基于吡啶基三唑配体的镉配位聚合物的合成、结构和荧光性质
2020-02-18冰张淑慧杜继远孙
王 冰张淑慧杜继远孙 琳
(河南大学 化学化工学院,河南 开封475004)
In recent years, coordination polymers, which linked to metal centers, have been shown in the field of magnetism, gas storage, ion exchange,photocatalysis, biotechnology chemistry, etc.[1-5]Depending on the structure and architecture of coordination polymers (CPs), they have different applications and features. It is known that some kinds of aromatic carboxylic acids and their derivatives have widely been used as ligands to construct intriguing CPs with various architectures. Organic linkers are important in the design and synchronization of CPs with the desired functionality. Polycarboxylic acids play an important role in the construction of CPs, in which the carboxyl⁃rich coordination mode can meet the geometric requirements of different metal centers[6-11].
Luminescent materials based on CPs have been extensively investigated since the initial discovery of a bright and stable emission of tris⁃(8⁃hydroxyquinolinato)aluminum(III) (Alq3)[12]. Luminescent metal complexes have advantages such as the combination of emitting an electron transferring roles,higher environmental stability,and a better extent of diversity that is achievable through tuning of electronic properties by under structural and metal⁃center variability[13]. In recent years, a series of luminescent materials based on CPs and cadmium ions have been prepared and discussed. In 2017, YAN and coworkers reported a new Cd (II) CP, and the enhancement and the redshifts of luminescence emission maxima may be due to the chelating and bridging effects of the relevant ligands to the metal atoms, which effectively increases the rigidity and conjugation upon metal coordination and then affects the loss of energy by a radiationless pathway[14]. In 2019, MAJUMDAR and coworkers have explored the steady⁃state and time⁃resolved fluorescence properties of a bi⁃nuclear Cd(II)CP in solid⁃state conditions at room temperature which exhibits bi and tri⁃exponential decay. The fluorescence behaviors are predominantly intra⁃ligand in nature (π→π∗) with lifetimes in the range (1.16 -1.11 ns)[15].ZHANG and coworkers communicated a new bi⁃nuclear Cd(II) CP, which has some effects on the fluorescence properties to accelerate the quenching rate of Fe3+[16].
In view of the above considerations, as a continuation of our research in these studies[17-19], we have employed 1H⁃3⁃(3⁃pyridyl)⁃5⁃(3′⁃pyridyl)⁃1,2,4⁃triazole ( 3, 3′⁃Hbpt ) and 1, 2, 4, 5⁃benzenetetracarboxylic acid (H4bta) with cadmium transition metal ions to construct one cadmium based{[(3,3′⁃Hbpt)Cd(bta)0.5(H2O)]·(H2O)}n(1).Compound 1 was further characterized by X⁃ray single⁃crystal diffraction, elemental analysis, infrared spectroscopy ( IR), and power X⁃ray diffraction(PXRD). Structural analyses show that compound 1 displays Cd2+ions are complex and regular structure.The solid⁃state photoluminescence of 1 also has been investigated at room temperature.
1 Experimental
1.1 Material and physical measurements
The chemical reagents were of analytical grade and used as purchased without further purification.Infrared spectroscopy (IR) was performed in KBr pellets using a Brooke VERTEX 70 Fourier infrared spectrometer with a measurement range of 4 000 to 500 cm-1. Elemental analyses (C and H) were carried out on an Elementar Vario Elcube CHNS analyzer. The powder X⁃ray diffraction ( PXRD) patterns were recorded on a Bruker D8 ADVANCE instrument with Cu Kαradiation (λ=0.154 056 nm). The solid⁃state fluorescence spectroscopy was performed on a FSL980 fluorescence spectrophotometer at room temperature.
1.2 Synthesis of 1
3,3′⁃Hbpt (0.1 mmol), Cd(CH3COO)2·4H2O(0.1 mmol) and H4bta (0.1 mmol) were mixed in 7 mL distilled water. The light white solution was prepared,heated by hydrothermal method at 150 ℃for 4 h, and then filtered left for about one week at ambient temperature to give suitable light white block crystals for X⁃ray structure determination. The yield was 30%. Elemental analysis data (%), theoretical value: C, 41.07; H, 2.82; N, 14.09; O, 19.33;Cd, 22,63. Experimental value: C,41.10; H, 2.80;N, 14.07; O, 9.79; Cd, 22.72.
2 Results and discussion
2.1 Crystal structure
Single crystal X⁃ray analysis showed that 1 crystallizes in the monoclinic system, with space groupP21/n(Table 1). The relative geometric parameters are listed in Tables 2 and 3,respectively. As shown in Fig.1, Cd1 situates in a distorted pentagonal bipyramidal coordination geometry completed, the ligand atoms around the Cd1 center are the carboxyl oxygen atoms of adipic acid and the pyridine nitrogen atoms from the 3,3′⁃Hbpt ligand, respectively. The bond of Cd⁃O ranges from 0.232 2(2) to 0.251 1(2) nm, while the bond length of Cd⁃N ranges from 0.233 6(3) to 0.235 7(3)nm, and the bond length of Cd⁃C is 0.274 4(3) nm.The mental ions are bridged by 3,3′⁃Hbpt ligands to form a ring as shown in Fig.2. Compound 1 is additionally interlinked to give a 1D coordination polymers chains running along theb⁃axis (Fig.3). Fig.4, which can also be further simplified to gwg topology if the ligands are considered as linkers.
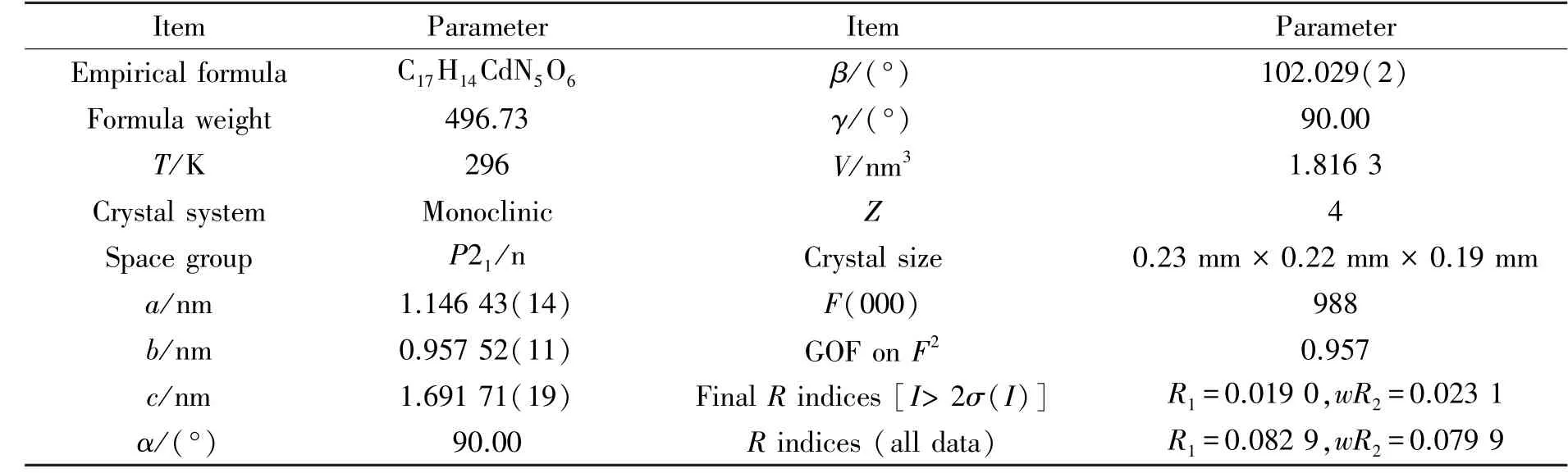
Table 1 Crystallographic data and structure refinement parameters for 1
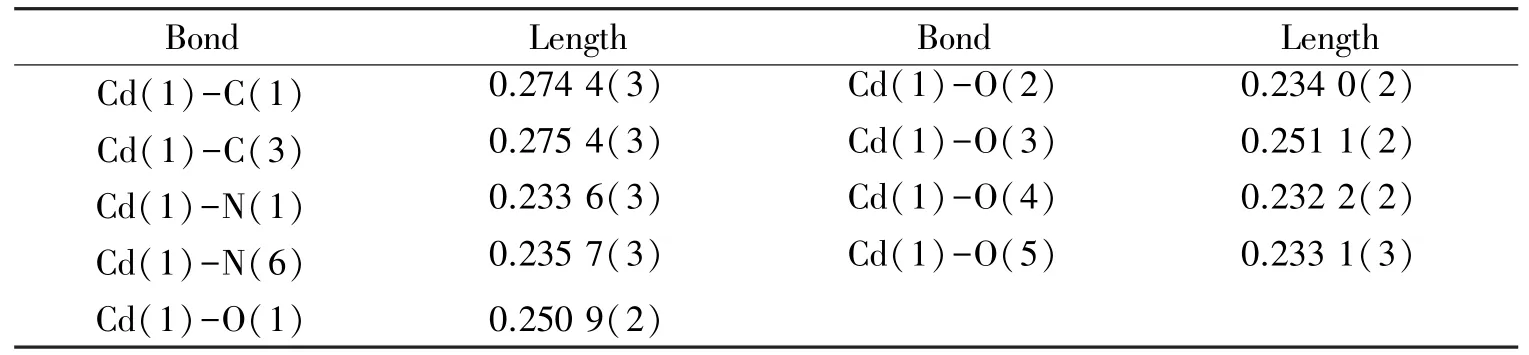
Table 2 Selected bond lengths (nm) of compound 1
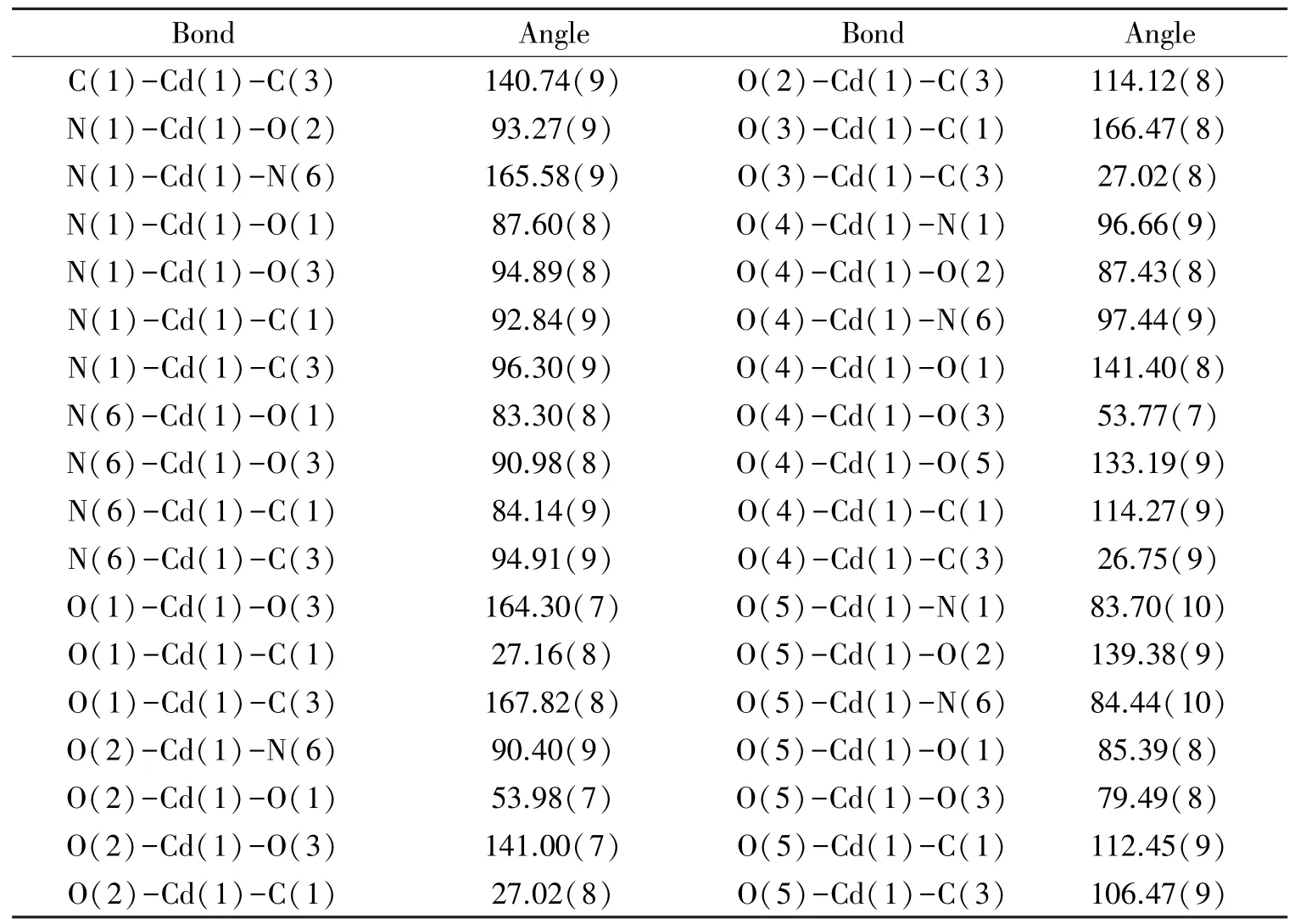
Table 3 Selected bond angles (°) of compound 1
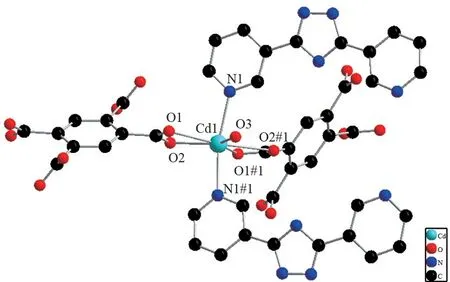
Fig.1 Coordination environment of the Cd(II) atom in compound 1
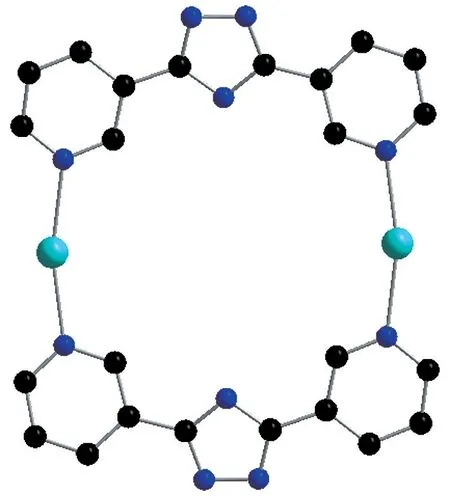
Fig.2 Ring unit in 1
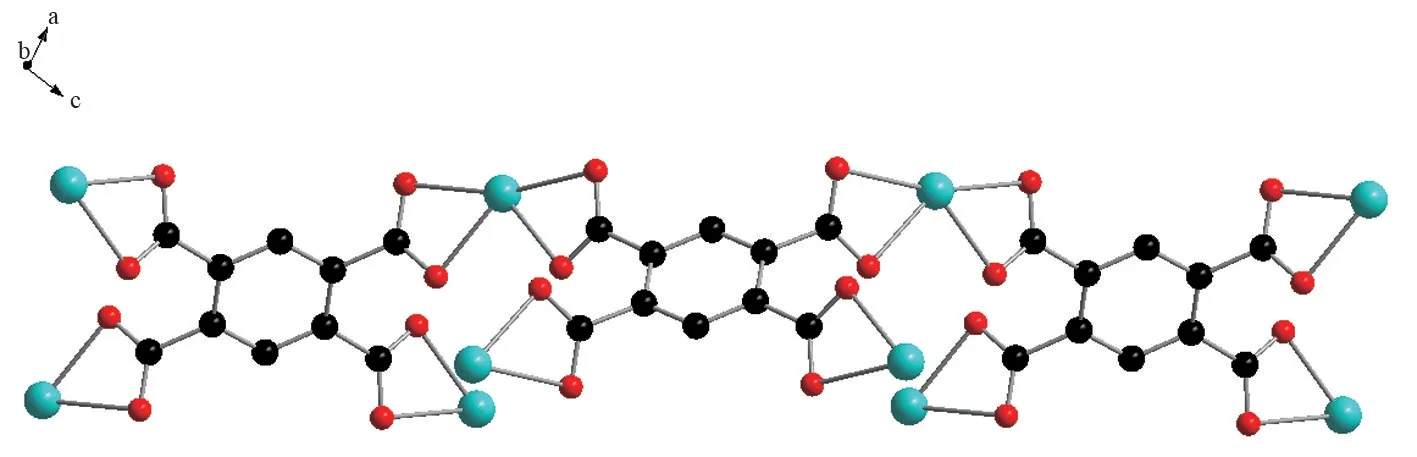
Fig.3 Simplified chain structure of 1
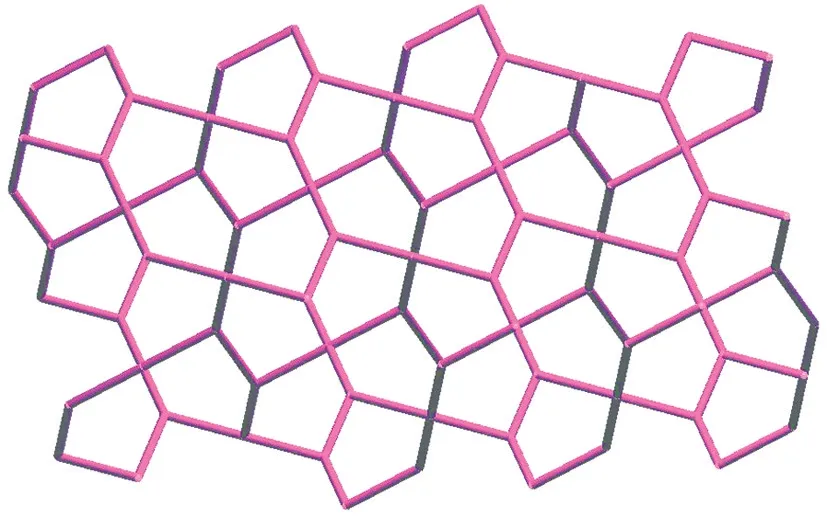
Fig.4 A drawing showing the gwg topology of 1
2.2 PXRD patterns
The purity of complex 1 was determined by PXRD in the range of 5° ≤2θ≤50°. As shown in Fig.5,the experimental PXRD patterns of 1 are in good agreement with the simulated PXRD patterns of 1 that originated from the single⁃crystal X⁃ray diffraction structural analyses, indicating that the experimental samples are in good phase purity.
2.3 Fluorescence analysis
Metal ions with d10electron configurations, such as Cu(I), Ag(I), Zn(II), and Cd(II), as well as complexes of unsaturated heterocyclic⁃ligands, usually exhibit interesting fluorescence properties[20-22].Therefore, the fluorescence properties of compound 1 were preliminarily studied.
The solid⁃state fluorescence spectrum of 1 has been investigated under an excitation wavelength of 350 nm at room temperature. As shown in Fig. 6, the emission spectrum of 1 had a small acromion peak at 406 nm and a maximum emission band at 433 nm. To further explain the essence of the emission band,fluorescence measurements were performed on solid samples of 3,3′⁃Hbpt ligand. The shape of 3,3′⁃Hbpt ligand is similar to that of compound 1, and the emission peak appears at 410 nm and 464 nm. It can be seen that the emission band of compound 1 is due to the transition in the 3,3′⁃Hbpt ligand, and the red shift in the emission peak position compared to the ligand, which should be caused by the metal⁃ligand charge transfer (MLCT). In 1, Cd2+ions are bridged by H4bta and 3,3′⁃Hbptligand to form a complex structure. Furthermore, the solid⁃state fluorescence spectrum of 1 also has been investigated, indicating that 1displays characteristic emissions of 3,3′⁃Hbpt ligand.
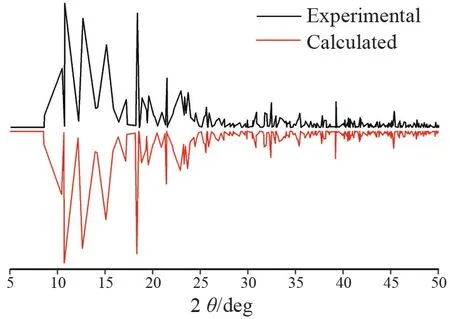
Fig.5 PXRD of compound 1
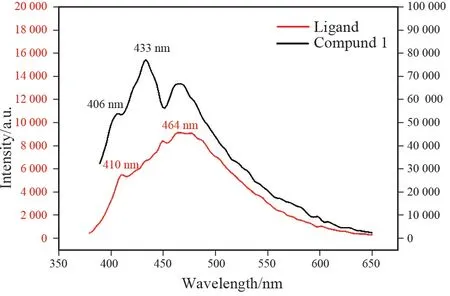
Fig.6 Solid⁃state fluorescence spectrum of 3,3′⁃Hbpt ligand (excitation wavelength: 370 nm)and 1 (excitation wavelength: 350 nm)
3 Conclusions
In summary, one cadmium⁃based CP{[(3,3′⁃Hbpt) Cd (bta)0.5(H2O)] · (H2O)}n(1) was successfully synthesized by the reaction of 3, 3′⁃Hbptligand, H4bta and cadmium transition metal ions,which was further characterized by X⁃ray single⁃crystal diffraction, elemental analysis, and Power X⁃ray diffraction ( PXRD). The solid⁃state fluorescence spectrum of 1 has been investigated. The results indicate the emission band of 1 is due to the transition in the 3,3′⁃Hbpt ligand, and MLCT could be likely to cause red shift in the emission peak position compared to the ligand.
