丙种球蛋白无反应型川崎病免疫指标的变化分析
2020-02-22赵雅淇黄宏琳
赵雅淇 黄宏琳


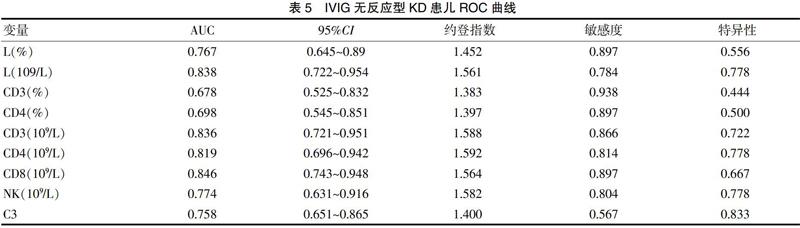
[摘要] 目的 探討丙种球蛋白(IVIG)无反应型川崎病(KD)患儿急性期淋巴细胞亚群及免疫球蛋白的变化及临床价值。 方法 回顾分析2018年7月~2020年5月厦门市儿童医院收治的115例KD患儿临床资料,IVIG无反应型18例,IVIG敏感型97例,正常对照组27例,应用单因素分析KD组与对照组、IVIG无反应组与IVIG敏感组患儿各实验室指标的变化,应用多元逐步回归分析及受试者工作特征曲线(ROC)分析筛查IVIG无反应型KD的独立危险因素并预测其临床效能。 结果 KD组外周血白细胞(WBC)、淋巴细胞(L)计数、CD19百分比、CD4、CD19绝对值、CD4/CD8比值、补体C3均高于对照组,KD组L比例、CD3、CD8、NK百分比、IgA均低于对照组,差异有统计学意义(P<0.05)。IVIG无反应组WBC、L比例及计数、CD3、CD4百分比、CD3、CD4、CD8、CD19、NK绝对值、补体C3均低于IVIG敏感组,差异有统计学意义(P<0.05)。CD3绝对值及补体C3降低为IVIG无反应型KD的独立危险因素,且CD3≤1.588×109/L预测IVIG无反应的灵敏度为86.6%、特异度为72.2%,ROC曲线下面积为0.836,补体C3≤1.40 g/L预测IVIG无反应的灵敏度为56.7%、特异度为83.3%,ROC曲线下面积为0.758。 结论 外周血淋巴细胞亚群和补体C3可作为早期识别IVIG无反应型KD的实验室指标,并为探索难治型KD的治疗提供依据。
[关键词] 川崎病;丙种球蛋白无反应;淋巴细胞亚群;免疫功能
[中图分类号] R725.4 [文献标识码] B [文章编号] 1673-9701(2020)32-0075-06
[Abstract] Objective To investigate the changes and clinical value of lymphocyte subpopulation and immunoglobulin in children with intravenous immune globulin (IVIG) resistant Kawasaki disease (KD) at acute phase. Methods A total of 115 children with KD admitted to Xiamen Children's Hospital from July 2018 to May 2020 were retrospectively analyzed. Among them, there were 18 cases with resistant IVIG, 97 cases with sensitive IVIG, and 27 cases with the normal control group. The changes of laboratory indexes in the KD group and the control group, the IVIG resistant group and the IVIG sensitive group were analyzed by univariate analysis, and the independent risk factors for KD in resistant IVIG group were screened by multivariate stepwise regression analysis and receiver operating characteristic curve (ROC) analysis to predict its clinical efficacy. Results The peripheral white blood cell (WBC), lymphocyte (L) count, CD19 percentage, CD4, absolute values of CD19, CD4/CD8 ratio and complement C3 in KD group were all higher than those in the control group. The L ratio, CD3, CD8, NK percentage and IgA in the KD group were all lower than those in the control group, and the differences were statistically significant(P<0.05). The WBC, L ratio and count, CD3, CD4 percentage, CD3, CD4, CD8, CD19, the absolute value of NK, and complement C3 in the IVIG resistant group were all lower than those in the IVIG sensitive group, and the differences were statistically significant(P<0.05). The absolute value of CD3 and reduction of complement C3 were the independent risk factors for resistant IVIG KD, and the sensitivity and specificity of CD3≤1.588×109/L to predict IVIG resistant were 86.6% and 72.2%, respectively. The area under ROC curve was 0.836, and the sensitivity and specificity of complement C3≤1.40 g/L to predict IVIG resistant were 56.7% and 83.3%, respectively. The area under the ROC curve was 0.758. Conclusion Peripheral blood lymphocyte subpopulation and complement C3 can be used as laboratory indexes for early recognition of resistant IVIG resistant KD and provide the basis for exploring the treatment of refractory KD.
3 讨论
川崎病易引起严重冠状动脉损害,因此成为儿童后天心脏病的危险因素。外文报道,以IVIG作为一线药物的免疫、炎症性疾病多为难治性疾病[6],其中对首剂大剂量IVIG无反应的KD患儿是并发CAL的主要群体,因此急性期通过异常指标识别IVIG无反应型KD、了解其免疫作用机制,可指导临床早期联合其他免疫抑制剂治疗以降低CAL发生率,并为探索新型KD敏感靶标药物提供帮助。
KD本质是以全身血管炎性病变为主的免疫性疾病,大多学者认为[7],其机制与细胞、体液免疫应答异常导致调控血管内皮的炎症细胞因子失衡有关。本研究发现,与对照组相比,KD组淋巴细胞计数、CD4绝对值、CD4/CD8比值、CD19百分比与绝对值显著升高,而CD3、CD8百分比明显降低,提示KD急性期存在T淋巴细胞及B淋巴细胞的异常激活,这可能成为KD患儿后期血管损伤的始动环节。
国外文献指出[8-9],T淋巴细胞主要包括调节性T细胞和辅助性T细胞,在特定条件下分别发挥代偿性减轻致病物质反应的抗炎症介质作用,以及导致肿瘤坏死因子(Tumor necrosis factor-α,TNF-α)、白细胞介素(Interleukin,IL)等炎症细胞因子、介质释放,引起血管内皮炎性病变的细胞毒性作用[10]。目前认为,调节性 T 细胞在调控和维持机体自身免疫耐受中发挥重要作用,FOXP3作为特异性的转录因子,表达减少或突变均可导致调节性 T细胞减少或功能障碍[11]。既往文献指出,重症肌无力等自身免疫性疾病活动期CD4+CD25+FOXP3+T细胞表达显著低于恢复期及健康儿童,而免疫抑制剂糖皮质激素和IVIG治疗后调节性T细胞比例明显上升[12]。针对KD的研究也发现IVIG治疗KD的主要机制与降低淋巴细胞凋亡、增强调节性T淋巴细胞功能有关[13]。Yu等[14]检测KD患儿CD4细胞中CD25、FOXP3的表达,结果显示缺乏CD4(+)CD25(+)FOXP3(+)调节性T细胞的患儿更易对IVIG治疗无反应。本研究结果表明,IVIG无反应组白细胞计数、淋巴细胞比例及计数、CD3、CD4百分比、CD3、CD4、CD8绝对值较IVIG敏感组降低,推测KD早期IVIG无反应组调节性T细胞比例低于IVIG敏感组,首剂IVIG治疗不能有效地延缓淋巴细胞凋亡、加强调节性T淋巴细胞功能,因此不能在急性期抑制炎症介质生成、降低炎症反应,进一步说明,在KD早期,IVIG无反应与T淋巴细胞抗炎、促炎双作用的失衡相关。
本研究还发现,尽管KD急性期B淋巴细胞异常活化,但IVIG无反应组较IVIG敏感组CD19绝对值明显降低、CD19百分比无明显差异,提示IVIG无反应患儿并不存在较IVIG敏感患儿更活跃的体液免疫应答反应,KD早期IVIG能有效结合B细胞受体并激活B细胞抑制通道,加速B细胞功能失调[15],推断B淋巴细胞紊乱对IVIG无反应影响可能不大。既往研究在KD早期损伤的冠状动脉壁可见大量NK细胞聚集浸润[16]。本研究结果显示,KD组NK细胞百分比较对照组明显下降,且IVIG无反应组NK绝对值较IVIG敏感组明显下降,推测KD急性期NK细胞过度消耗以参与血管炎症损伤,且IVIG无反应组NK细胞消耗更明显。
免疫球蛋白和补体研究结果显示,KD组IgA水平较对照组明显降低,与既往文献发现的IgA浆细胞易促成KD抗体产生的结论不相符[17],考虑系对照组个案数偏少,建议后期扩大样本量进一步验证。而免疫球蛋白在IVIG无反应组和IVIG敏感组间表达差异不显著。补体C3在KD组明显高于对照组,与CD19在急性期的表达一致,表明体液免疫在KD发病机制中发挥作用。外国文献指出,IVIG有明显的补体抑制作用,且呈剂量依赖性[18],因此在一般KD患儿早期能有效抑制体液免疫的异常激活。但补体C3在IVIG无反应组明显低于IVIG敏感组,且是IVIG无反应的独立危险因素,推测可能与补体C3参与某种损伤机制消耗增加或基因变异有关[19,20],但目前无IVIG无反应KD相关文献报道。此外,KD组白细胞计数、淋巴细胞计数显著升高但淋巴细胞比例显著降低,提示除细胞免疫抑制外,还有其他中性粒细胞相关因子参与免疫炎症反应[21],与小林评分中将中性粒细胞百分比≥80%作为IVIG无反应及KD合并CAL的预测指标相符[22]。
至今KD患儿IVIG无反应的病因及机制尚不明确,研究表明可能与遗传易感性有关。陈丽琴等[23]通过靶向捕获测序技术发现,CARD11、CHUK等T细胞受体信号通路相关基因与IVIG无反应有关,此外KIR2DS4及GZMB的位点基因型与IVIG疗效相关,其中杀伤细胞免疫球蛋白样受体(Killer cell immunoglobulin-like receptors,KIRs)是由T细胞和NK细胞表达的跨膜糖蛋白,而GZMB基因编码的蛋白能被毒性T细胞和NK细胞活化,诱导靶细胞凋亡[24]。本研究也显示IVIG,无反应与淋巴细胞特别是T淋巴细胞、NK细胞功能失衡相关,进一步证实了免疫紊乱在IVIG无反应KD中的作用。
本研究结果表明,IVIG无反应KD患儿与IVIG敏感型及健康儿童间最显著的差异是淋巴细胞比例及计数,CD3、CD4百分比、CD3、CD4、CD8、NK绝对值,以及补体C3,其曲线下面积均大于0.5,说明以上实验指标对IVIG无反应KD均有较高的预判价值,且CD3绝对值及补体C3降低是IVIG无反应的独立危险因素。
本研究的不足之处,IVIG无反应病例数偏少,这主要与排除了所有IVIG診断性治疗的疑似病例以及部分数据不完整的确诊病例相关,但与IVIG无反应发生率也相吻合,另外补体C3在IVIG无反应KD中的机制尚无有力文献可依,有待后续收集更多样本数据进一步探讨。
[参考文献]
[1] Newburger JW,Takahashi M,Burns JC.Kawasaki disease[J].J Am Coll Cardiol,2016,67(14):1738-1749.
[2] Lin MT,Sun LC,Wu ET,et al.Acute and late coronaryoutcomes in 1073 patients with Kawasaki disease with andwithout intravenous γ-immunoglobulin therapy[J].Arch Dis Child,2015,100(6):542-547.
[3] 谢利剑,黄敏.川崎病诊治的新观念[J].临床儿科杂志,2015,33(7):675-677.
[4] Mccrindle BW,Rowley AH,Newburger JW,et al.Diagnosis,treatment,and long-term management of Kawasaki disease:a scientific statement for health professionals From the American Heart Association[J].Circulation,2017, 135(17):e927-e999.
[5] 富洋,刘芳.静脉丙种球蛋白无反应型川崎病的早期识别与治疗进展[J].国际儿科学杂志,2018, 5:333-337.
[6] Galeotti C,Kaveri SV,Bayry J.Molecular and immunologicalbiomarkers to predict IVIg response[J].Trends Mol Med,2015,21(3):145-147.
[7] Kuo HC,Yang KD,Chang WC,et al.Kawasaki disease:an update on diagnosis and treatment[J].Pediatr Neonatol,2012,3:4-11.
[8] Jia S,Li C,Wang G,et al.The T helper type 17/regulatory Tcell imbalance in patients with acute Kawasaki disease[J].Clin Exp Immunol,2010,162(1):131-137.
[9] Yujia Wang,Wei Wang,Fangqi Gong,et al.Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease[J].Arthritis&Rheumatism,2013,65:805-814.
[10] 马乐,杜忠东.川崎病血管内皮细胞损伤机制的研究进展[J].中华儿科杂志,2016,54(2):158-160.
[11] Sakaguchi S,Mikami N,Wing JB,et al.Regulatory T Cells and Human Disease[J].Annu Rev Immunol,2020, 38:541-566.
[12] 许文华,任明山,王芳,等.重症肌无力患者外周血中调节性T细胞的变化及免疫抑制剂对调节性T细胞的影响[J].中国临床保健杂志,2013,16(5):479-481+562.
[13] Franco A,Shimizu C,Tremoulet AH,et al.MemoryT-cells and characterization of peripheral T-cell clones in acute Kawasaki disease[J].Autoimmunity,2010,43(4):317-324.
[14] Yu Hirabayashi,Yoshiyuki Takahashi.Lack of CD4+CD25+FOXP3+ regulatory T cells is associated with resistance to intravenous immuno globulin therapy in patientswith Kawasaki disease[J].Eur J Pediatr,2013,172:833-837.
[15] Xu M,Jiang Y,Wang J,et al.Distinct variations of antibody secreting cells and memory B cells during the course of Kawasaki disease[J].BMC Immunol,2019,20(1):16.
[16] Charles Jennette,Janiece Sciarrotta,Kei Takahashi,et al.Predominance of monocytes and macrophages in the inflammatory infiltrates of acute Kawasaki disease arteritis[J].Pediatrics Research,2003,53:173.
[17] Rowley AH.Kawasaki disease:novel insights into etiologyand genetic susceptibility[J]. Annu RevMed,2011, 62:69-77.
[18] Watanabe J,Scornik JC.IVIG and HLA antibodies. Evidence for inhibition of complement activation but not for anti-idiotypic activity[J].Am J Transplant,2005,5(11):2786-2790.
[19] Petri MA,Conklin J,O'Malley T,Dervieux T.Platelet-bound C4d,low C3 and lupus anticoagulant associate with thrombosis in SLE[J].Lupus Sci Med,2019,6(1):e000318.
[20] Rhodes B,Hunnangkul S,Morris DL,et al.The heritability and genetics of complement C3 expression in UK SLE families[J].Genes Immun,2009,10(5):525-530.
[21] Zenshiro Onouchi,Kenji Hamaoka.Neutropenia in the acute phase of Kawasakidiseaseand prevention of coronary artery aneurysm[J].Pediatrics International,2009,51:448-452.
[22] Tohru Kobayashi,Yoshinari Inoue,Kazuo Takeuchi,et al.Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease[J].Circulation,2006,113:2606-2612.
[23] 陳丽琴,宋思瑞,张晗,等.川崎病丙种球蛋白无反应型易感基因研究[J].临床儿科杂志,2019,10:721-726.
[24] Burns JC,Franco A.The immunomodulatory effects ofintravenous immunoglobulin therapy in Kawasaki disease[J].Expert Rev Clin Immunol,2015,11(7):819-825.
(收稿日期:2020-07-12)
