一种新型铈嵌入的轮状四聚同多钨酸盐的合成与表征
2020-02-18刘肖依张琰罗沛佳李艳周
刘肖依张 琰罗沛佳李艳周
(河南大学 化学化工学院, 河南 开封475004)
Polyoxometalates (POMs) as an excellent class of anionic metal⁃oxygen clusters have appealed to increasing research enthusiasm in recent years on account of their structural diversities and potential applications in catalysis[1-3], medicine[4-5],magnetism[6-8], optics[9-10], electronic sensors[11-12].Isopolyoxotungstates (IPOTs), as a subset of POMs,have been greatly developed due to their fascinating physical properties and amazing structural features[13].For example, CRONIN's group reported two complicated {W11}⁃bridging “S”⁃shaped [H4W22O74]12-and “ §”⁃shaped [H10W34O116]18-IPOTs[14].On the base of the linkages of three ‘cis⁃edge⁃sharing'{W11} units, a threefold symmetric Celtic IPOT [H12W36O120]12-was also isolated by them[15]. Moreover,except for a gigantic [H64W200O684]104-, three very large and structurally diverse IPOTs [H12W48O164]28-{W48}, [H20W56O190]24-{W56} and [H12W92O311]58-{W92} were also obtained[16]. {W48} and {W56}clusters are dimeric species incorporating two {W21}units and {W56} species is the first example of a molecular metal oxide cluster containing a chiral“double⁃stranded” motif. Importantly, the synthesized IPOTs may be also subsequently used as building blocks to construct larger architectures or functional networks.
In this context, IPOTs can function as multidentate inorganic ligands to bind rare⁃earth (RE)cations forming functional materials, which have outstanding physical and chemical properties in luminescence,magnetism,catalysis,and electrochemistry[17]. Since the first series of RE⁃substituted IPOTs [ RE ( W5O18)2]9-were communicated by PEACOCK and WEAKLEY in 1971[18], only a few examples of RE⁃substituted IPOTs have been known including [Ce(H2O)(DMF)6(W10O32)][19], [H6Ce2(H2O)Cl(W5O18)3]7-[20], [RE4(WO4) (H2O)16{W7O22(O2)2}4]14-(RE =La3+,Pr3+)[21], [Ce2(H2O)6W22O72(OH)4]10-[22], [RE2(H2O)10W22O71(OH)2]8-(RE =La3+, Ce3+, Tb3+,Dy3+, Ho3+, Er3+, Tm3+, Yb3+, Lu3+, Y3+)[23]and[RE2(H2O)10W28O93(OH)2]14-(RE = Sm3+,Eu3+)[24]. However, the large ionic radius of the RE ions hinder them effectively to implant to lacunary sites of POM units. Hence, the creation of novel RE⁃substituted IPOTs remains a serious challenge. In this context, we have begun to synthesize neoteric RE⁃substituted IPOTs. In 2014, two types of oxalate⁃bridging tetra⁃RE substituted lacunary Lindqvist IPOTs{[RE2(C2O4)(H2O)4(OH)W4O16]2}10-and {[RE(C2O4) W5O18]4}20-(RE = Eu3+, Ho3+, Er3+,Tb3+), in which the double⁃oxalate⁃connecting di⁃RE encapsulated divacant Lindqvist dimers and single⁃oxalate⁃ connecting RE substituted monovacant Lindqvist tetramers are observed for the first time[25].Furthermore, by reacting Na2WO4·2H2O with RE(NO3)3·6H2O in the presence of dimethylamine hydrochloride in acidic aqueous solution, three types of novel RE⁃containing iso⁃POTs {[RE4(H2O)22W28O94H2]2}12-(RE =Pr3+, Nd3+, Sm3+), {[Eu(H2O)7]2[Eu(H2O)5]2[W22O74H2]}2-and {[RE(H2O)4][RE(H2O)5]2[W22O74H2]}5-(RE =Gd3+, Tb3+,Er3+, Tm3+, Yb3+, Lu3+) have been obtained[26].{[RE4(H2O)22W28O94H2]2}12-displays a 1⁃D chain alignment constructed from hexameric multi⁃RE containing {[RE4(H2O)22W28O94H2]2}12-units via[RE(H2O)5]3+linkers.
Herein, we aimed to extend the family of RE⁃substituted IPOTs using a combination of pH control and organic component ( 1, 2⁃diminopropane) to unravel the influence factors on the self⁃assembly of RE⁃substituted IPOTs. We demonstrate that it is possible to discover new cluster architectures in RE⁃substituted IPOTs by the one⁃step self⁃assembly strategy in acidic medium, thus, a tetrameric Ce3+⁃bridging tetrameric IPOT [ H2dap ]5Na2H24[ Ce(H2O)3W11O39]4·40H2O (1) (CCDC 2 008 588)at pH 4.5 was isolated. 1 can be isolated in good yield and was characterized by single⁃crystal X⁃ray diffraction, IR spectrum and thermal gravimetric analysis.
1 Experimental
1.1 Physical measurements
In order to obtain the structure information of 1,Nicolet FT⁃IR 360 spectrometer was used to measure the IR spectrum by using the pressed KBr pellets in the range from 4 000 to 400 cm-1. The thermodynamic stability of 1 was acquired from thermogravimetric(TG) analysis using a Mettler Toledo TGA/SDTA 851einstrument under N2atmosphere with 10 ℃·min-1as heating rate. A Bruker APEX⁃II CCD diffractometer at 296(2) K (Mo Kαradiation,λ= 0.071 073 nm,graphite monochromator) was used to record the single crystal diffraction data of 1.
1.2 Synthesis of 1
Na2WO4·2H2O (2.500 g, 7.579 mmol), K2TeO3(0.200 g,0.788 mmol),2⁃picolinic acid (0.050 g,0.406 mmol),oxalic acid (0.050 g, 0.555 mmol) and 1,2⁃diaminopropane (0.15 mL, 1.746 mmol) were dissolved in 20 mL of water. The pH of the solution was adjusted to 4.5 by a dilute HCl solution (6 mol·L-1). Then, Ce(NO3)3·6H2O (0.250 g,0.576 mmol) was successively added. The final pH was adjusted to 4.5 by a prepared NaOH solution (4 mol·L-1). The synthetic solution was stirred for 30 min at room temperature. After that, the resulting solution was heated in a water bath for two hours in 30 ℃. After slow evaporation at room temperature for about three days, yellow rod⁃shaped crystals of 1 were obtained. Yield:0.282 g (16% based on Ce(NO3)3·6H2O).
1.3 X⁃ray crystallography
In order to get the single⁃crystal structure information of 1, the multi⁃scan technique and SADABS program were used to apply and perform the absorption corrections. Structure of 1 was solved by SHELXTL⁃97 program package[27-28]with direct methods and refined onF2by full⁃matrix least squares methods. For hydrogen atoms of 1 in its structure, on one hand, they were geometrically attached to C and N atoms; on the other hand, they were not located for lattice water molecules. The crystallographic data and structure refinements were shown in Table 1.
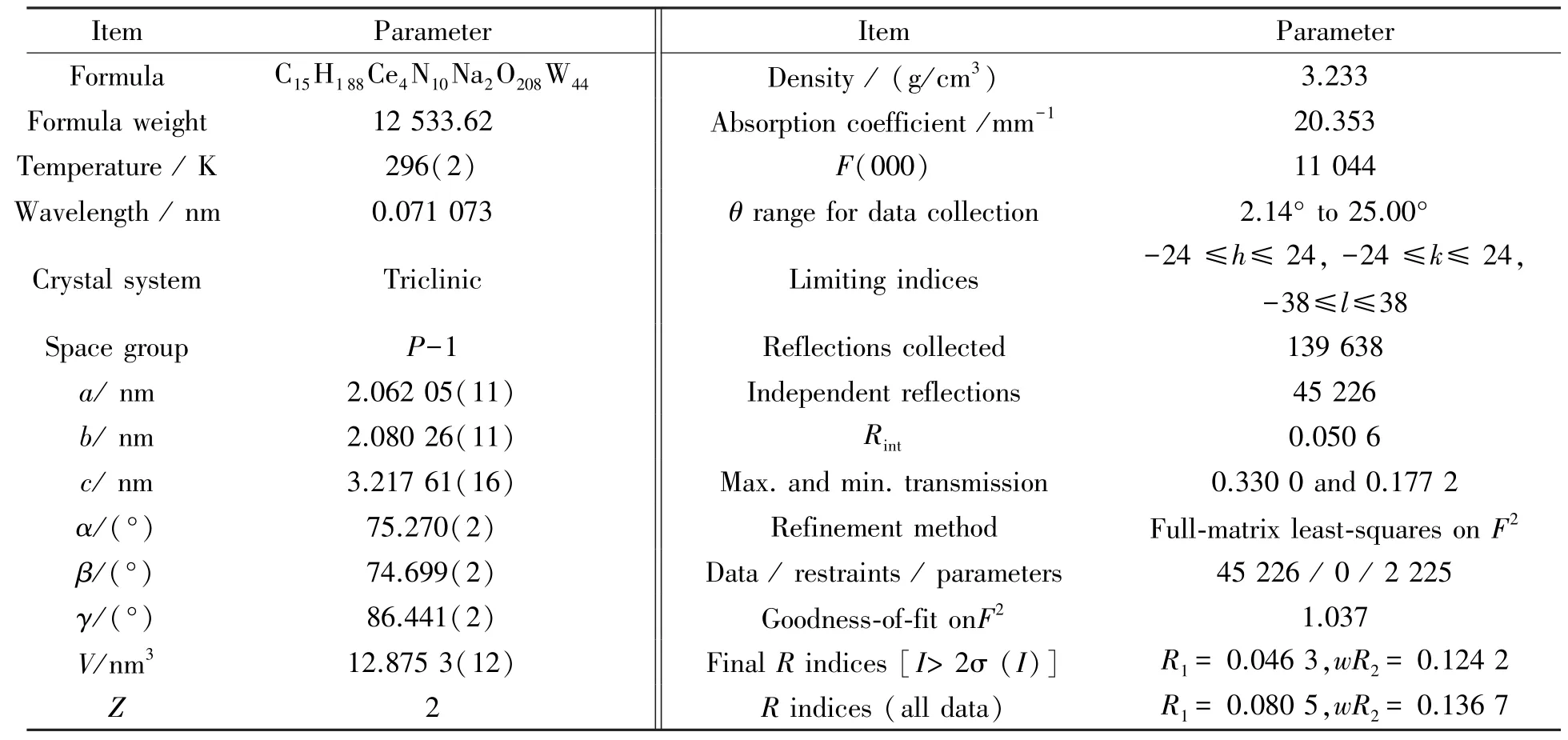
Table 1 Crystal data and structural refinements of 1
2 Results and discussion
2.1 Crystal structure
X⁃ray single⁃crystal diffraction indicates that 1 crystallize in the triclinic space groupP⁃1. The remarkable structural feature of 1 is that the tetrameric{[ Ce ( H2O )3W11O39]4}36-polyoxoanion is constructed from four monovacant [Ce (H2O)3W11O39]9-units bridged through four [Ce(H2O)3]3+cations (Fig.1a-1b). Alternatively, the tetrameric{[Ce ( H2O)3W11O39]4}36-polyoxoanion can be considered as a fusion of two dimeric {[Ce(H2O)3W11O39]2}18-subunits linked by two [Ce(H2O)3]3+cations (Fig.1c). In each [Ce(H2O)3W11O39]9-subunit, the Ce3+cation is located at the vacant site of the [W11O39]12-subunit (Fig.1d). From the viewpoint of crystallography,four crystallographically independent Ce3+cations in the architecture all exhibit the similar distorted mono⁃capped square antiprism geometry. Only the Ce13+cation is described here( Fig.1e). In the mono⁃capped square antiprism geometry of the Ce13+cation, the O70 atom from a{WO6} octahedron [W(44)-O(70): 0.180 6(9)nm] and O1W, O2W and O3W from the coordination water ligands constitute the top plane of the square antiprism while the bottom plane of the square antiprism is composed of the O59, O62, O56 and O46 atoms from the mono⁃lacunary [CeW11O39]9-subunit[Ce(1)-O(59): 0.253 0(10) nm, Ce(1)-O(62):0.2501(11) nm, Ce(1)-O(56): 0.255 4(12) nm,Ce(1)-O(46): 0.253 0(11) nm] and the O53 atom coming from a {WO6} octahedron is located at the top of the square antiprism geometry [Ce(1)-O(53):0.254 4(10) nm]. Importantly, as illustrated in Fig.1f, this characteristic coordination configuration of a Ce3+cation also promotes its ability to link two monovacant [W11O39]12-subunits (Fig.1g) to form a dimeric {[Ce(H2O)3W22O39]2}18-subunit.
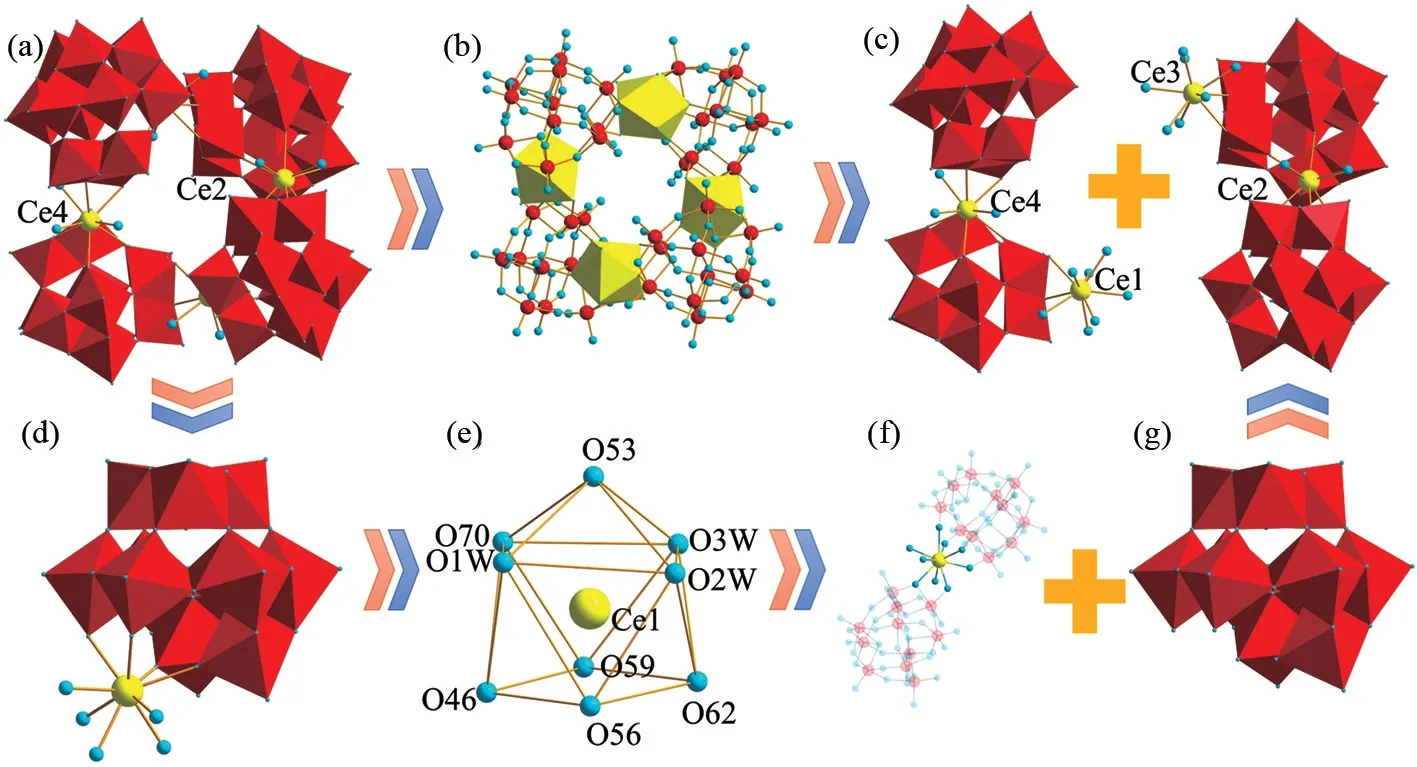
Fig.1 (a-b) Tetrameric {[Ce(H2O)3W11O39]4}36- polyoxoanion in 1;(c) View of two dimeric {[Ce(H2O)3W11O39]2}18- subunits;(d) [Ce(H2O)3W11O39]9- subunit;(e) Mono⁃capped square antiprism geometry of the Ce13+ cation;(f) Ball⁃and⁃stick view of dimeric {[Ce(H2O)3W11O39]2}18- subunit;(g) Polyhedral view of the monovacant [W11O39]12- fragment. Color codes:{WO6} octahedra: red, Ce atom: yellow, O: sky blue, W: red
Ulteriorly, the 3D packing arrangements of tetrameric {[Ce(H2O)3W11O39]4}36-polyoxoanions along thea,bandcaxis are quite interesting (Fig.2a-c).Viewing along thea,bandcaxis , the tetrameric{[Ce(H2O)3W11O39]4}36-polyoxoanions are arranged in three kinds of different stagger fashions with the -ABABpattern (Fig.2d-2f),respectively, which are contributed to reducing the steric hindrance as much as possible.
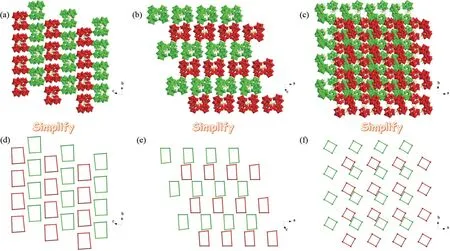
Fig.2 (a-c) Views of the 3D packing arrangements of tetrameric {[Ce(H2O)3W11O39]4}36-polyoxoanions along the a, b and c axis; (d-f) Simplified view of the 3D packing arrangements of tetrameric{[Ce(H2O)3W11O39]4}36- polyoxoanions along a,b and c axis
2.2 IR spectrum
The structure of 1 was further corroborated by IR spectrum (Fig. 3). The IR spectrum shows strong absorptions at 3 300-3 500, 1 300-1 700, and 500-1 000 cm-1. In the 3 300-3 500 cm-1region, the band at about 3 422 cm-1was assigned toν(O - H)stretching vibration of water molecules[29], which was confirmed the existence of H2O molecules in the structure. In the 1 300-1 700 cm-1region, N-H at 1 611 cm-1and C-H at 1 490 cm-1bending vibration bands appear, respectively[30-31]. In addition, their weak stretching vibrations were observed at ~3 160 cm-1and 2 800 cm-1. The appearance of these characteristic peaks indicates the presence of dap in 1.The absorption peaks at 500 - 1 000 cm-1are the feature ones for IPOT framework in the structure[26,32].Specially, the vibration of three characteristic vibration bands appear (927 cm-1for terminalv(W-Ot), 855 and 758 cm-1for corner⁃sharingν(W-Ob), 674 cm-1for edge⁃sharingν(W-Oc), respectively)[33].
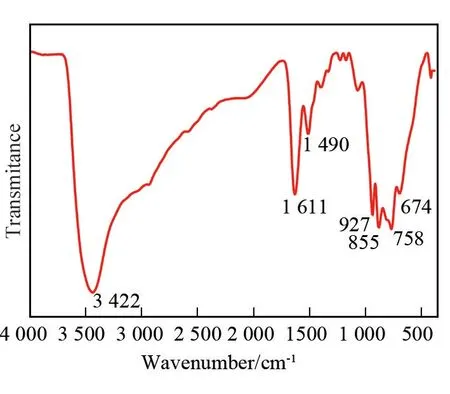
Fig.3 IR spectrum of 1
2.3 Thermogravimetric (TG) analysis
1 was characterized by TG analysis to determine its thermal behaviour. Fig.4 shows the TG curves for 1 in N2in the temperature range from 25 to 1 000 ℃. As evident from the weight loss in Fig.4, there is weight loss in three temperature regions. In the low temperature region from 25 to 200 ℃, the first weight loss of 5.70% ( calcd. 5.74% ) was observed,corresponding to the release of 40 lattice water molecules. In the mid temperature region, the dehydration of 34 protons, the removal of five dap and three coordinated water were observed (5.90%, calcd.5.83%) in the temperature range from 200 to 700 ℃.The third weight loss indicates the collapse of the crystal skeleton from 700 to 1 000 ℃. Apparently, this compound shows high stability at inert gas atmosphere.
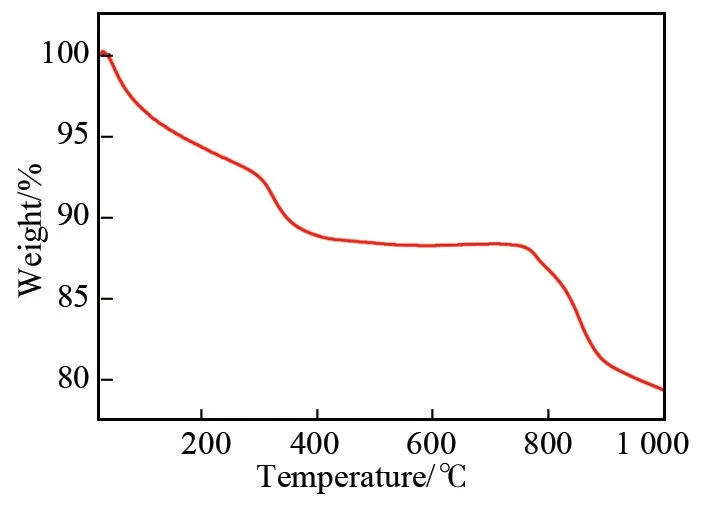
Fig.4 TG curve of 1
Conclusions
In summary, a type of Ce3+⁃bridging tetrameric IPOT is successfully synthesized in aqueous solution via the one⁃step self⁃assembly reaction of simple starting materials. It should be noted that the tetrameric{[Ce(H2O)3W11O39]4}36-polyoxoanion is constructed from four mono⁃ vacant [Ce(H2O)3W11O39]9-units bridged through four [Ce(H2O)3]3+cations, which is rather rare in IPOTs. The successful preparation of the tetrameric {[ Ce ( H2O)3W11O39]4}36-polyoxoanion not only is conducive to enrich the variety of IPOT structures, but also provides a feasible and convenient synthetic method for designing and assembling more novel IPOT frameworks.