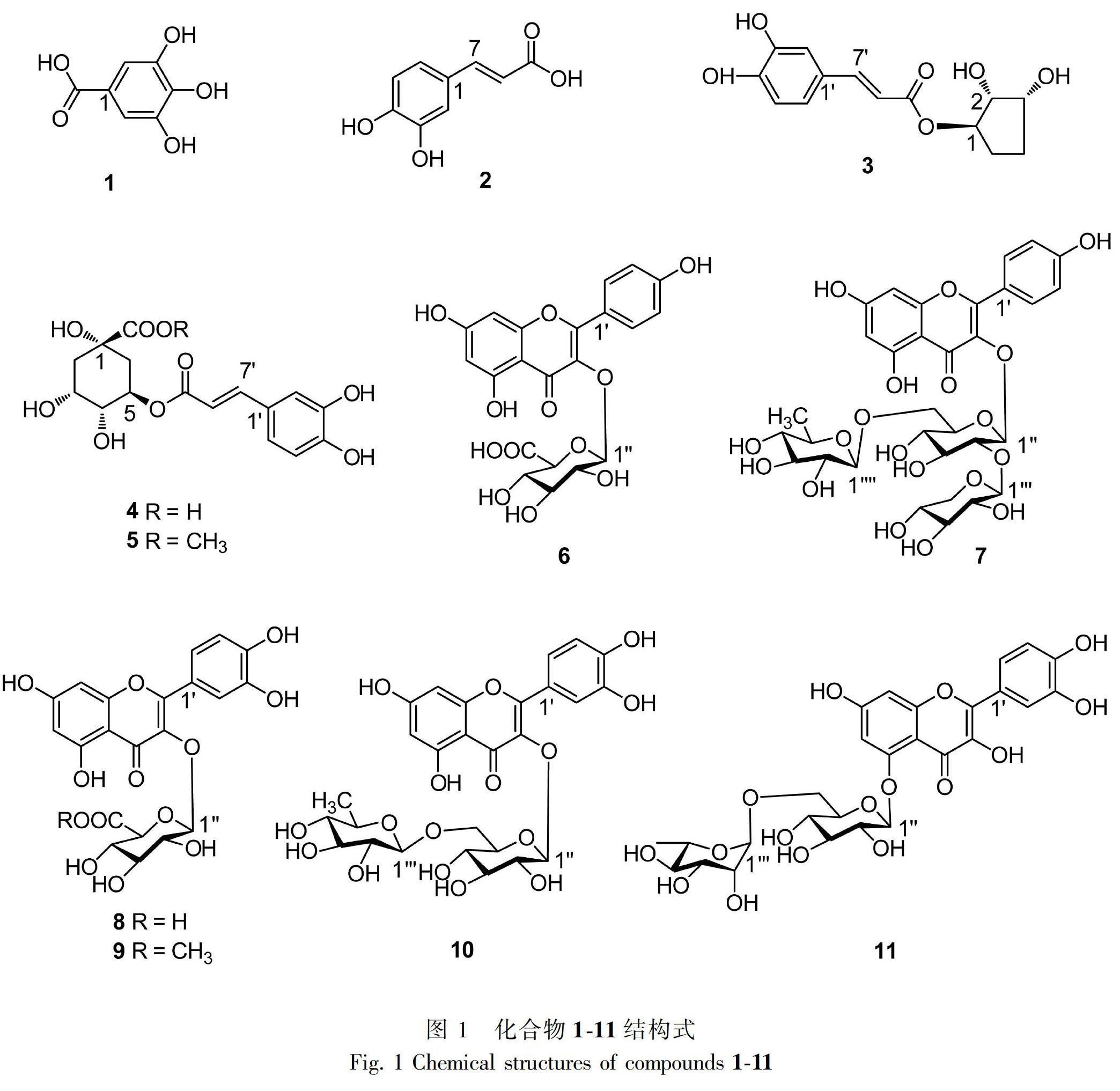
摘 要: 瓦山锥植物富含植物多酚类成分且资源丰富,目前尚无该植物化学成分及生物活性方面的报道。为了明确瓦山锥的物质基础,为该植物资源的合理开发与可持续利用提供科学依据,该研究采用Sephadex LH-20、Diaion HP20SS、Toyopearl HW-40F等多种柱层析方法对瓦山锥树叶乙醇提取物进行分离纯化,从中得到11个单体化合物,它们的结构经波谱数据分析及文献对照鉴定为没食子酸(1)、咖啡酸(2)、1-(3′, 4′-二羟基肉桂酰)-环戊-2,3-二酚(3)、绿原酸(4)、绿原酸甲酯(5)、kaempferol 3-O-β-D-glucuronopyranoside(6)、kaempferol 3-O-{β-D-xylopyranosyl-(1→2)- [α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside}(7)、quercetin 3-O-β-D-glucuronopyranoside(8)、quercetin 3-O-β-glucuronide-6″-methyl ester(9)、蘆丁(10)、quercetin 5-O- [α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside(11)。所有化合物均为首次从瓦山锥中分离得到,其中化合物3,7-11为首次从锥属植物中分离得到。
关键词: 瓦山锥, 化学成分, 结构鉴定, 植物多酚
中图分类号: Q946
文献标识码: A
文章编号: 1000-3142(2020)05-0648-06
Chemical constituents from Castanopsis ceratacantha (Ⅰ)
LI Siwen1,2, WANG Yafeng2, HE Ruijie2, DAI Tiange1,LI Dianpeng2, HUANG Yonglin2*
( 1. Guilin Medical University, Guilin 541004, Guangxi, China; 2. Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization, Guangxi Institute of Botany, Guangxi Zhuang Autonomous Region and Chinese Academy of Sciences, Guilin 541006, Guangxi, China )
Abstract: Castanopsis ceratacantha contains large amounts of polyphenols and abounds in natural resources, but its chemical constituents and biological activities have not been reported. In order to clarify the chemical basis of the plant and provide scientific basis for the rational development and sustainable utilization of the plant resources. The ethanol extracts of C. ceratacantha were isolated and purified by various chromatographic methods such as Sephadex LH-20, Diaion HP20SS, and Toyopearl HW-40F to yield 11 compounds. Their structures were elucidated by spectroscopic data and comparison with literatures as gallic acid (1), caffeic acid (2), 1-(3′,4′-dihydroxycinnamoyl)-cyclopenta-2,3-diol (3), chlorogenic acid (4), chlorogenic acid methyl ester (5), kaempferol 3-O-β-D-glucuronopyranoside (6), kaempferol 3-O-{β-D-xylopyranosyl-(1→2)- [α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside} (7), quercetin 3-O-β-D-glucuronopyranoside (8) , quercetin 3-O-β-glucuronide-6″-methyl ester (9), rutin (10), and quercetin 5-O- [α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside (11). All these compounds were obtained from this plant for the first time,and compounds 3, 7-11 were isolated from the genus Castanopsis for the first time.
Key words: Castanopsis ceratacantha, chemical constituents, structural identification, polyphenols
壳斗科(Fagaceae)锥属(Castanopsis)植物为常绿乔木,我国约有63种2变种,产长江以南各地(中国科学院中国植物志编辑委员会,1998)。现有研究表明,锥属植物主要含有多酚类成分,具有很好的抗氧化生理活性及市场开发前景(王亚凤等, 2016)。瓦山锥(Castanopsis ceratacantha)为壳斗科锥属(Castanopsis)植物,又名瓦山栲、黄山栲等,乔木,通常高8~15 m,在我国主要分布于云南、贵州、四川等省,在老挝、泰国东北部也有分布(中国科学院中国植物志编辑委员会,1998)。瓦山锥民间广泛用于止血、止渴、止泻(坚果)、慢性溃疡(叶)等疾病的治疗,主要应用在医药行业、食品行业、日化行业以及生化行业等领域。
本实验选取锥属植物瓦山锥作为研究对象,目的在于丰富锥属植物的化学成分,掌握瓦山锥的药效物质基础,为瓦山锥的合理开发与可持续利用奠定基础。此外,通过对锥属植物瓦山锥化学成分的研究,为在经典分类中系统位置有争议的壳斗科植物提供依据。
本研究以瓦山锥叶为原料,80%乙醇作为提取溶剂,通过Sephadex LH-20、Chromatorex C18、Diaion HP20SS、Toyopearl HW-40F等柱色谱层析,从瓦山锥叶乙醇提取物中分离得到11个单体化合物,经波谱数据分析及与文献比较鉴定了化合物的结构,所分离得到的成分主要为植物多酚类成分。化合物1-15结构式见图1所示。
1 材料与方法
1.1 材料和仪器
材料于2017年8月采自云南省景洪市,经广西壮族自治区中国科学院广西植物研究所丁涛副研究员鉴定为壳斗科锥属植物瓦山锥(Castanopsis ceratacantha)的树叶。
Brucker Avance 500 MHz超導核磁共振波谱仪(瑞典Bruker);N-1100 旋转蒸发仪(东京理化);CA-1111 冷却水循环仪(东京理化);自动接收仪(日本Advantec);F254硅胶薄层板(德国默克);Sephadex LH-20(GE Healthcare Bio-Science AB);Chromatorex C18(日本Fuji Silysia Chemical);Diaion HP20SS(Mistubishi Chemical);Toyopearl HW-40F(日本TOSOH公司);Sephadex LH-20(GE Healthcare Bio-Science AB);提取、分离所用试剂均为分析纯。
1.2 提取和分离
取干燥瓦山锥叶3.5 kg,切成碎片后用80%乙醇室温浸提2次,每次32 L,每次7 d,合并提取液并过滤,滤液经减压浓缩后得浸膏481.3 g。浸膏水溶解后经Sephadex LH-20柱(8.5 cm × 40 cm)层析分离,甲醇-水(0~100%,每20%为1梯度)和60%丙酮-水溶液洗脱(每1梯度2 L),经薄层层析分析合并,得到8个流份:Fr.1~8。Fr.1(10.0 g)经Diaion HP20SS、Chromatorex C18、Sephadex LH-20等色谱柱反复层析分离纯化得到化合物6(88 mg)、8(800 mg)、10(48 mg)。Fr.2(69.1 g)经Diaion HP20SS、Toyopearl HW-40F等色谱柱反复层析分离纯化得到化合物1(28 mg)、2(132 mg)、3(13 mg)、4(148 mg)、5(214 mg)、7(49 mg)、9(74 mg)、11(26 mg)。
2 结构鉴定
化合物1 C7H6O5, 白色粉末。HR-ESI-MS
BINA SS, NASIMA K, SABIRA B, et al., 2012. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity [J]. Phytochemistry, 77: 238-244.
CHEN QH, HUANG Y, ZHOU JJ, et al., 2017. Chemical constituents from leaves of Camellia nitidissima var. longistyla (II) [J]. Chin Trad Herb Drugs, 48(23): 4845-4850. [陈秋虹, 黄艳, 周洁洁, 等, 2017. 长柱金花茶叶的化学成分研究(II) [J]. 中草药, 48(23): 4845-4850.]
Chinese Botanical Society Editorial Board of Chinese Academy of Sciences, 1998. Flora Reipublicae Popularis Sinicae [M]. Beijing: Science Press, 22:13, 69. [中国科学院中国植物志编辑委员会, 1998. 中国植物志 [M]. 北京: 科学出版社, 22: 13, 69.]
DU ZZ, YANG XW, HAN H, et al., 2010. A new flavone C-glycoside from Clematis rehderiana [J]. Molecules, 5(1): 15: 672-679.
HARI PD, SENA M, SHOJI Y, 2017. Amentoflavone and kaempferol glycosides from the aerial parts of Cissampelos pareira [J]. Biotechnology, 5(1): 1-4.
HE ZN, LIAN WW, LIU JW, et al., 2017. Isolation, structural characterization and neuraminidase inhibitory activities of polyphenolic constituents from Flos caryophylli [J]. Phytochem Lett, 19: 160-167.
Jiangsu New Medical School, 1977. Traditional Chinese Medicine Dictionary: The first and the second [M]. Shanghai: Shanghai Peoples Publishing House. [江苏新医学院, 1977, 中药大辞典(上、下两册) [M]. 上海:上海人民出版社.]
LI CD, BEI LW, JIAN MC, 2008. Flavonoid triglycosides from the seeds of Camellia oleifera Abel [J]. Chin Chem Lett, 19: 1315-1318.
LI S, LIU YH, 2015. Chemical constituents with antioxidative activity from the flower buds of Lonicera serreana [J]. Guihaia, 35(4): 586-589. [李树, 刘玉衡, 2015. 毛药忍冬花蕾抗氧化活性部位化学成分研究 [J]. 广西植物, 35(4): 586-589.]
PU SC, GUO YQ, GAO WY, et al., 2010. Chemical constituents from Hydrocotyle sibthorpioides [J]. Chin Trad Herb Drugs, 41(9): 1440-1442. [蒲首丞, 郭远强, 高文远, 2010. 天胡荽化学成分的研究 [J]. 中草药, 41(9): 1440-1442.]
WANG YF, HUANG YL, LIU JL, et al., 2016. Content and antioxidant capacity of polyhenols from the Fagaceae plants [J]. Guangxi Sci, 23(2):180-183. [王亚凤, 黄永林, 刘金磊, 等, 2016. 壳斗科植物种子的多酚类含量及抗氧化能力 [J]. 广西科学, 23(2):180-183.]
WEI H, YANG JW, YAN XJ, et al., 2018. Chemical constituents from walnut green husk: Phenols [J]. Guihaia, 38(4): 463-468. [魏欢, 杨建文, 颜小捷, 等, 2018. 核桃青皮的化学成分研究——酚类化合物 [J]. 广西植物, 38(4): 463-468.]
XU J, ZHANG TJ, GONG SX, et al., 2010. Chemical constituents from the effective hemostatic of Cirsium setosum [J].Chin Trad Herb Drugs, 41(4): 542-544. [許浚, 张铁军, 龚苏晓, 等, 2010. 小蓟止血活性部位的化学成分研究 [J]. 中草药, 41(4): 542-544.]
ZHU TF, LI P, SUN QW, et al., 2019. Chemical constituents from leaves of Sabia parviflora [J]. Guihaia, 39(4): 511-515. [朱仝飞, 李萍, 孙庆文, 等, 2019. 小花清风藤叶的化学成分研究 [J]. 广西植物, 39(4): 511-515.]
(责任编辑 何永艳)
m/z: 171.0245 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 7.03 (2H, s, H-2, 6)。上述所有数据与参考文献(魏欢等, 2018)比对相同,经HPLC分析,与没食子酸标准品保留时间一致,故鉴定化合物1为没食子酸。
化合物2 C9H8O4,白色粉末。1H NMR (500 MHz, CD3OD) δ: 7.64 (1H, d, J=15.9 Hz, H-7), 7.08 (1H, d, J=1.8 Hz, H-2), 6.99 (1H, dd, J=8.2, 1.8 Hz, H-6), 6.75 (1H, d, J=8.2 Hz, H-5), 6.37 (1H, d, J=15.9 Hz, H-8); 13C NMR (125 MHz, CD3OD) δ: 115.2 (C-2), 115.5 (C-5), 116.5 (C-8), 123.3 (C-6), 127.9 (C-1), 146.5 (C-7), 147.4 (C-3), 149.6 (C-4), 168.7 (C-9)。上述所有数据与参考文献(朱仝飞等, 2019)比对相同,故鉴定化合物2为咖啡酸。
化合物3 C14H16O6,淡黄色粉末。1H NMR (500 MHz, CD3OD) δ: 7.64 (1H, d, J=15.9 Hz, H-7′), 7.05 (1H, d, J=1.9 Hz, H-2′), 6.98 (1H, dd, J=8.2, 1.9 Hz, H-6′), 6.76 (1H, d, J=8.2 Hz, H-5′), 6.38 (1H, d, J=15.9 Hz, H-8′), 4.81 (1H, dd, J=9.3, 2.9 Hz, H-1), 4.31-4.26 (2H, m, H-2, 3), 2.22-1.99 (4H, m, H-4a, 4b, 5a, 5b); 13C NMR (125 MHz, CD3OD) δ: 38.5 (C-4), 42.7 (C-5), 65.6 (C-3), 69.3 (C-1), 79.5 (C-2), 115.2 (C-2′), 115.6 (C-8′), 116.1 (C-5′), 123.0 (C-6′), 127.9 (C-1′), 146.8 (C-7′), 147.1 (C-4′), 149.5 (C-3′), 169.0 (C-9′)。上述所有数据与参考文献(许浚等, 2010)比对相同,故鉴定化合物3为1-(3′,4′-二羟基肉桂酰)-环戊-2,3-二酚。
化合物4 C16H18O9,白色粉末。1H NMR (500 MHz, CD3OD) δ: 7.53 (1H, d, J=15.9 Hz, H-7), 7.04 (1H, d, J=2.0 Hz, H-2′), 6.97 (1H, dd, J=8.2, 2.0 Hz, H-6′), 6.75 (1H, d, J=8.2 Hz, H-5′), 6.23 (1H, d, J=15.9 Hz, H-8), 5.27 (1H, m, H-5), 4.11 (1H, m, H-3), 3.75 (1H, dd, J=7.6, 3.1 Hz, H-4), 2.22-2.17 (2H, m, H-2a, 6a), 2.13 (1H, dd, J=13.3, 8.3 Hz, H-2b), 2.02 (1H, dd, J=13.3, 6.6 Hz, H-6b); 13C NMR (125 MHz, CD3OD) δ: 38.0 (2C, C-2, 6), 70.4 (C-3), 72.0 (C-5), 72.6 (C-4), 75.9 (C-1), 115.1 (C-2′), 115.3 (C-8′), 116.5 (C-5′), 123.1 (C-6′), 127.2 (C-1′), 146.7 (C-3′), 147.3 (C-7′), 149.7 (C-4′), 168.6 (C-9′), 175.3 (C-7)。上述所有数据与参考文献(李树和刘玉衡,2015)比对相同,故鉴定化合物4为绿原酸。
化合物5 C17H20O9,灰白色粉末。HR-ESI-MS m/z: 369.0948 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 7.52 (1H, d, J=15.9 Hz, H-7), 7.05 (1H, d, J=1.9 Hz, H-2), 6.97 (1H, dd, J=8.2, 1.9 Hz, H-6), 6.75 (1H, d, J=8.2 Hz, H-5), 6.22 (1H, d, J=15.9 Hz, H-8), 5.33 (1H, m, H-3), 4.14 (1H, dd, J=6.6, 3.3 Hz, H-5), 3.74 (1H, dd, J=7.5, 3.1 Hz, H-4), 3.69 (3H, s, -OCH3), 2.24-2.01 (4H, m, H-2a, 2b, 6a, 6b); 13C NMR (125 MHz, CD3OD) δ: 37.8 (C-2), 38.0 (C-6), 53.0 (-OCH3), 70.4 (C-5), 72.0 (C-3), 72.6 (C-4), 75.9 (C-1), 115.0 (C-8′), 115.4 (C-2′), 116.3 (C-5′), 123.0 (C-6′), 127.2 (C-1′), 146.9 (C-3′), 147.2 (C-7′), 149.3 (C-4′), 168.6 (C-9′), 175.4 (C-7)。上述所有數据与参考文献(蒲首丞等,2010)比对相同,故鉴定化合物5为绿原酸甲酯。
化合物6 C21H18O12,淡黄色粉末。HR-ESI-MS m/z: 463.1262 [M+H]+。1H NMR (500 MHz, acetone-d6) δ: 8.16 (2H, d, J=8.9 Hz, H-2′, 6′), 6.93 (2H, d, J=8.9 Hz, H-3′, 5′), 6.56 (1H, d, J=1.9 Hz, H-8), 6.29 (1H, d, J=1.9 Hz, H-6), 5.46 (1H, d, J=7.6 Hz, H-1″), 3.87 (1H, d, J=9.7 Hz, H-5″), 3.67-3.50 (3H, m, H-2″, H-3″, 4″); 13C NMR (125 MHz, acetone-d6) δ: 72.4 (C-4″), 75.2 (C-2″), 76.4 (C-3″), 77.4 (C-5″), 94.3 (C-8), 99.9 (C-6), 104.1 (C-1″), 105.4 (C-10), 116.0 (2C, C-3′, 5′), 122.3 (C-1′), 132.2 (2C, C-2′, 6′), 135.3 (C-3), 158.0 (C-2), 158.4 (C-9), 161.0 (C-4′), 162.7 (C-5), 165.2 (C-7), 169.9 (C-6″), 178.9 (C-4)。上述所有数据与参考文献(Hari et al., 2017)比对相同,故鉴定化合物6为kaempferol 3-O-β-D-glucuronopyranoside。
化合物7 C32H38O19,黄色粉末。HR-ESI-MS m/z: 727.1855 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 8.06 (2H, d, J=8.8 Hz, H-2′, 6′), 6.92 (2H, d, J=8.8 Hz, H-3′, 5′), 6.43 (1H, d, J=2.1 Hz, H-8), 6.21 (1H, d, J=2.1 Hz, H-6), 5.41 (1H, d, J=7.5 Hz, H-1″), 4.78 (1H, d, J=7.0 Hz, H-1), 4.50 (1H, s, H-1″″), 1.09 (3H, d, J=6.2 Hz, H-6″″); 13C NMR (125 MHz, CD3OD) δ: 17.8 (C-6″″), 66.6 (C-5), 68.1 (C-6″), 69.7 (C-5″″), 71.0 (C-4″), 71.4 (C-4), 72.1 (C-3″″), 72.3 (C-2″″), 73.9 (C-4″″), 74.7 (C-2), 76.9 C-3), 77.1 (C-5″), 78.2 (C-3″), 82.0 (C-2″), 94.2 (C-8), 99.5 (C-6), 100.7 (C-1″), 102.4 (C-1″″), 105.2 (C-1), 105.7 (C-10), 116.2 (2C, C-3′, 5′), 122.9 (C-1′), 132.4 (2C, C-2′, 6′), 134.8 (C-3), 158.5 (C-2), 158.7 (C-9), 161.2 (C-4′), 163.4 (C-5), 165.7 (C-7), 179.5 (C-4)。上述所有数据与参考文献(Li et al., 2008)比对相同,故鉴定化合物7为kaempferol 3-O-{β-D-xylopyranosyl-(1→2)- [α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside}。
化合物8 C21H18O13,黄色粉末。HR-ESI-MS m/z: 479.0548 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 7.66 (1H, d, J=2.2 H-2′), 7.64 (1H, dd, J=8.6, 2.2 Hz, H-6′), 6.87 (1H, d, J=8.6 Hz, H-5′), 6.38 (1H, d, J=2.0 Hz, H-8), 6.19 (1H, d, J=2.0 Hz, H-6), 5.34 (1H, d, J=7.7 Hz, H-1″), 3.76 (1H, d, J=9.7 Hz, H-5″), 3.61-3.46 (3H, m, H-2″, 3″, 4″); 13C NMR (125 MHz, CD3OD) δ: 72.8 (C-4″), 75.4 (C-2″), 77.0 (C-3″), 77.6 (C-5″), 94.8 (C-8), 100.0 (C-6), 104.2 (C-1″), 105.6 (C-10), 116.0 (C-2′), 117.3 (C-5′), 122.8 (C-6′), 123.5 (C-1′), 135.4 (C-3), 145.9 (C-3′), 150.2 (C-4′), 158.1 (C-2), 159.1 (C-9), 162.6 (C-5), 165.9 (C-7), 172.3 (C-6″), 179.2 (C-4)。上述所有数据与参考文献(Du et al., 2010)比对相同,故鉴定化合物8為quercetin 3-O-β-D-glucuronopyranoside。
化合物9 C22H20O13,黄色无晶形粉末。HR-ESI-MS m/z: 493.0742 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 7.60 (1H, d, J=2.2 H-2′), 7.56 (1H, dd, J=8.5, 2.2 H-6′), 6.83 (1H, d, J=8.5 Hz, H-5′), 6.40 (1H, br s, H-8), 6.20 (1H, br s, H-6), 5.25 (1H, d, J=7.8 Hz, H-1″), 3.80 (1H, d, J=9.7 Hz, H-5″), 3.68 (3H, s, 6″-OCH3), 3.61-3.47 (3H, m, H-2″, 3″, 4″); 13C NMR (125 MHz, CD3OD) δ: 52.9 (6″-OCH3), 72.7 (C-4″), 75.3 (C-2″), 77.0 (C-5″), 77.3 (C-3″), 94.5 (C-8), 99.9 (C-6), 104.6 (C-1″), 105.5 (C-10), 115.9 (C-5′), 117.3 (C-2′), 122.8 (C-1′), 123.5 (C-6′), 135.4 (C-3), 145.9 (C-3′), 149.8 (C-4′), 158.1 (C-9), 159.3 (C-2), 162.9 (C-5), 166.4 (C-7), 170.9 (C-6″), 179.2 (C-4)。上述所有数据与参考文献(He et al., 2017)比对相同,故鉴定化合物9为quercetin 3-O-β-glucuronide-6″-methyl ester。
化合物10 C27H30O16,淡黄色粉末。HR-ESI-MS m/z: 611.1935 [M+H]+。1H NMR (500 MHz, acetone-d6) δ: 7.72 (1H, d, J=2.2 Hz, H-2′), 7.67 (1H, dd, J=8.5, 2.2 Hz, H-6′), 6.93 (1H, d, J=8.5 Hz, H-5′), 6.49 (1H, d, J=2.1 Hz, H-8), 6.31 (1H, d, J=2.1 Hz, H-6), 5.12 (1H, d, J=7.3 Hz, H-1″), 4.53 (1H, d, J=1.8 Hz, H-1), 1.06 (3H, d, J=6.5 Hz, H-6), 3.74-3.30 (10H, m, H-2″, 3″, 4″, 5″, 6a″, 6b″, 2, 3, 4, 5); 13C NMR (125 MHz, acetone-d6) δ: 17.7 (C-6), 67.5 (C-6″), 69.0 (C-5), 70.3 (C-4″), 71.2 (C-3), 71.6 (C-2), 73.0 (C-4), 74.9 (C-3″), 76.3 (C-2″), 77.4 (C-5″), 94.5 (C-8), 99.9 (C-6), 101.7 (C-10), 104.4 (C-1), 104.8 (C-1″), 115.8 (C-5′), 117.4 (C-2′), 122.3 (C-1′), 123.1 (C-6′), 135.0 (C-3), 145.2 (C-3′), 149.2 (C-4′), 157.7 (C-2), 158.6 (C-5), 161.9 (C-9), 165.3 (C-7), 178.6 (C-4)。上述所有数据与参考文献(陈秋虹等,2017)比对相同,故鉴定化合物10为芦丁。
化合物11 C27H30O16,淡黄色粉末。HR-ESI-MS m/z: 611.1472 [M+H]+。1H NMR (500 MHz, CD3OD) δ: 7.67 (1H, d, J=1.9 Hz, H-2′), 7.61 (1H, dd, J=8.5, 1.9 Hz, H-6′), 6.85 (1H, d, J=8.5 Hz, H-5′), 6.40 (1H, d, J=1.9 Hz, H-8), 6.21 (1H, d, J=1.9 Hz, H-6), 5.11 (1H, d, J=7.7 Hz, H-1″), 4.52 (1H, br s, H-1), 3.80 (1H, d, J=10.9 Hz, H-6a″), 3.63 (1H, m, H-3), 3.52-3.26 (8H, m, H-2″, 3″, 4″, 5″, 6b″, 2, 4, 5); 13C NMR (125 MHz, CD3OD) δ: 17.9 (C-6), 68.6 (C-6″), 69.7 (C-5), 71.4 (C-4″), 72.1 (C-3), 72.3 (C-2), 73.9 (C-4), 75.7 (C-2″), 77.2 (C-5″), 78.2 (C-3″), 94.9 (C-8), 100.0 (C-6), 102.4 (C-1), 104.6 (C-1″), 105.8 (C-10), 116.1 (C-5′), 117.9 (C-2′), 123.1 (C-1′), 123.6 (C-6′), 135.6 (C-3), 145.9 (C-4′), 149.8 (C-3′), 158.7 (C-9), 159.1 (C-2), 163.0 (C-7), 166.4 (C-5), 179.1 (C-4)。上述所有数据与参考文献(Bina et al., 2012)比对相同,故鉴定化合物11为quercetin 5-O- [α-L-rhamnopyranosyl- (1→6)]-β-D-glucopyranoside。
3 讨论
本研究从瓦山锥干燥的树叶醇提取物中分离鉴定了11个植物多酚类成分,包括1个没食子酸类化合物、2个奎宁酸类化合物、2个咖啡酸类化合物、6个黄酮类化合物,所有化合物均为首次从瓦山锥植物中分离得到。本课题组系统地进行了广西常见的壳斗科植物南岭栲、红栲、甜槠、饭甑青冈、钩锥、瓦山锥等植物的化学成分研究,从南岭栲、红栲、甜槠、钩锥中得到锥属植物中特有的特征性三萜鞣花单宁成分,为在经典分类中系统位置有争议的壳斗科植物锥属的系统位置提供了直接的化学证据。瓦山锥中暂未分离得到特征性三萜鞣花单宁成分,但含有大量原花青素类多聚体化合物。此类多聚体多为结构相近、极性相似的化合物,较难分离,本实验通过Sephadex LH-20、Chromatorex C18等柱色谱反复分离提取得到三个此类化合物,目前化合物的结构正在鉴定当中。本实验为植物多酚類成分的分离纯化积累经验,为瓦山锥植物的合理开发与可持续利用提供了科学依据。
参考文献:
BINA SS, NASIMA K, SABIRA B, et al., 2012. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity [J]. Phytochemistry, 77: 238-244.
CHEN QH, HUANG Y, ZHOU JJ, et al., 2017. Chemical constituents from leaves of Camellia nitidissima var. longistyla (II) [J]. Chin Trad Herb Drugs, 48(23): 4845-4850. [陈秋虹, 黄艳, 周洁洁, 等, 2017. 长柱金花茶叶的化学成分研究(II) [J]. 中草药, 48(23): 4845-4850.]
Chinese Botanical Society Editorial Board of Chinese Academy of Sciences, 1998. Flora Reipublicae Popularis Sinicae [M]. Beijing: Science Press, 22:13, 69. [中国科学院中国植物志编辑委员会, 1998. 中国植物志 [M]. 北京: 科学出版社, 22: 13, 69.]
DU ZZ, YANG XW, HAN H, et al., 2010. A new flavone C-glycoside from Clematis rehderiana [J]. Molecules, 5(1): 15: 672-679.
HARI PD, SENA M, SHOJI Y, 2017. Amentoflavone and kaempferol glycosides from the aerial parts of Cissampelos pareira [J]. Biotechnology, 5(1): 1-4.
HE ZN, LIAN WW, LIU JW, et al., 2017. Isolation, structural characterization and neuraminidase inhibitory activities of polyphenolic constituents from Flos caryophylli [J]. Phytochem Lett, 19: 160-167.
Jiangsu New Medical School, 1977. Traditional Chinese Medicine Dictionary: The first and the second [M]. Shanghai: Shanghai Peoples Publishing House. [江苏新医学院, 1977, 中药大辞典(上、下两册) [M]. 上海:上海人民出版社.]
LI CD, BEI LW, JIAN MC, 2008. Flavonoid triglycosides from the seeds of Camellia oleifera Abel [J]. Chin Chem Lett, 19: 1315-1318.
LI S, LIU YH, 2015. Chemical constituents with antioxidative activity from the flower buds of Lonicera serreana [J]. Guihaia, 35(4): 586-589. [李树, 刘玉衡, 2015. 毛药忍冬花蕾抗氧化活性部位化学成分研究 [J]. 广西植物, 35(4): 586-589.]
PU SC, GUO YQ, GAO WY, et al., 2010. Chemical constituents from Hydrocotyle sibthorpioides [J]. Chin Trad Herb Drugs, 41(9): 1440-1442. [蒲首丞, 郭远强, 高文远, 2010. 天胡荽化学成分的研究 [J]. 中草药, 41(9): 1440-1442.]
WANG YF, HUANG YL, LIU JL, et al., 2016. Content and antioxidant capacity of polyhenols from the Fagaceae plants [J]. Guangxi Sci, 23(2):180-183. [王亚凤, 黄永林, 刘金磊, 等, 2016. 壳斗科植物种子的多酚类含量及抗氧化能力 [J]. 广西科学, 23(2):180-183.]
WEI H, YANG JW, YAN XJ, et al., 2018. Chemical constituents from walnut green husk: Phenols [J]. Guihaia, 38(4): 463-468. [魏欢, 杨建文, 颜小捷, 等, 2018. 核桃青皮的化学成分研究——酚类化合物 [J]. 广西植物, 38(4): 463-468.]
XU J, ZHANG TJ, GONG SX, et al., 2010. Chemical constituents from the effective hemostatic of Cirsium setosum [J].Chin Trad Herb Drugs, 41(4): 542-544. [许浚, 张铁军, 龚苏晓, 等, 2010. 小蓟止血活性部位的化学成分研究 [J]. 中草藥, 41(4): 542-544.]
ZHU TF, LI P, SUN QW, et al., 2019. Chemical constituents from leaves of Sabia parviflora [J]. Guihaia, 39(4): 511-515. [朱仝飞, 李萍, 孙庆文, 等, 2019. 小花清风藤叶的化学成分研究 [J]. 广西植物, 39(4): 511-515.]
(责任编辑 何永艳)
