水飞蓟中黄酮和木脂素类化学成分研究
2017-09-03陈效忠张艳
陈效忠,张艳
(黑龙江中医药大学佳木斯学院,黑龙江 佳木斯 154007)
水飞蓟中黄酮和木脂素类化学成分研究
陈效忠,张艳*
(黑龙江中医药大学佳木斯学院,黑龙江 佳木斯 154007)
目的:阐述水飞蓟种子的化学成分。方法:95%乙醇提取药材,综合运用多种色谱技术(如硅胶柱色谱、葡聚糖凝胶柱色谱、高效液相制备色谱等)分离得到5个化合物,经NMR鉴定化合物结构。结果:从水飞蓟种子中分离得到1个黄酮和4个木脂素类化合物。结论:分离得到的5个化合物为(1R,5R,6R)-6-{4-O-[2-(1-(4-hydroxyphenyl-3-methoxy))glycerol]-3,5-dimethoxyphenyl}-3,7-dioxabicyclo[3.3.0]octan-2-one (1), acutissimalignan B (2), (+)-(7S,8S)-1′,4-dihydroxy-3,3′,5′-trimethoxy-7′,8′,9′-trinor-8,4′-oxyneoligna-7,9-diol (3), (+)-(7S,8S)-4-hydroxy-3,3′,5′-trimethoxy- 8′,9′-dinor-8,4′-oxyneoligna-7,9-diol-7′-oic acid (4), (7′S,8′S)-Bilagrewin (5),以上化合物均为从该植物中首次发现。
水飞蓟;黄酮;木脂素
水飞蓟(SilybummarianumL.Gaertn)又称水飞雉、乳雉子,是菊科水飞蓟属植物[1]。水飞蓟有效成分在果实内,水飞蓟的干燥果实经提取、精制而得到的有效的黄酮类成分称为水飞蓟素[2]。国内外学者还对水飞蓟植物的其他化学成分进行了研究,发现了一些非黄酮类成分,如糖、氨基酸、木脂素、三萜、甾醇、有机酸等化学成分[3]。为了进一步研究水飞蓟的化学成分, 本文采用多种色谱法从95%乙醇提取物中分离并鉴定了1个黄酮和4个木脂素类化合物。
1 仪器与材料
NMR-600型核磁共振波谱仪;岛津公司制备型HPLC;C18反相硅胶柱色谱。
药材经黑龙江中医药大学佳木斯学院陈效忠副教授鉴定为水飞蓟(SilybummarianumL.Gaertn),标本保存于黑龙江中医药大学佳木斯学院标本馆。
2 提取与分离
水飞蓟种子10 kg压榨法去油,95%乙醇提取得到浸膏0.8 kg,浸膏经硅胶柱色谱,以二氯甲烷-甲醇洗脱,得到Fr.A-Fr.F六个部位,Fr.E经葡聚糖凝胶Sephadex LH-20柱色谱,甲醇-水(10:1~100:0)洗脱, 得到S1~S10组分,S6用制备型HPLC分离纯化得到化合物1 (7 mg), 2 (6 mg)和3 (5 mg), S10用制备型HPLC分离纯化得到化合物4(6 mg)和5(8mg)。
3 结构鉴定
化合物1,白色粉末。1H-NMR (CDCl3, 500 MHz): δ 3.50 (1H, m, H-1),4.40 (1H, d, J=10.0 Hz, H-4a), 4.25 (1H, dd, J=10.0, 3.5 Hz, H-4b), 3.16 (1H, dd, J=10.0, 3.5 Hz, H-5), 4.68 (1H, d, J=7.0 Hz, H-6), 4.54 (1H, dd, J=10.0, 7.0 Hz, H-8a), 4.40 (1H, dd, J=10.0, 1.0 Hz, H-8b), 6.66 (2H, s,H-2′和H-6′),6.98 (2H, s,H-2″),6.88 (1H, d,J=8.5, H-5″),6.77 (1H, d,J=8.5, H-6″),5.00 (1H, d,J=4.0, H-7″),4.18 (1H, dd,J=6.5, 3.9, H-8″),3.90 (1H, m, H-9″a),3.50 (1H, m, H-9″b),3.90 (9H, s, OMe);13C-NMR (CDCl3, 125 MHz): δ46.5 (C-1), 177.9 (C-2), 70.5 (C-3), 36.5 (C-4), 49.0 (C-5), 86.2 (C-6), 70.1 (C-8), 135.0 (C-1′), 103.0 (C-2′和C-6′), 153.7 (C-3′和C-5′), 133.7 (C-4′), 131.0 (C-1″), 108.0 (C-2″), 145.0 (C-3″), 146.8 (C-4″), 114.3 (C-5″), 118.6 (C-6″), 72.5 (C-7″), 87.5 (C-8″), 60.2 (C-9″), 56.0 (C-3′和C-5′-OMe), 56.2 (C-3″-OMe)。以上数据与文献[4]报道一致,故鉴定化合物为(1R,5R,6R)-6-{4-O-[2-(1-(4-hydroxyphenyl-3-methoxy))glycerol]-3,5-dimethoxyphenyl}-3,7-dioxabicyclo[3.3.0]octan-2-one (图1)。
化合物2,白色粉末。1H-NMR (CDCl3, 500 MHz): δ7.56 (1H, d, J=1.8 Hz, H-6), 7.20 (1H, dd, J=8.3, 1.9 Hz, H-6′), 7.05 (1H, d, J=1.9 Hz, H-2′), 7.00 (1H, d, J=8.3 Hz, H-5′), 6.85 (1H, d, J=8.0 Hz, H-5″), 6.75 (1H, dd, J=8.0, 1.9 Hz, H-6″), 6.65 (1H, d, J =1.9 Hz, H-2″), 4.29 (2H, overlap, H-4a, H-4b), 3.93 (3H, s, OMe-3′), 3.85 (3H, s, OMe-3″), 3.82 (1H,m, H-3), 3.08 (1H, dd, J=14.5, 4.3 Hz, H-5a), 2.65 (1H, dd, 14.5, 10.0 Hz, H-5b);13C-NMR (CDCl3, 125 MHz): δ172.3 (C-1), 147.6 (C-4′), 146.9 (C-3′), 146.3 (C-3″), 144.5 (C-4″), 137.6 (C-6), 129.6 (C-1″), 126.5 (C-1′), 125.7 (C-2), 124.0 (C-6′), 121.4 (C-6″), 115.4 (C-5′), 114.3 (C-5″), 112.6 (C-2′), 111.9 (C-2″), 69.3 (C-4), 56.0 (OMe-3′), 55.9 (OMe-3″), 39.7 (C-3), 37.5 (C-5)。以上数据与文献[5]报道一致,故鉴定化合物为acutissimalignan B (图1)。
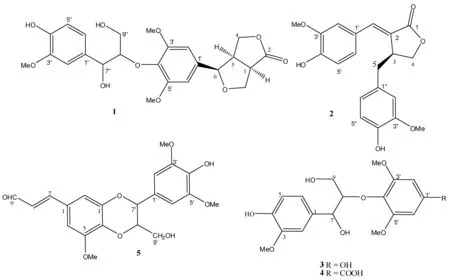
图1 化合物的结构
化合物3,白色粉末。1H-NMR (CDCl3, 500 MHz): δ 7.10 (1H, d, J=2.0, H-2),6.78 (1H, d, J=8.0, H-5),6.80 (1H, dd, J=8.0, 2.0, H-6),5.00 (1H, d, J=7.0, H-7),3.74 (1H, m, H-8),3.59 (1H, dd, J=12.0, 3.0, H-9a),3.22 (1H, d, J=12.0, 3.0, H-9b),6.20 (1H, brs, H-2′),6.20 (1H, brs, H-6′), 3.81 (6H, s, OMe), 3.82 (6H, s, OMe);13C-NMR (CDCl3, 125 MHz):δ 133.9 (C-1), 111.8 (C-2), 147.9 (C-3), 148.3 (C-4), 115.0 (C-5), 120.9 (C-6), 74.0 (C-7), 90.8 (C-8), 61.0 (C-9), 156.0 (C-1′), 94.0 (C-2′), 154.8 (C-3′), 130.0 (C-4′), 154.3 (C-5′), 94.0 (C-6′), 56.0 (C-3和C-5-OMe), 56.4 (C-3′和C-5′-OMe。以上数据与文献[6]报道一致,故鉴定化合物为(+)-(7S,8S)-1′,4-dihydroxy-3,3′,5′-trimethoxy-7′,8′,9′-trinor-8,4′-oxyneoligna-7,9-diol (图1)。
化合物4,白色粉末。1H-NMR (CDCl3, 500 MHz): δ 7.00 (1H, d, J=2.0, H-2),6.70 (1H, d, J=8.0, H-5),6.85 (1H, dd, J=8.0, 2.0, H-6), 4.95 (1H, d, J=7.0, H-7),4.06 (1H, m, H-8),3.78 (1H, dd, J=12.0, 3.0, H-9a),3.25 (1H, d, J=12.0, 3.0, H-9b),7.20 (1H, brs, H-2′),7.20 (1H, brs, H-6′), 3.87 (6H, s, OMe), 3.85 (6H, s, OMe);13C-NMR (CDCl3, 125 MHz):δ 133.5 (C-1), 111.5 (C-2), 148.9 (C-3), 148.3 (C-4), 115.0 (C-5), 120.9 (C-6), 74.0 (C-7), 90.8 (C-8), 61.0 (C-9), 123.0 (C-1′), 107.0 (C-2′), 153.8 (C-3′), 138.0 (C-4′), 152.3 (C-5′), 108.0 (C-6′), 156.5 (C-7′), 56.0 (C-3和C-5-OMe), 56.4 (C-3′和C-5′-OMe)。以上数据与文献[6]报道一致,故鉴定化合物为(+)-(7S,8S)-4-hydroxy-3,3′,5′-trimethoxy- 8′,9′-dinor-8,4′-oxyneoligna-7,9-diol-7′-oic acid (图1)。
化合物5,黄色粉末, HCl-Mg 反应阳性, FeCl3反应阳性。1H-NMR (CDCl3, 500 MHz): 6.67 (2H, s, H-2′ 和 H-6′), 3.96 (6H, s, OMe-3′ 和 OMe-5′), 4.95 (1H, d, J=8.0 Hz, H-7′), 4.08 (1H, dt, J=8.0, 3.0 Hz, H-8′), 3.60 (1H, m, H-9′a), 3.92 (1H, m, H-9′b), 6.92 (1H, d, J=2.0 Hz, H-2), 3.93 (3H, s, OMe-5), 6.75 (1H, d, J=2.0 Hz, H-6), 7.35 (1H, d, J=16.0 Hz, H-7), 6.62 (1H, dd, J=16.0, 7.5 Hz, H-8), 9.68 (1H, d, J=7.6 Hz, CHO);13C-NMR (CDCl3, 125 MHz):δ126.9 (C-1′), 104.8 (C-2′), 147.8 (C-3′), 135.6 (C-4′), 147.3 (C-5′), 104.5 (C-6′), 76.8 (C-7′), 79.3 (C-8′), 61.5 (C-9′), 127.0 (C-1), 111.8 (C-2), 144.5 (C-3), 136.5 (C-4), 149.6 (C-5), 104.5 (C-6), 152.3 (C-7), 127.8 (C-8), 193.6 (C-9) 56.7 (OMe-3′), 56.6 (OMe-5), 56.7 (OMe-5′)。以上数据与文献[7]报道一致,故鉴定化合物为(7′S,8′S)-Bilagrewin (图1)。
[1] 陈毓荃,王春梅.水飞蓟综合利用基础研究[J].西北农业学报,1997,6(4):91-93.
[2] 柯铭清.中草药有效成分理化与药理特性[M].长沙:湖南科学技术出版社,1982:266.
[3] 石玉生,吴鹏程,张艳.水飞蓟宾对UVA致人表皮角质形成细胞光老化保护作用的研究[J].中医药信息,2014,31(3):20-21.
[4] Xian-Wen Yang, Pei-Ji Zhao, Yan-Lin Ma,et al.Mixed Lignan-Neolignans from Tarenna attenuate[J].J. Nat. Prod,2007,70(4):521-525.
[5] Patoomratana Tuchinda, Jittra Kornsakulkarn, Manat Pohmakotr, et al.Dichapetalin-Type Triterpenoids and Lignans from the Aerial Parts of Phyllanthus acutissima[J].J. Nat. Prod,2008,71,655-663.
[6] Liang Xiong, Cheng-geng Zhu, Yan-ru Li, et al. Lignans and Neolignans from Sinocalamus affinis and Their Absolute Configurations[J].J. Nat. Prod,2011,74,1188-1200.
[7] Jih-Jung Chen, Tzu-Ying Wang, Tsong-Long Hwang. Neolignans, a Coumarinolignan, Lignan Derivatives, and a Chromene: Anti-inflammatory Constituents fromZanthoxylum a Wicennae[J].J. Nat. Prod, 2008,71(2):212-217.
Flavonoid and Lignins fromSilybummarianumL.Gaertn
CHEN Xiao-zhong, ZHANG Yan
(JiamusiCollegeofHeilongjiangUniversityofChineseMedicine,Jiamusi154007,China)
Objective:To investigate the chemical constituents from the seeds ofSilybummarianumL.Gaertn. Methods:The seeds ofSilybummarianumL.Gaertn were extracted with 95% ethanol. Five compounds were extracted from the plant by various isolation methods (including silica gel column chromatography, sephadex-LH20, and HPLC), and compound structures were determined by NMR spectra. Results:Five compounds were separated fromSilybummarianumL.Gaertn for the first time, including one flavonoid and four lignins, which were (1R,5R,6R)-6-{4-O-[2-(1-(4-hydroxyphenyl-3-methoxy))glycerol]-3,5-dimethoxyphenyl}-3,7-dioxabicyclo[3.3.0]octan-2-one(1), acutissimalignan B(2), (+)-(7S,8S)-1′,4-dihydroxy-3,3′,5′-trimethoxy-7′,8′,9′-trinor-8,4′-oxyneoligna-7,9-diol(3), (+)-(7S,8S)-4-hydroxy-3,3′,5′-trimethoxy- 8′,9′-dinor-8,4′-oxyneoligna-7,9-diol-7′-oic acid(4), and (7′S,8′S)-Bilagrewin(5).
SilybummarianumL.Gaertn; Flavonoid; Lignins
2016-08-26
2016-09-15
黑龙江中医药大学佳木斯学院自主课题
陈效忠(1962-),男,副教授,主要从事常用中草药药效物质基础研究。
*通讯作者:张艳(1982-),女,硕士,讲师,主要从事常用中草药微量活性成分研究。
R284.1
A
1002-2392(2017)04-0064-03
