苯并咪唑基Irバ配合物的合成、结构和发光行为的调控或转换
2019-08-08吴石山曹登科
芮 凯 吴石山 曹登科
(南京大学化学化工学院,南京 210093)
0 Introduction
It is well known that an imidazole unit can coordinate to a metal ion through its neutral-N=donor or deprotonated-N--donor.In this regard,some cyclometalated Irバcomplexes incorporate imidazole units,leading to various structures and the related switching of photophysical properties.For example,Williams group firstly reported the proton-switchable behavior from a pair of benzoimidazole-based cyclometalated Irバ complexes[Ir(ppy)2(pybzH)]PF6and[Ir(ppy)2(pybz)] (Scheme S1)[1],in which ligands pybzH and pybz-coordinate with Irバions through-N=donor and deprotonated-N--donor,respectively.It was found that the two complexes in CH2Cl2revealed structural interconversion upon addition of acid/base(i.e.proton-switchable behavior,Scheme S1),resulting in luminescence switching between the emission at 521 nm from[Ir(ppy)2(pybzH)]PF6and the emission at 496 nm from[Ir(ppy)2(pybz)].After this report,the other imidazole/benzoimidazole-based[Ir(C^N)2(N^N)]+complexes were designed and synthesized[2-6],in which the acid/base-induced transition of coordination mode between-N=mode and-N--mode resulted in the significant variations in electronic absorption spectra[3],luminescence intensity[4-5],and emission color[6].
Recently,our group reported complexes[Ir(dfppy)(qbiH)]PF6(1F·PF6)and[Ir(dfppy)(qbi)] (2F)(Scheme 1)[6],in which an{Ir(dfppy)2}+unit is chelated by a benzoimidazole-based neutral ligand qbiH using the coordination mode N^N in 1F·PF6,while anion ligand qbi-using the coordination mode N^N-in 2F.Their distinct structures result in the significant differences in luminescence,strong emission at 558 nm for 1F·PF6in CH2Cl2while weak emission at 546 nm for 2F.Upon addition of NEt3/TFA,the two complexes can switch their luminescence between strong emission at 558 nm and weak emission at 546 nm,due to their acid-/base-induced structural interconversion between the protonation state and the deprotonation state of qbiH ligand.We synthesized complexes [Ir(ppy)(qbiH)]NO3(1·NO3) and[Ir(ppy)(qbi)] (2),which incorporate cyclometalated ligand ppy-instead of the dfppy-ligands in the reported complexes 1F·PF6and 2F (Scheme 1).The aims of this study mainly include the below two aspects.わWe explore the influence of ligands ppy-on the structures and associated luminescence of complexes 1·NO3and 2.ぷ The neighboring molecules in both 1F·PF6and 2F stack through π…π interactions (Fig.S1 and S2),due to the incorporation of some fluorine substituents[7].In contrast,complexes 1·NO3and 2 without any fluorine substituent are expected to be lack of inter-molecularπ…π stacking interactions.The resultant loose packing structures of both 1·NO3and 2 would facilitate their possible solid-state luminescence switching.Herein,we discuss the structuresand luminescence modulation/switching of 1·NO3and 2.
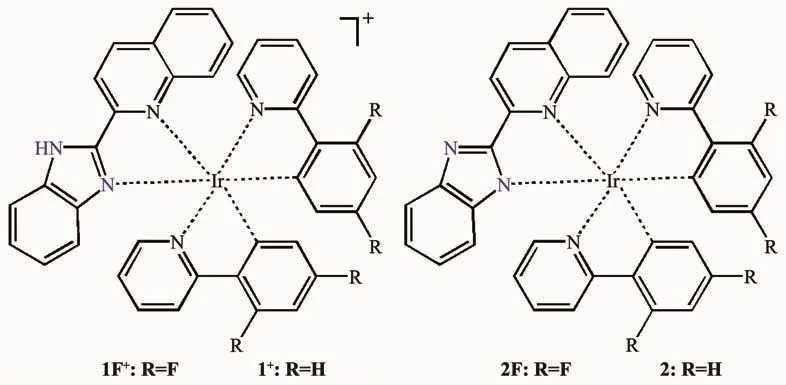
Scheme 1 Molecular structures of cations 1F+and 1+,and complexes 2F and 2
1 Experimental
1.1 M aterials and physical measurements
Compounds qbiH and[Ir(ppy)2Cl]2were prepared according to the literature methods[6,8].All other reagents were commercially available and used without further purification.Elemental analyses were performed on a Perkin Elmer 240C elemental analyzer.The IR spectra were obtained as KBr disks on a VECTOR 22 spectrometer.The1H NMR spectra were recorded at room temperature with a 400 MHz BRUKER spectrometer.UV-Vis absorption spectra were measured on a Cary 100 spectrophotometer.The luminescence spectra at room temperature and at 77 K were measured on a Hitachi F-4600 fluorescence spectrometer.The luminescence lifetimes of 1·NO3and 2 both in CH2Cl2and in solid state were measured at room temperature on a HORIBA FL-3 Spectrofluorometer with a 374 nm LED pulsed from a NanoLED resource.The photoluminescence quantum yields of 1·NO3and 2 in CH2Cl2were measured by using a relative method by comparing with a standard,a solution of quinine sulfate in 0.5 mol·L-1H2SO4(Φ=54.6%,λex=366 nm)[9].The quantum yields of these complexes in the solid state were measured at room temperature on a HORIBA FL-3 spectrofluorometer.
1.2 Synthesis of[Ir(ppy)(qbiH)]NO 3 (1·NO3)
A mixture of[Ir(ppy)2Cl]2(0.15 mmol,0.161 1 g),qbiH (0.3 mmol,0.073 5 g),CH2Cl2(12 mL)and CH3OH (12 mL)was heated in an oil bath (50 ℃)under argon for one day,and then was evaporated under vacuum.To a solution of the obtained residue in CH2Cl2(30 mL)was added a solution of AgNO3(0.6 mmol,0.102 0 g)in CH3CN (10 mL),and this mixture was stirred at room temperature overnight.After removing the resultant AgCl, the filtrate was evaporated.The residue was dissolved in a mixture of CH2Cl2(40 mL)and H2O (15 mL).The CH2Cl2layer was separated and evaporated.The resultant solid was purified through silica column chromatography using CH3OH-CH2Cl2(VCH3OH/VCH2Cl2=0~0.01)solution,obtaining orange-red solid (Fig.S3)with a yield of 194.4 mg(80%based on[Ir(ppy)2Cl]2).Anal.Calcd.for C38H27N6O3Ir(%):C,56.50;H,3.37;N,10.40.Found(%):C,56.57;H,3.63;N,10.61.IR (KBr,cm-1):3 444(w),3 043(w),2 923(w),2 853(w),1 604(s),1 583(m),1 562(w),1 520(m),1 477(s),1 420(m),1 384(s),1 338(m),1 321 (s),1 267 (m),1 227 (w),1 161 (w),1 116(w),1 062(w),1 030(w),993(w),873(w),842(w),793(w),756 (s),731 (s),630 (w),438 (w).1H NMR (400 MHz,CDCl3): δ5.91 (d,J=8.4 Hz,1H),6.23 (d,J=7.6 Hz,1H),6.48 (d,J=7.2 Hz,1H),6.83~6.91 (m,4H),6.96~7.05 (m,2H),7.10 (t,J=7.4 Hz,1H),7.19~7.22 (m,2H),7.44~7.48 (m,2H),7.58~7.89 (m,9H),8.16 (d,J=7.6 Hz,1H),8.51 (d,J=6.4 Hz,1H)and 9.12 (broad,1H) (5.91~7.22 and 7.44~9.12:total 26H from two ppy-units and one qbiH ligand).
1.3 Synthesis of[Ir(ppy)(qbi)] (2)
A mixture of[Ir(ppy)2Cl]2(0.15 mmol,0.161 1 g),qbiH (0.3 mmol,0.073 5 g),K2CO3(0.6 mmol,0.082 8 g),CH2Cl2(12 mL)and CH3OH (12 mL)was heated in an oil bath (50 ℃)under argon for one day.After evaporation under vacuum,the resultant residue was dissolved in a mixture of CH2Cl2(30 mL)and H2O (10 mL).The CH2Cl2layer was separated,dried with MgSO4,and filtered.The filtrate was evaporated under vacuum.The resultant solid was purified through silica column chromatography using CH3OH-CH2Cl2(VCH3OH/VCH2Cl2=0~0.005)solution,obtaining an orangeyellow solid (Fig.S3)with a yield of 181 mg (81%based on[Ir(ppy)2Cl]2).Anal.Calcd.for C38H26N5Ir(%):C,61.27;H,3.52;N,9.40.Found(%):C,61.42;H,3.84;N,9.40.IR (KBr,cm-1):3 420 (w),3 047(w),2 924(w),1 604(m),1 583(w),1 561(w),1 521(w),1 477(s),1 338(w),1 321(w),1 267(w),1 161(w),1 116(w),1 062(w),1 030(w),866(w)and 755(w).1H NMR (400 MHz,CDCl3):δ5.86 (d,J=8.4 Hz,1H),6.26 (d,J=8.4 Hz,1H),6.57 (d,J=8.4 Hz,1H),6.67~6.73 (m,3H),6.85 (t,J=8.2 Hz,1H),6.93~7.00 (m,2H),7.05 (t,J=8.0 Hz,2H),7.13 (t,J=8.0 Hz,1H),7.34~7.40 (m,2H),7.49 (t,J=8.0 Hz,1H),7.56 (t,J=8.0 Hz,1H),7.61~7.67 (m,2H),7.72~7.75 (m,2H),7.80 (d,J=8.0 Hz,1H),7.84 (d,J=8.0 Hz,1H),7.91 (d,J=6.4 Hz,1H),8.12 (d,J=8.8 Hz,1H),8.28 (d,J=8.4 Hz,1H)and 8.80 (d,J=8.8 Hz,1H) (5.86~7.13 and 7.34~8.88:total 26H from two ppy-units and one qbi-ligand).
1.4 X-ray crystallographic studies
The single crystals of 1·NO3and 2 were grown from the corresponding CH2Cl2-CH3OH solution.Single crystals of dimensions 0.21 mm×0.13 mm×0.10 mm for 1·NO3,and 0.18 mm×0.15 mm×0.11 mm for 2 were used for structural determination on a Bruker SMART APEX CCD diffractometer using graphitemonochromated Mo Kα radiation (λ=0.071 073 nm)at room temperature.A hemisphere of data were collected in the θrange of 1.50°~26.00°for 1·NO3,and 1.70°~28.24°for 2 using a narrow-frame method with scan widths of 0.30°in and an exposure time of 10 s per frame.Numbers of observed and unique reflections are 48 567 and 6 096 (Rint=0.037 1)for 1·NO3,and 21 076 and 7 412 (Rint=0.0430)for 2,respectively.The data were integrated using the Siemens SAINT program[10],with the intensities corrected for Lorentz factor,polarization,air absorption,and absorption due to variation in the path length through the detector faceplate.Multi-scan absorption corrections were applied.The structures were solved by direct methods and refined on F2by full matrix least squares using SHELXTL[11-12].All the non-hydrogen atomswere located from the Fourier maps,and were refined anisotropically.All H atoms were refined isotropically,with the isotropic vibration parameters related to the non-H atom to which they are bonded.The crystallographic data for complexes 1·NO3and 2 are listed in Table 1,and selected bond lengths and bond angles are given in Table 2 and 3.
CCDC:1907283,1·NO3;1907284,2.3
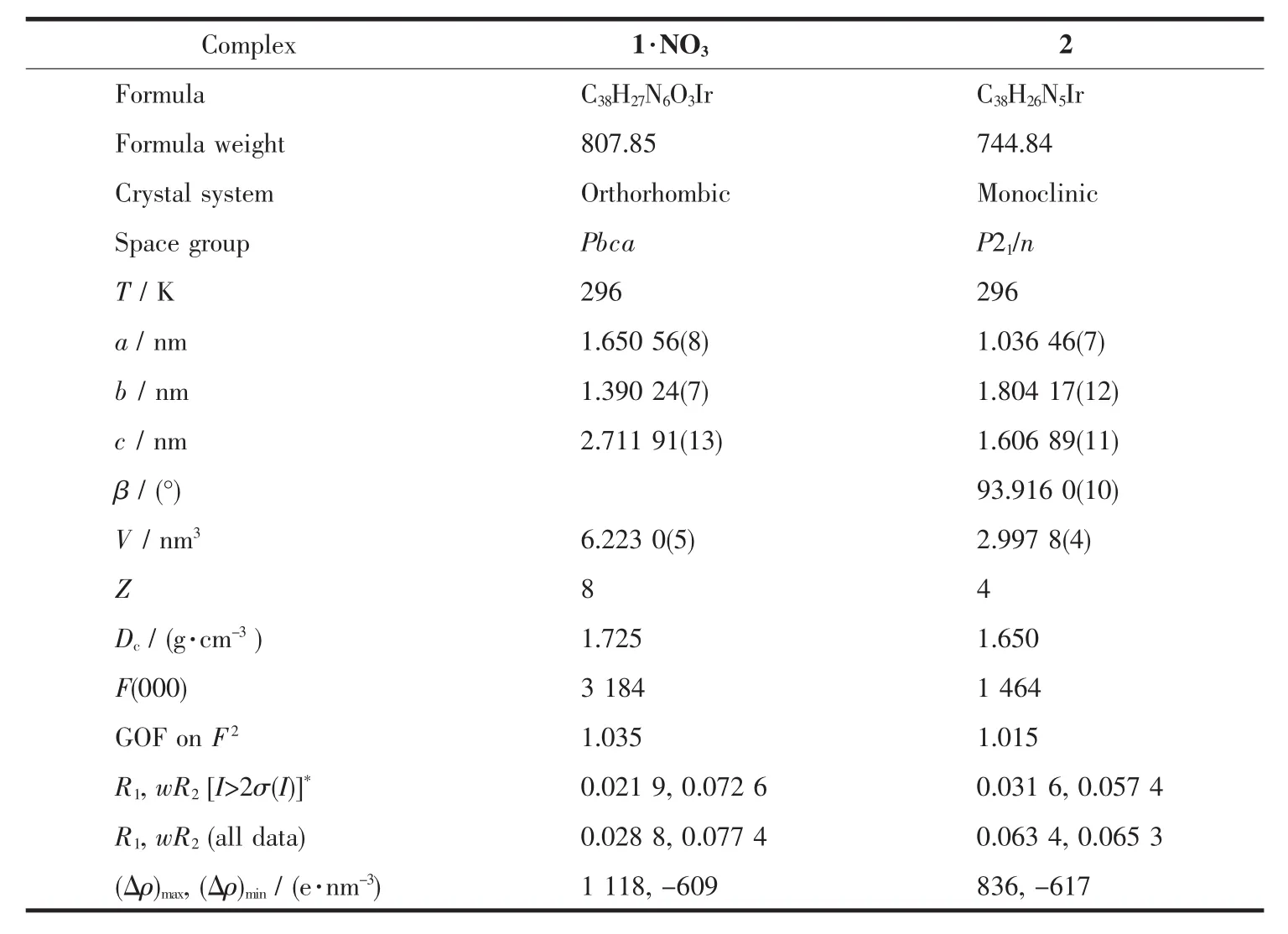
Table 1 Crystallographic data for 1·NO and 2

Table 2 Selected bond lengths (nm)and bond angles (°)for 1·NO3

Table 3 Selected bond lengths(nm)and bond angles(°)for 2
2 Results and discussion
2.1 Syntheses and structural transformation
Complex 1·NO3were synthesized through the reaction of[Ir(ppy)2Cl]2and qbiH in a CH2Cl2-CH3OH solution at 50℃for 24 hours,and followed by the anion exchange of Cl-with NO3-for 1·NO3.In contrast,complex 2 was obtained by the reaction of[Ir(ppy)2Cl]2and qbiH in the presence of K2CO3.Thus,an[Ir(ppy)2]+unit is chelated by a qbi-anion in complex 2,while a neutral ligand qbiH in complex 1·NO3,which was confirmed by the crystal structures of these complexes.
Complexes 1·NO3and 2 can interconvert in solution upon addition of an acid or a base,due to the structural transformation between ligand qbiH and ligand qbi-(Scheme 1),which was supported by1H NMR spectra.After the CDCl3solution of 1·NO3was fully mixed with a D2O solution of NaOH,its1H NMR spectrum clearly changed to that of 2 (Fig.1).After adding some DCl in the CDCl3solution of 2,although the measured1H NMR spectrum is different from that of 1·NO3probably due to the influence of DCl,it is in agreement with the1H NMR spectrum of 1·NO3in CDCl3containing DCl(Fig.2).
2.2 Crystal structures of 1·NO3 and 2

Fig.1 1H NMR spectra of 1·NO3 in CDCl3 after adding NaOH and 2 in CDCl3

Fig.2 1H NMR spectra of 2 in CDCl3 after adding DCl and 1·NO3 in CDCl3 containing DCl
In order to clarify the structures of complexes 1·NO3and 2,their crystal structures were measured.In complex 1·NO3,an[Ir(ppy)2]+unit is coordinated by a neutral qbiH ligand (Fig.3).The resultant[Ir(ppy)2(qbiH)]+cation connects a NO3-anion through hydrogen bond N4-H…O1-NO2(N4…O1 0.270 8(1)nm).In this cation,the Irバion shows a distorted octahedral coordination geometry.Four of the six coordination sites around the Irバion are occupied by two pyridine nitrogen atoms (N1,N2)and two carbon atoms (C1,C12)from two nonequivalent cyclometalated ppyligands.The remaining two coordination sites are filled with atoms N3 and N5 from a qbiH ligand.In the molecular structure of 1·NO3,two cyclometalated ppy-ligands adopt the C,C-cis and N,N-trans arrangement as those in[Ir(ppy)2Cl]2[13].Ligand qbiH isarranged with their nitrogen atoms N3 and N5 lying in the trans-position forσ-bond carbon atoms (C12 and C1)in the ppy-ligands (i.e.N,C-trans arrangement),leading to much longer distances for Ir1-N3 bond (0.214 2(2)nm)and Ir1-N5 bond (0.224 9(2)nm)compared to Ir-C(N)ppybonds (0.200 8(3)~0.204 8(2)nm)[14].

Fig.3 Molecular structure of 1·NO3
Compared to 1·NO3,complex 2 is a neutral complex (Fig.4),in which a qbi-anion uses its N4 and N5 atoms to coordinate with an[Ir(ppy)2]+unit.In the molecular structure of 2,ligands ppy-and qbi-adopt the same structural arrangements as those in 1·NO3,i.e.C,C-cis,N,N-trans and N,C-trans arrangements.The Ir-C(N)distances in 2 (0.2014(4)~0.2260(3)nm)are comparable to those in 1·NO3(0.200 8(3)~0.224 9(2)nm).
Clearly,complexes 1·NO3and 2 have different structures,a neutral ligand qbiH in the former while anion ligand qbi-in the latter,which would lead to their distinct photophysical properties.Additionally,in the packing structures of both 1·NO3and 2,neighboring Irバfragments are held together by van der Waals interactions (Fig.S7 and S8),and there is no inter-molecularπ…πstacking interactions as those in the reported complexes 1F·PF6and 2F.This indicates that the cyclometalated ligands ppy-in both 1·NO3and 2 can significantly affect the molecular arrangements of these complexes.

Fig.4 Molecular structure of 2
2.3 Electronic absorption spectra
The UV-Vis spectra of complexes 1·NO3and 2 were measured in CH2Cl2at room temperature(Fig.5,Table 4).The complexes showed similar high-energy absorption bands at~252 and 297 nm,which could be due to their ligand-centered (1LC)transitions (ppy-and qbiH/qbi-ligands).However,the low-energy absorption bands of 2 (368 and 390 nm)show significant red shift compared to that of 1·NO3(368 nm).These lowenergy absorption bands in the complexes are likely to be a combination of metal-to-ligand charge transfer(1MLCT)and ligand-centered (1LC)transition,because of their high extinction coefficients in a range of 1.5×104~2.0×104L·mol-1·cm-1[5,14].In addition,both 1·NO3and 2 exhibited weaker absorption tails towards 490 nm,which are mainly attributed to3MLCT absorptions in the complexes[15-16].

Fig.5 UV-Vis absorption spectra of 1·NO3 and 2 inCH2Cl2
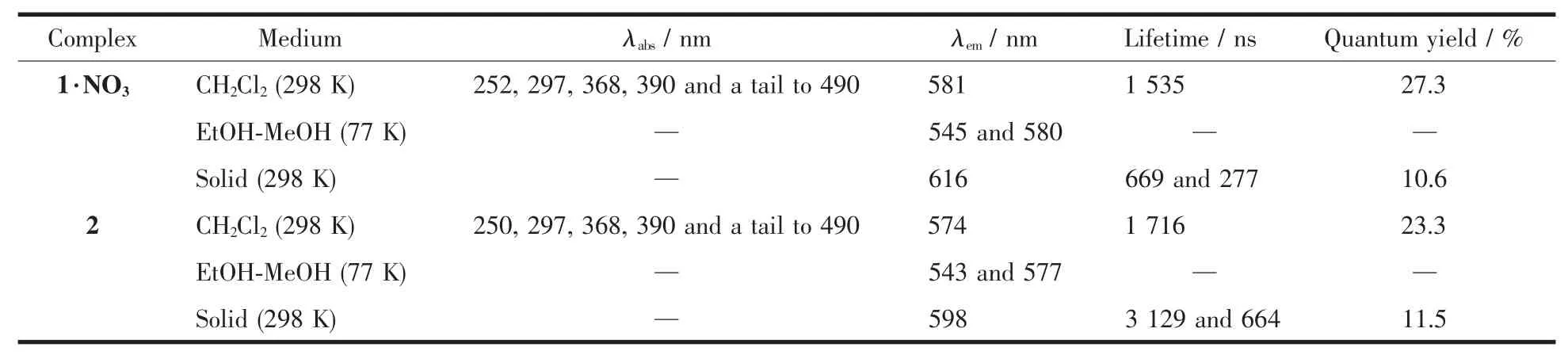
Table 4 Photophysical data of 1·NO3 and 2
2.4 Lum inescence properties

Fig.6 Luminescence spectra of 1·NO3 and 2 in CH2Cl2
We measured the luminescence spectra of both 1·NO3and 2 in CH2Cl2at room temperature under the excitation with 398 nm (Fig.6).Compared to 1·NO3with an emission at 581 nm,complex 2 revealed a slightly blue-shifted emission at 574 nm,due to the fact that the deprotonated ligand qbi-leads to higher energy of MLCT (from Irバ ion to ligand qbi-).At 77 K,the complexes showed blue-shifted emissions with respect to their emissions at room temperature,occurring at 545 and 580 nm for 1·NO3,and 543 and 577 nm for 2 (Fig.S9,Table 4).This rigidochromism is characteristic of a CT character for the luminescence of these complexes[17-18].In addition,the luminescence quantum yields (Φ)and the emission lifetimes (τ)of both 1·NO3and 2 were measured in degassed CH2Cl2at room temperature,Φ=27.3%and τ=1 535 ns for 1·NO3,andΦ=23.3%andτ=1 716 ns for 2.
Clearly,complexes 1·NO3and 2 in CH2Cl2have different luminescence,due to their different ancillary ligands (qbiH and qbi-,respectively).On the other hand,compared to their analogous complexes 1F·PF6and 2F (Scheme 1),both 1·NO3and 2 incorporate cyclometalated ligands ppy-,which leads to their significantly different luminescence behaviors,mainly including the below three aspects.わThe emission wavelengths of 1·NO3(581 nm)and 2 (574 nm)were longer than those of 1F·PF6(558 nm)and 2F (546 nm),because the molecular structures of 1·NO3and 2 have no electron-drawing fluorine group (Scheme 1).ぷ Complexes 1·NO3and 2 revealed higher luminescence quantum yields (27.3%and 23.3%,respectively)than both 1F·PF6(14%)and 2F (3.2%). ぺ The quantum yield of 2F (3.2%)was significantly lower than that of 1F·PF6(14%),but the similar quantum yields for 1·NO3(27.3% )and 2 (23.3% ).This suggests that ligand qbi-in complex 2 has less contribution to the excited state of this complex.
Although complexes 1·NO3and 2 revealed the similar emission color in CH2Cl2(Fig.6),they exhibited significantly different solid-state luminescence (Fig.7).A red emission at 611 nm was observed for 1·NO3,while an orange emission at 598 nm for complex 2.The emissions of both 1·NO3and 2 in solid state reveal significantly red shift compared to the corresponding emission in CH2Cl2,with Δλ=35 nm and 24 nm,respectively,which could be due to molecular aggregation in solid state[19].For the solid-state samples of both 1·NO3and 2,we further measured their luminescence quantum yields and lifetimes.The complexes revealed similar solid-state luminescence quantum yield,Φ=10.6%for 1·NO3and 11.5%for 2.However,the solid-state emission lifetimes of 1·NO3(τ1=669 ns,79%contribution and τ2=277 ns,21%contribution)are significantly shorter than those of 2(τ1=3 129 ns,77%contribution and τ2=664 ns,23%contribution).The solid-state luminescence behaviors of 1·NO3and 2 are clearly different from those of complexes 1F·PF6(emissions at 542,572 and 611 nm)and 2F (emissions at 595 and 633 nm).This is in agreement with their distinct molecular structures and stacking structures (Scheme 1).

Fig.7 Luminescence spectra of 1·NO3 and 2 in solid state at room temperature
It is interesting that complexes 1·NO3and 2 in solid state show acid/base-induced emission switching(Fig.8).Upon meeting the vapor of Et3N,complex 1·NO3changed its emission color from red to orange,indicating Et3N-induced structural transition from 1+to 2 (i.e.from[Ir(ppy)(qbiH)]+to[Ir(ppy)(qbi)]).On the other hand,complex 2 showed emission-color change from orange to red upon meeting TFA vapor,which could be due to structural transition from 2 to 1+.These experimental results indicate that solid-state complexes 1·NO3and 2 can undergo TFA-/NEt3-induced structural interconversion,leading to their emission color switching between red and yellow.It should be noted that the similar solid-state emission switching behavior has not been observed for 1F·PF6and 2F,which could be due to their close molecular stacking through π…π interactions (Fig.S1 and S2).Moreover,we found that the anion NO3-in complex 1·NO3could be partly replaced by CF3COO-in TFA vapor,which was confirmed by the measurement of IR spectra (Fig.S14).After the treatment by TFA vapor,complex 1·NO3showed two new peaks at 1 672 and 1 202 cm-1from CF3COO-anion,indicating the replacement of NO3-by CF3COO-.Before and after meeting TFA vapor,complex 1·NO3always revealed the absorption peaks of NO3-(1 425 and 842 cm-1),suggesting that only a part of NO3-anions are replaced by CF3COO-anions.
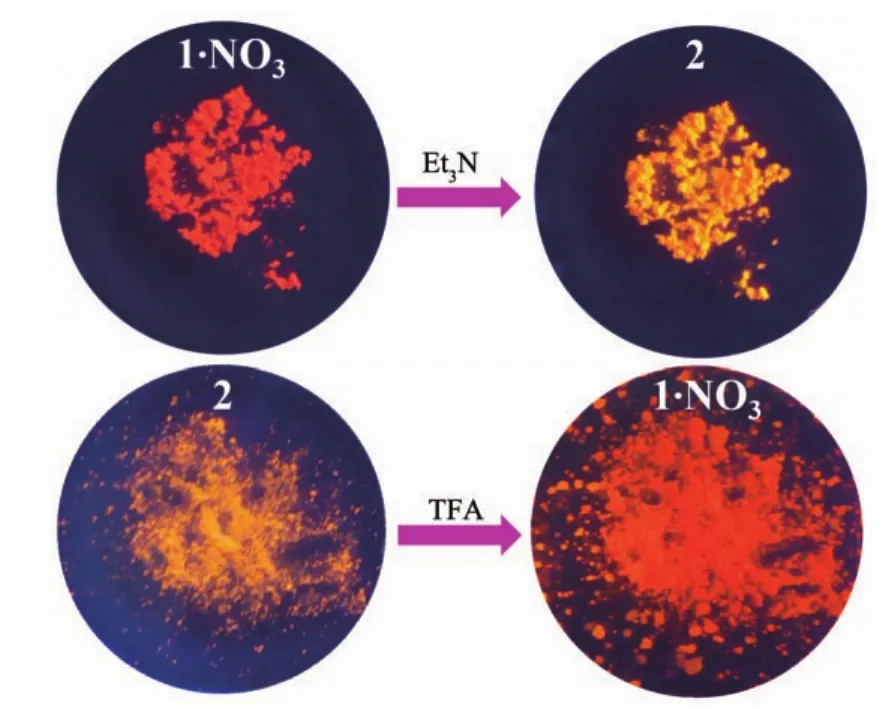
Fig.8 TFA-/Et3N-induced emission switching for solidsate 1·NO3 and 2 under 365 nm light at room temperature
3 Conclusions
In summary,we synthesized two new cyclometalated Irバ complexes[Ir(ppy)(qbiH)]NO3(1·NO3)and[Ir(ppy)(qbi)] (2).Their crystal structures indicate that an [Ir(ppy)2]+unit is chelated by a neutral benzoimidazole-based ligand qbiH in 1·NO3,while anion ligand qbi-in 2.Neighboring Irバfragments in both 1·NO3and 2 are held together only by van der Waals interaction.The distinct molecular structures and packing structures between 1·NO3and 2 lead to their different luminescence both in CH2Cl2and in solid state.In CH2Cl2,an emission at 581 nm withΦ=27.3%was observed for 1·NO3and 574 nm with Φ=23.3%for 2.In solid state,complexes 1·NO3and 2 exhibit a red emission at 611 nm and an orange emission at 598 nm,respectively.Complexes 1·NO3and 2 revealed structural interconversion upon addition of acid/base (i.e.DCl/NaOD)in their CDCl3solution,which is assigned to acid/base-induced structural transformation between ligand qbiH and ligand qbi-.This structural transformation can even occur in solidstate 1·NO3and 2,probably due to the loose intermolecular stacking in the two complexes.Upon meeting Et3N/TFA vapor,the solid-state samples of 1·NO3and 2 revealed luminescence switching between red emission and orange emission.
Supporting information is available at http://www.wjhxxb.cn
