CD133在卵巢黏液性肿瘤中的作用探讨
2019-06-28张乾王海欧林凤
张乾 王海欧 林凤
[摘要] 目的 研究CD133在卵巢黏液性肿瘤中的表达,并探讨其临床意义。 方法 采用免疫组织化学PV9000两步法分别检测CD133在25例卵巢黏液性囊腺癌、19例卵巢交界性黏液性肿瘤和22例卵巢黏液性囊腺瘤的表达水平,并分析其与卵巢黏液性肿瘤的临床病理特征的关系。 结果 CD133在卵巢黏液性腺癌、卵巢交界性黏液性肿瘤和卵巢黏液性囊腺瘤的阳性表达率分别为72.0%、42.1%、9.1%,三组比较,差异有统计学意义(P<0.05)。在卵巢黏液腺癌中,CD133在中低分化组的阳性表达率高于高分化组(P<0.05),在临床分期Ⅲ~Ⅳ期高于Ⅰ~Ⅱ期(P<0.05)。 结论 CD133可能参与卵巢黏液性肿瘤的发生、发展,其蛋白检测可能用于肿瘤恶性程度的判断,可能对判断卵巢黏液性癌的恶性增殖和患者预后有一定的临床意义。
[关键词] 肿瘤干细胞;CD133;卵巢黏液性肿瘤;免疫组织化学
[中图分类号] R737.31 [文献标识码] A [文章编号] 1673-9701(2019)11-0020-04
The role of CD133 in ovarian mucinous tumors
ZHANG Qian WANG Haiou LIN Feng
Department of Gynecology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, China
[Abstract] Objective To study the expression of CD133 in ovarian mucinous tumors and to explore its clinical significance. Methods The expression levels of CD133 in 25 cases of ovarian mucinous cystadenocarcinoma, 19 cases of ovarian borderline mucinous tumor and 22 cases of ovarian mucinous cystadenoma were detected by immunohistochemical PV9000 two-step method, and its relationship with the clinicopathological features of ovarian mucinous tumors was analyzed. Results The positive expression rate of CD133 in ovarian mucinous adenocarcinoma, ovarian borderline mucinous tumor and ovarian mucinous cystadenoma was 72.0%, 42.1%, and 9.1%, respectively. There were significant differences among the three groups(P<0.05). In ovarian mucinous adenocarcinoma, the positive expression rate of CD133 in the moderately and poorly differentiated group was higher than that in the highly differentiated group(P<0.05), and the positive expression rate of CD133 in clinical stage with stage Ⅲ-Ⅳ was higher than that with stageⅠ-Ⅱ(P<0.05). Conclusion CD133 may be involved in the occurrence and development of ovarian mucinous tumors. Its protein detection may be used to judge the malignant degree of tumor, which may have certain clinical significance for judging the malignant proliferation and prognosis of ovarian mucinous carcinoma.
[Key words] Cancer stem cells; CD133; Ovarian mucinous neoplasms; Immunohistochemistry
卵巢黏液性腫瘤是常见的卵巢肿瘤之一,其中卵巢黏液性腺癌恶性程度高,多数患者就诊时已属于晚期,复发、死亡率高[1]。有研究表明肿瘤干细胞(cancer stem cells,CSCs)可能是肿瘤发生、发展、侵袭、转移及发生耐药的根源[2]。CD133是常见的肿瘤干细胞表面标志物之一[3],目前CD133在多种肿瘤中的表达及其功能研究已成为热点,但在卵巢黏液性肿瘤中尚缺乏系统性的研究。本研究应用免疫组织化学方法检测CD133在卵巢黏液性肿瘤中表达情况,并探讨其在卵巢黏液性肿瘤中的潜在临床作用,现报道如下。
1 资料与方法
1.1 临床资料
病理标本为2010年1月~2016年12月在温州医科大学附属第一医院妇科手术的患者,收集卵巢黏液性肿瘤石蜡病理标本共66例,入选标准:排除既往有恶性肿瘤病史的患者,年龄18~69岁。所有病理标本均经2位病理医师生阅片确定;根据病理分为三组,其中卵巢黏液性囊腺癌(mucinous ovarian adenocarcinoma,MOA)组25例,年龄22~69岁,其中年龄≥50岁16例,<50岁9例;病理类型高分化11例,中低分化14例;Ⅰ~Ⅱ期16例,Ⅲ~Ⅳ期9例。卵巢交界性黏液性肿瘤(mucinous borderline ovarian tumor,M-BOT)组19例,年龄20~50岁。卵巢良性黏液性囊腺瘤(mucinous ovarian cystadenoma MOC)组22例,年龄18~50岁。
1.2 实验试剂
PV-9000两步法免疫组织化学检测试剂盒,鼠抗人CD133单克隆抗体、10 mmol/L枸橼酸盐缓冲液(pH 6.0)、DAB显色试剂盒等购自北京市中杉金桥生物技术公司。
1.3 实验方法
将石蜡病理标本切片,厚4 μm,脱蜡水化,置于10 mmol/L柠檬酸盐缓冲液中,微波修复抗原20 min,3.0%双氧水阻断内源性过氧化酶,血清封闭后滴加抗CD133抗体(1:100)4 ℃过夜,加二抗后37℃温箱孵育30 min,其余步骤常规按PV-9000方法操作,以PBS代替一抗做阴性对照,已知阳性结果作为阳性对照。
1.4 结果判断
由2位病理医师用双盲法评估病理切片。CD133定位于细胞浆和细胞膜上。判断标准[4]:肿瘤细胞浆、细胞膜呈棕黄色颗粒。400倍镜下随机观察5个视野,每个视野计数100个细胞中的阳性细胞数,阳性细胞数>75%为+++,51%~75%为++,26%~50%为+,≤25%为阴性。
1.5统计学方法
采用SPSS 20.0统计软件进行数据分析,各组间计数资料比较,采用χ2检验,P<0.05为差异有统计学意义。
2 结果
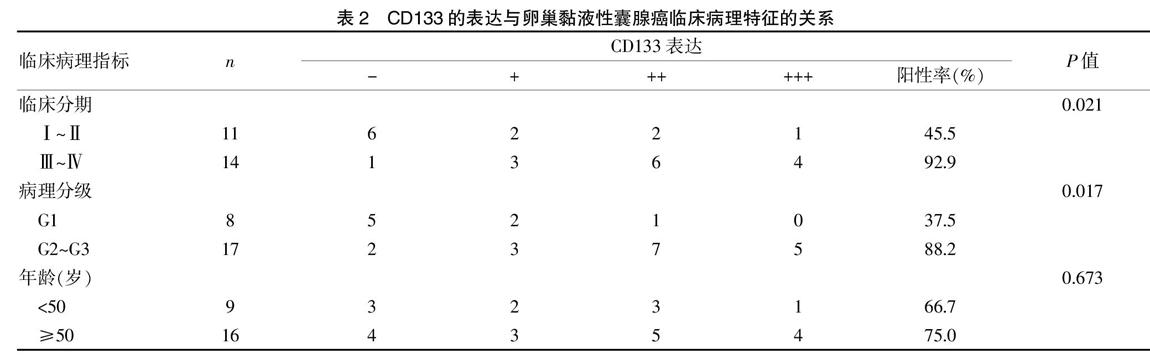
2.1 CD133在各组卵巢黏液性肿瘤的阳性表达
CD133在MOA、M-BOT、MOC的阳性表达率分别为72.0%、42.1%、9.1%,三组间比较,差异有统计学意义(P<0.05),见表1、封三图4。
2.2 CD133表达与临床病理特征的关系
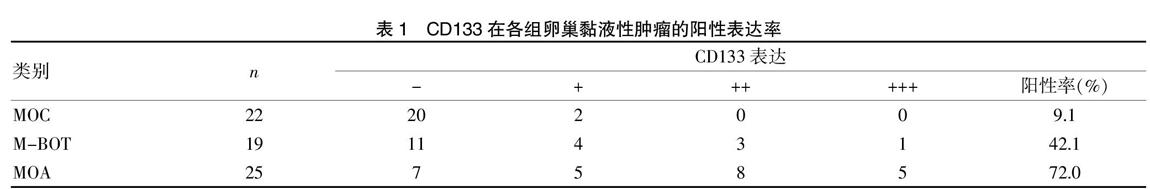
在卵巢黏液性囊腺癌组(MOA)中,CD133的表达水平在不同年龄段之间比较,差异无统计学意义(P>0.05),但在不同的临床分期和病理分级之间比较,差异有统计学意义(P<0.05),见表2。
3 讨论
卵巢黏液性腺癌是常见的卵巢恶性肿瘤之一,恶性程度高,易复发,预后差,且大部分卵巢黏液性癌术后需要化学药物治疗,但研究表明卵巢黏液性腺癌对铂类等一线化疗药物容易产生耐药性[5]。Shimada M等[6]研究发现,卵巢浆液性腺癌的预后显著高于黏液性腺癌,对铂类化疗药物敏感性分别为67.7%及12.5%,说明卵巢黏液性腺癌对铂类的反应明显差于卵巢浆液性腺癌。因此,研究对耐药的癌细胞起作用的新的化疗药物是未来卵巢黏液性腺癌研究的重要方向之一。最新提出的肿瘤干细胞理论为临床研究提供新途径,肿瘤干细胞具有多向分化潜能和自我更新能力,还具有药物免疫性和高致瘤性,是形成不同分化程度肿瘤细胞和促使肿瘤不断生长的根源,大量临床研究表明化学药物能杀死大部分卵巢癌细胞,却不能完全杀死卵巢癌中存在肿瘤干细胞,故认为卵巢癌中残存的肿瘤干细胞是卵巢癌復发、产生耐药性的重要原因之一[7-9]。Bapat SA等[10]在卵巢癌患者腹水中分离获得了干细胞样细胞克隆,能表达多种干细胞标志物,具有极强自我更新能力,并能够在裸鼠体内形成与原发肿瘤组织和细胞结构相似的移植瘤,由此肯定了卵巢癌干细胞的存在,及其在卵巢癌发生和发展中的重要作用。
CD133是目前肿瘤干细胞标志物的热点研究的标志物之一,是一种5次跨膜糖蛋白,含有865个氨基酸的单链多肽,分子质量为120kD,最早发现于造血干细胞和神经干细胞表面,在各类原始细胞如间质干细胞、内皮祖细胞等中均有特异性表达。研究发现CD133在肿瘤及其他组织中均有发现,如食管癌[11]、结肠癌[12]、胰腺癌[13]、子宫内膜腺癌[14]等,且CD133表达阳性的肿瘤干细胞表现更强的成瘤性及不良预后。近年来研究发现CD133在多种肿瘤组织中存在,包括肝癌[15]、肺癌[16]、卵巢癌[17-18]等,具有极强致瘤能力及增殖能力。大量研究表明在卵巢癌中CD133阳性表达可能与上皮性卵巢癌的侵袭性生物学性有关[19]。O'Brien CA等[20]用流式细胞仪分离出CD133+和CD133-结肠癌细胞,并将它们移植到小鼠肾被膜下观察成瘤性,发现占肿瘤细胞极少部分的CD133阳性细胞均具有成瘤性,而占大多数的CD133阴性细胞却没有致瘤性,由此可见,虽然CD133+细胞数目极少,却对肿瘤的增殖、分化及复发有着极为重要的作用。
本研究发现,MOC组中CD133表达阳性率显著低于M-BOT组,而M-BOT组中CD133表达阳性率又显著低于MOA组,提示在MOA、M-BOT和MOC三组中CD133表达阳性率逐步增高,可能提示检测CD133阳性表达率有助于判断卵巢黏液性肿瘤的恶性潜能。据一项CD133高表达与卵巢癌的预后及临床病理特征关系的Meta分析显示,CD133高表达与减少2年生存率(OR=1.67,95%CI:1.06~2.63,P=0.03)及肿瘤分期(OR=0.26,95%CI: 0.12~0.58,P=0.001)密切相关,结果表明CD133过表达与卵巢癌的预后差及临床分期密切相关[21]。李笠[4]研究发现CD133在卵巢癌组织表达阳性率高,与正常卵巢组织相比,差异有统计学意义(P<0.05),随着肿瘤分化程度的不同,从高分化到低分化,CD133表达阳性率逐渐增高。
本研究結果也显示,随着卵巢黏液性腺癌组(MOA)织分化程度降低、临床分期增高,CD133阳性表达率也增高,与卵巢黏液性腺癌恶化程度呈正相关,提示CD133的表达可能与MOA的分化程度相关,在不同年龄段之间CD133的阳性表达却无明显差异,因此,我们猜想检测CD133的表达可能对判断卵巢黏液腺癌的恶性增殖程度和患者预后具有一定的临床价值。
[参考文献]
[1] Siegel RL,Miller KD,Jemal A. Cancer statistics[J]. CA Cancer J Clin,2015,65(1):5-29.
[2] Gil J,Stembalska A,Pesz KA,et al. Cancer stem cells:the theory and perspectives in cancer therapy[J]. J Appl Genet,2008,49(2):193-199.
[3] Arndt K,Grinenko T,Mende N,et al. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells[J]. Proc Natl Acad Sci U S A,2013,110(14):5582-5587.
[4] 李笠. 肿瘤干细胞标志物巢蛋白和CD133在卵巢癌中的表达及临床意义[J]. 中国医师进修杂志,2014,37(9):60-62.
[5] Pisano C,Greggi S,Tambaro R,et al. Activity of chemotherapy in mucinous epithelial ovarian cancer:A retrospective study[J]. Anticancer Res,2005,25(5):3501-3505.
[6] Shimada M,Kigawa J,Ohishi Y,et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary[J]. Gynecol Oncol,2009,113(3):331-334.
[7] Ahmed N,A bubaker K,Findlay J,et al. Cancerous ovarian stem cells:obscure targets for therapy but relevant to chemoresistance[J]. J Cell Biochem,2013,114(1):21-34.
[8] LI C,Liu B,Wen Z,et al. Inhibition of CD44 expression by small interfering RNA to suppress the growth and metastasis of ovarian cancer cells in vitro and in vivo[J]. Folia Biol(Praha),2008,54(6):180-186.
[9] Valen P,Bonnet D,De Maria R,et al. Cancer stem cell definitions and terminology:the devil is in the details[J]. Nat Rev Cancer,2012,12(11):767-775.
[10] Bapat SA,Mali AM,Koppikar CB,et a1.Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer[J]. Cancer Res,2005,65(8):3025-3029.
[11] Mokrowiecka A,Veits L,Falkeis C,et al. Expression profiles of cancer stem cell markers:CD133,CD44,Musashi-1 and EpCAM in the cardiac mucosa-Barrett's esophagus-early esophageal adenocarcinoma-advanced esoph-ageal adenocarcinoma sequence[J]. Pathol Res Pract,2017, 213(3):205-209.
[12] Lee YM,Yeo MK,Seong IO. Nuclear Expression of CD133 Is Associated with Good Prognosis in Patients with Colorectal Adenocarcinoma[J]. Anticancer Res,2018,38(8):4819-4826.
[13] Oakie A,Li J,Fellows GF,et al. Characterization and Differentiation of Sorted Human Fetal Pancreatic ALDH and ALDH/CD133 Cells Toward Insulin-Expressing Cells[J]. Stem Cells Dev,2018,27(4):275-286.
[14] Park JY,Hong D,Park JY,et al. Association between Morphological Patterns of Myometrial Invasion and Cancer Stem Cell Markers in Endometrial Endometrioid Carcinoma[J]. Pathol Oncol Res,2019,25(1):123-130.
[15] Yuan CW,Wang ZC,Liu K,et al. Incomplete radiofrequency ablation promotes the development of CD133 cancer stem cells in hepatocellular carcinoma cell line HepG2 via inducing SOX9 expression[J].Hepatobiliary Pancreat Dis Int,2018,17(5):416-422.
[16] Sarvi S,Mackinnon AC,Avlonitis,et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist[J]. Cancer Res,2014,74(5):1554-1565.
[17] Ruscito I,Cacsire Castillo-Tong D,Vergote I,et al. Exploring the clonal evolution of CD133/aldehyde-dehydrogenase-1(ALDH1)-positive cancer stem-like cells from primary to recurrent high-grade serous ovarian cancer(HGSOC). A study of the Ovarian Cancer Therapy-Innovative Models Prolong Survival(OCTIPS) Consortium[J].Eur. J Cancer,2017,7(79):214-225.
[18] Roy M,Connor J,Al-Niaimi A,et al. Aldehyde dehydrogenase 1A1(ALDH1A1)expression by immunohistochemistry is associated with chemo-refractoriness in patients with high-grade ovarian serous carcinoma[J]. Hum. Pathol,2018,3(73):1-6.
[19] Zhao L,Li J,Liu M,et al. The clinicopathological parameters significance of CD133 and Nestin in epithelial ovarian cancer:a meta-analysis[J]. Future Oncol,2017,13(28):2555-2570.
[20] O'Brien CA,Pollett A,Gallinger S,et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice[J]. Nature,2007,445(7123):106-110.
[21] Zhou Q,Chen A,Song H,et al. Prognostic value of cancer stem cell marker CD133 in ovarian cancer:a meta-analysis[J]. Int J Clin Exp Med,2015,8(3):3080-3088.
(收稿日期:2018-12-21)
