Experiment study of the effects of hydrodynamic disturbance on the interaction between the cyanobacterial growth and the nutrients*
2016-10-18JianHUANG黄健BeidouXI席北斗QiujinXU许秋瑾XixiWANG王喜喜WeipingLI李卫平LianshengHE何连生HongliangLIU刘鸿亮
Jian HUANG (黄健), Bei-dou XI (席北斗), Qiu-jin XU (许秋瑾), Xi-xi WANG (王喜喜),Wei-ping LI (李卫平), Lian-sheng HE (何连生), Hong-liang LIU (刘鸿亮)
1. School of Environment, Beijing Normal University, Beijing 100875, China
2. Chinese Research Academy of Environmental Sciences, Beijing 100012, China
3. Department of Civil and Environmental Engineering, Old Dominion University, Virginia, USA,
E-mail: jianhuang225@gmail.com
4. School of Environment, Inner Mongolia University of Science and Technology, Baotou 014010, China
Experiment study of the effects of hydrodynamic disturbance on the interaction between the cyanobacterial growth and the nutrients*
Jian HUANG (黄健)1,2,3, Bei-dou XI (席北斗)2, Qiu-jin XU (许秋瑾)2, Xi-xi WANG (王喜喜)3,Wei-ping LI (李卫平)4, Lian-sheng HE (何连生)2, Hong-liang LIU (刘鸿亮)2
1. School of Environment, Beijing Normal University, Beijing 100875, China
2. Chinese Research Academy of Environmental Sciences, Beijing 100012, China
3. Department of Civil and Environmental Engineering, Old Dominion University, Virginia, USA,
E-mail: jianhuang225@gmail.com
4. School of Environment, Inner Mongolia University of Science and Technology, Baotou 014010, China
The eutrophication of shallow lakes is sensitive to dynamic currents (i.e., disturbances) because of their shallow depths and high contents of nutrients in bed sediments. The relation between the sediment resuspension and the algae bloom is not well understood in the field scale because the interwoven influencing factors cannot be examined individually. By combining the laboratory experiment and the field observation, this paper proposes a sediment-water-algae concept to simulate the effects of hydrodynamic disturbances on the algae growth in the Taihu Lake located in east China. The sediments are sampled from the Taihu Lake while the Microcystis aeruginosa (M. aeruginosa) algae is cultured in the laboratory and then transplanted into the experiment cylinders. The temperature and the light intensity in the experiment are adjusted to be similar with the prevalent in situ conditions. The results indicate that the M. aeruginosa populations under the disturbance condition of the rotational speedin the experiment (corresponding to the bottom velocity flow, the shear stress, or the wind speedin the field) are higher than those under the disturbance condition of the rotational speed is 400 rad/min (corresponding to the bottom flow velocity 0.079 m/s, the shear stress 0.124 N/m2). It is suggested that a low to moderate disturbance prompts the release of the nitrogen as well as the phosphate nutrients from the bed sediments, amplifying the eutrophication of the Taihu Lake.
bed sediment, eutrophication, hydrodynamics, Taihu Lake, M. aeruginosa
Introduction
The eutrophication in lakes and reservoirs is a worldwide environmental problem that threatens the ecosystem health and results in imbalances among different biological processes as well as a decrease of ecosystem biodiversity[1]. The state of shallow lakes is relatively easy to change because they: (1) have shallow depths with high concentrations of sediment,(2) lack a stable long-term thermal stratification[2,3], (3)incur a frequent mixing of the entire water column and the resuspension of unconsolidated sediments[4], and(4) have a substantial internal loading of nutrients from the sediments to the water column[5]. In lakes with such features, the water quality conditions are likely to have a complex relationship with human activities as well as climate and hydrology conditions.
Studies of shallow lakes usually aim to cope with the eutrophication and the harmful algae bloom due to nutrient inputs[6,7], human activities[8,9], and climate change[10,11]. The algae is a diverse group of simple and typically autotrophic organisms ubiquitous in the source water. The algae bloom can degrade the water quality (e.g., the disinfection by products, the unpleasant taste and ordor). In a eutrophic shallow lake, its bed sediment can be the dominant source of nutrients(e.g., nitrogen and phosphorus) of the lake water once the exogenous inputs are effectively controlled[12]. The hydrodynamic condition is a key factor affecting the occurrence of chemicals in the lake water. A number of studies[13,14]revealed that the water currents can propel the resuspension of the bed sediments and help to release chemicals. The dynamic turbulence can reduce the thickness of the diffusion boundary layer and thus enhance the mass transport (i.e., molecular diffusion flux) of chemicals across the sediment-water interface[15,16]. However, because it is not easy to examine in the field scale how individual factors (e.g.,hydrodynamic disturbance) influence such a mass transport process, few studies were documented with quantitative relations between the eutrophication of shallow lakes and the dynamic disturbance.
For a given nutrient level, the algae growth is closely related to the hydrodynamic conditions[17]. Low to moderate disturbances are found to be beneficial for the algae growth[18], whereas strong disturbances have a restrictive effect[19]. Herein, the question is whether a critical hydrodynamic condition exists,beyond which the algae growth will be restricted. A scientific answer to this question is crucial for the environmental considerations related to the lake eutrophication, but it is not yet available in the existing literature. In fact, previous simulation studies of the algae growth in the nutrient medium[19-21]and the lake water[22,23], more often than not, did not consider the influences of the hydrodynamic disturbance on the release of chemicals at the sediment-water interface,likely to give biased or even erroneous results for lakes(including the typical shallow lake of the Taihu Lake in southeast China illustrated in Fig.1) with bed sediments of high contents of nutrients.
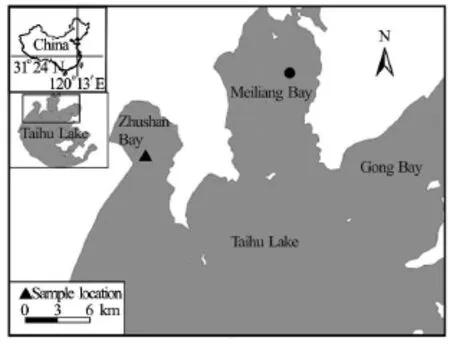
Fig.1 The location of Zhushan Bay within the Taihu Lake basin,where samples are taken for this study
The objective of this study is to integrate the laboratory experiment with the field observation to reveal the effects of the hydrodynamic disturbance on the nutrient release and the algae growth in the Taihu Lake. In the laboratory experiment, the sedimentwater-algae specimens with similar characteristics of the Taihu Lake are used, and the water temperature and the light intensity are adjusted to be similar with the prevalent in situ conditions. The field observations include the flow velocity and the wind speed (in place of hydrodynamic disturbances), the primary production (Chl-a), and the nutrient species of the total nitrogen (TN) and the total phosphorus (TP). In order to isolate the effects of the hydrodynamic disturbance from those of other factors (e.g., the light and the temperature), the laboratory experiments are highly controlled and the experiment results are interpreted in terms of the field data.
1. Experiment
1.1 Taihu Lake
The Taihu Lake is a typical shallow lake located in the south of the Yangtze River Delta in China(Fig.1). The lake’s average water depth is 1.9 m, with a total surface area of 2 338 km2[7]. In spite of being important regional fresh water source[24], the Taihu Lake is infamous for its widespread algae bloom and thus the water quality is seriously degraded.
The Taihu Lake is frequently influenced by the winds, with wind-induced currents to transport dissolved matters (e.g., nutrients) and to exchange water[25]. The dominant wind direction on the lake is southeast in the summer and northwest in the winter, with a mean wind speedof 3.5 m/s to 5.0 m/s[26]. As a result, nutrients tend to be accumulated, and the algae bloom is usually the severest, in the semi-enclosed Zhushan Bay and Meiliang Bay[12]. These two bays are similar in terms of the bed sediment characteristics[27,28]and the hydrology conditions[29]. Thus, it is assumed in this study that these two bays share common physical mechanisms of sediment resuspension and algae growth, and sediment samples from the Zhushan Bay are used in the laboratory experiments and field observations are made in the Meiliang Bay. The reason for taking sediment samples from the Zhushan Bay is that we have no logistic access to the Meiliang Bay.
1.2 Sampling location and methods
Common estimates of the “active” sediment depth vary between the top 0 m and 0.1 m in shallow lakes[30]. For this reason, the bed sediment samples taken from a 0.1 m thick layer in a typical region (shown as the solid triangle in Fig.1 of this bay) are used. The samples are collected using a Petersen grab sampler from the Wuhan Hengling Technology Company, Ltd.All samples are stored in a refrigerator atuntil subsequent laboratory operations (e.g., makingspecimens).
The samples are used to make the experimental specimens to be discussed in detail in the following sections. On the sampling day of June 2012, the sampling region, with a water depth of 1.8m, sees severe pollutions from industrial wastewater discharge and ship transportation wastes. An analysis of the samples indicate that the bed sediments consist of sandy-silty particles with a mean diameter of around 17 µm, and have a TP content of 0.49 g⋅P/kg dry solid and a TN content of 1.58 g∙N/kg dry solid.
1.3 Algae
Chen et al.[31]found that from 1992 to 2002, the phytoplankton assemblage in the Taihu Lake is mainly composed of four abundant algae groups, namely the cyanobacteria, the diatoms, the green algae, and the cryptophyceae. The cyanobacteria, which accounts for 38.3% of the total phytoplankton biomass, is dominated by the microcystis, which in turn accounts for 85.7% of the total cyanobacteria biomass[32]. The microcystis and the oscillatoria, two genera of bloomforming blue-green algae, can often be found in shallow eutrophic lakes[33]. In the Taihu Lake, the microcystis is found to be more prevalent than the oscillatoria in most years[24,34]. Thus, the microcystis aeruginosa (M. aeruginosa) is selected as the indicating algae in this study.
The M. aeruginosa, obtained from the Chinese Research Academy of Environmental Sciences(CRAES) Innovation Base of Lake Eco-environment,is raised in M11 medium (the pH value is adjusted to 8.0) with composites per liter solution of: 100 mg NaNO3, 10 mg K2HPO4, 75 mg MgSO4·7H2O, 40 mg CaCl2·2H2O, 20 mg Na2CO3, 6 mg ferric citrate, and 1 mg Na2EDTA·2H2O[35,36]. Stock cultures (approximately 105cells m/L) of the M. aeruginosa are maintained in 500 mL flasks sealed by membrane. When the population density of the cultures grows to about approximately 107to 108cells m/L, for about 10 d, the stock algae is transferred to experiment cylinders. Before used in the experiments, all equipment and medium are sterilized at 121oC for 0.5 h. All cultures are cultivated in an illumination incubator Safe PGX under the conditions of, 2 000 lux, and light/ darkcycles[37,38]. The incubator is from Ningbo Haishu Apparatus Company of China(http://www.hktdc.com/en-buyer).
1.4 Sediment-water interface dynamic simulator
1.4.1 Dynamic simulator
In this study, an innovative apparatus illustrated in Fig.2 is used to simulate the effects of various disturbances on the algae growth. The apparatus consists of a motor stirrer and a transparent reactive cylinder of 0.15 m in diameter and 0.25 m in height (equivalently an effective volumetric capacity of 4 L). The motor stirrer (Fig.2) operates at various rotational speeds to simulate different hydrodynamic conditions. For a given rotational speed, three identical apparatuses operate synchronically to obtain three trial values at any measurement time.
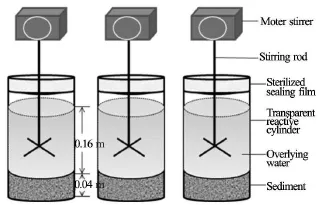
Fig.2 Experimental apparatus (phytotron)
In order to have a consistent initial state across all experimental trials, the sediments samples are uniformly mixed with a stirring rod. Subsequently, the uniformly mixed sediments are sterilized at 121oC for 1 h to eliminate any microorganisms[39]and then used to make experiment specimens. Limited by the volumetric capacity of the cylinder, the specimen in a cylinder is of 0.04 m in height. A 3 L deionized water(sterilized at 121oC for 0.5 h) sample is slowly (to avoid sediment resuspension) added into the cylinder through a siphon to a height of 0.16 m. A blade stirrer hangs above the cylinder. Based on several preliminary runs and literature values[40], the stirring rod is inserted into the water column at a position 0.05 m above the specimen top surface (Fig.2). Finally, the cylinder is sealed by a sterilized film. After 2 d when an equilibrium is established[40], the M. aeruginosa reaches its exponential growth phase and are inoculated into the cylinders at a density of 8×105cells m/L[36]. The phytotron conditions arelux, and light/darkcycles[37,38].
To simulate possible natural disturbances in the Zhusan Bay of the Taihu Lake, the blade stirrer operates at different rotational speeds to represent typical situations. For each rotational speed (i.e., in a given run), 100 mL water sample is extracted from each of the three trial cylinders on each of the 21 trial days. Because the extracted water is not replenished to minimize possible alternations of the algal density and the nutrient concentrations, the water volume in a cylinder at the end of a trial is reduced to 2/3 of that at the beginning of the trial, which is equivalent to a sediment-water ratio change of 67%. This change is judged to least impact the study because no abnormal (e.g.,sudden) jump or plump is observed in the measure-ments.
Each trial water sample is analyzed for the TN,the TP, the dissolved total nitrogen (DTN), the dissolved total phosphorus (DTP), the ammonium nitrogen(NH4-N), the ortho-phosphate (PO4-P), the Chl-a, and the algae density. The six types of nutrients are analyzed by following the standard methods described in Chen et al[31,41], while the Chl-a is extracted with the acetone (90%) and determined using a spectrophotometer-based method[42]. The algae density is determined by counting three times for each sample with a haemacytometer (TATAL in Japan, http://www.totallub.jp) under a microscope. In addition, the particulate phosphorus (PP) is estimated as the difference between the TP and the DTP (i.e.,), while the particulate nitrogen (TN) as the difference between the TN and the DTN (i.e.,
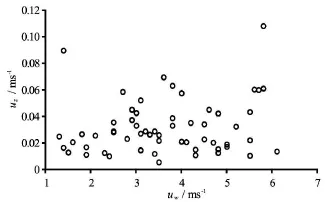
Fig.3 The relation between field flow velocity and wind speed
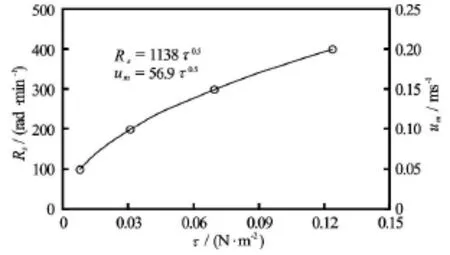
Fig.4 The rotational speeds used in the laboratory experiment and the responding bottom flow velocities in real Taihu Lake versus the resulting bottom shear stresses in real Taihu Lake
1.4.2 Selection of rotational speeds based on field data
The hydrodynamic stresses (i.e., the wind speed and the flow velocity), the chlorophyll-a (Chl-a) and the chemical parameters (TN and TP) in the Meiliang Bay of the Taihu Lake were synchronously measured by the Najing Institute of Geography and Limnology,Chinese Academy of Sciences (NIGLCAS) in 2010 and 2011 by following the guidance of Chinese Ministry of Environmental Protection. The measured data were checked for quality by experts in NIGLCAS. The wind speed was continuously measured/recorded at the Meiliang Bay station (solid circle in Fig.1) with a time interval of ten minutes. At this same station, the flow velocitynear the sediment bed, which induces erodible shear stresses under field conditions, was measured using a SonTek/YSI Argonaut (USA) positioned in the water 0.5 m above the bed to collect data ofwith a time interval of three days. Water samples were taken and analyzed for TN and TP concentrations using the standard methods described in Chen et al.[31,41], while Chl-a concentrations of the water samples were determined by filtering (GF/C,Whatman), extracting with ethanol (90%), and analyzing spectrophotometrically at 750 nm and 665 nm[42]. A statistical analysis of the field data (Fig.3) indicates that the occurrences of the flow velocity less than 0.05 m/s and the wind speed less than 4 m/s are both greater than 70%. This study examines the shear stress caused by the lake currents because they are considered to have similar dynamic effects of the stirring rod on the sediment resuspension and because the dynamic simulator could produce shear stresses of water currents in the laboratory. The shear stress (Fig.4)under the field condition is calculated using the observed bottom flow velocity(i.e., velocity 0.5 m above the bottom) of the Taihu Lake.
Shear stress under the field condition is computed as[43]

The bottom boundary velocity is computed as

Based on the similarity principle[46], the rotational speeds(Fig.4) are selected so that the Froude numberin the laboratory condition is equal tothatin the field condition.

Table 1 The shear stresses and rotational speeds computed by Eqs.(1) and (6)

Under the laboratory condition

Under the field condition

From the general relation between the tangential and angular speeds, the laboratory experimental rotational speed is

The field observed bottom flow velocity varies from 0.003 m/s to 0.37 m/s. As stated above, the occurrence of the bottom flow velocities less than 0.05 m/s is over 70%. The rotational speeds are selected to simulate the prevailing bottom flow velocities of 0 m/s to 0.2 m/s in the Taihu Lake. As a result, the blade stirrer operates at the rotational speeds of 0(CK), 100 (Cl), 200 (C2), 300 (C3), and 400 (C4) in the laboratory experiment (Table 1).
1.5 Data processing methods

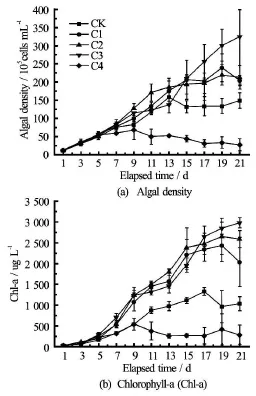
Fig.5 Plots showing mean (solid line) and range (vertical bar)of trial values concentration versus measurement/elapse time at rotational speeds (rad/min) of 0 (CK), 100 (C1),200 (C2), 300 (C3), and 400 (C4)
For each measured nutrient, its instantaneous sediment release rate is computed as[48]
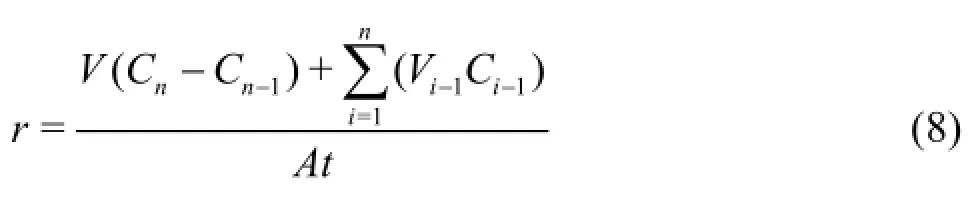
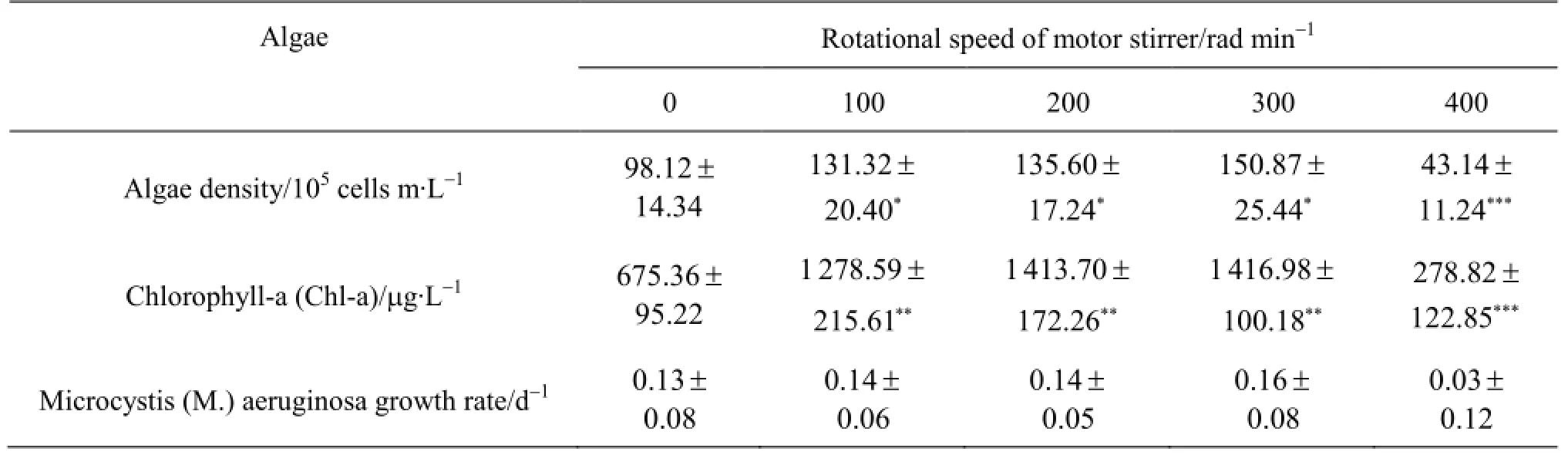
Table 2 The mean ± standard deviation of algae growth
1.6 Statistical analysis methods
For each experimental run, the measured values in all trials for a given parameter are pooled together to form one single dataset, which in turn is used to compute the mean and the standard deviation for this parameter in this run. On the other hand, for a given parameter, the datasets of all experimental runs are used to conduct a multiple comparison t-test in the16.0 to test the null hypothesis: the parameter values under the disturbance conditions (i.e., C1,C2, C3, and C4) are the same as those under the control/static condition (i.e., CK) at significance levels of0.05, 0.01, and 0.001. The results from those computations and tests are tabulated to detect any differences among the experimental runs. In addition,for a given experimental run, for each parameter and on each measurement day, the three trial values are arithmetically averaged to compute the average value of this parameter on that day. The maximum and minimum deviations of these three values from the average value are also computed. The average values along with the maximum and minimum deviations are plotted over the measurement day in the OriginLab®Origin 8.5 to visually examine whether and how the results of the runs are different. Further, the laboratory experiment and field observation data are plotted in the box graph at a typical hydrodynamic level.
2. Results
2.1 Effects on algae growth
Overally, the algae density and the Chl-a concentration under the disturbance condition of the rotational speedare insignificantly higher than those under the control condition (CK)(p-, whereas under the disturbance condition of the rotational speed is 400 rad/min they are insignificantly lower(Fig.5 and Table 2). In addition, the algae density and the Chl-a concentration increase with the disturbance intensity until the rotational speed reaches 300 rad/min, and when the rotational speed goes above 400 rad/min, the Chl-a starts to decrease. The algae density and the Chl-a concentration at the rotational speed is 400 rad/min change significantly as compared with those at the rotational speed
Averaged across the 21 measurement days, the algae density and the Chl-a concentration increase over time when the rotational speed(Fig.5) including under the static condition, but the indicators decrease over time when the rotational speed is 400 rad/min. Both indicators initially increase slowly and then either increase rapidly or decrease gradually,depending on the rotational speed. The peaks of the Chl-a concentration and algae density occur on 21d for the rotational speed of 300 rad/min but on 19 d for the speeds of 100 rad/min and 200 rad/min. The algae density and the Chl-a concentration reach the peak magnitudes of 1.51 × 107cells m/L and 1 417 g/L,respectively.
M. aeruginosa adapts itself to the disturbed sediment-water interface environment on 3 d, after which it starts to grow rapidly. M. aeruginosa starts to grow logarithmically from 5 d (Fig.5), when the algae density is 5.7×106cells m/L, the Chl-a concentration is 292 µg/L, and the water color is pale green. Its growth plateaus on 15 d, when the algae density is 2.1×107cells m/L, the Chl-a concentration is 1 963 µg/L, and the water color becomes dark green. The M.aeruginosa growth rate is statistically relative with the disturbance intensity, while it takes a maximum value of 0.16 d-1at 300 rad/min and a minimumvalue of 0.03 d-1at 400 rad/min.
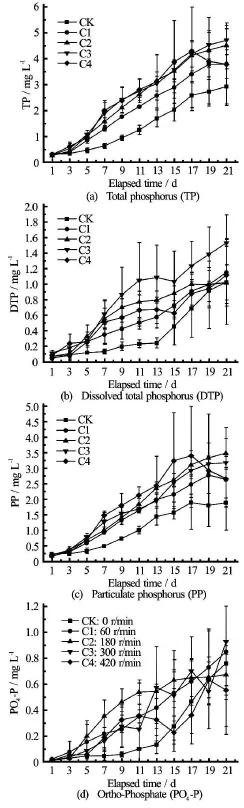
Fig.6 Plots showing mean (solid line) and range (vertical bar)of trial concentrations versus measurement/elapse time at rotational speeds (rad/min) of 0 (CK), 100 (C1), 200 (C2),300 (C3), and 400 (C4)
2.2 Effects on nutrients release
The results directly indicate that all phosphate nutrients under the disturbance conditions of this experiment are higher in content, but have either a larger or smaller variation, than under the control condition(Fig.6). TP, DTP and PP concentrations (Figs.6(a)-6(c)) under the disturbance conditions of the rotational speedand 300 rad/min are insignificantly higher than those under the control condition(Table 3). Similarly, the release rates of the phosphate nutrients under the disturbance condition are higher than those under the control condition. The phosphate nutrient release rates increase with the increase of the disturbance intensity when the rotational speed, and then start to decrease with the further increase of the disturbance intensity. Averaged across the 21 measurement days,the phosphate nutrient concentrations increase over time regardless of the disturbance or under the static condition. However, the phosphate nutrient release rate(Table 3) could reach the peak value when the rotational speed, its decrease above the rotational speed 400 rad/min. P04-P (Fig.6(d)) under the disturbance conditions is not more regular than others.
Similarly, the nitrogen nutrient concentration and release rate are also functions of the disturbance intensity and the time. They vary greatly with the disturbance intensities (Table 4 and Fig.7). The TN and PN concentrations (Figs.7(a), 7(c)) are higher than those under the control condition at the rotational speed, whereas they lower than those under the control condition at the rotational speed is 400 rad/min. This exception might indicate that an intense disturbance can facilitate the adsorption of the particulate nitrogen into the surface of the sediment particles and the resulting flocculates would become heavy enough to deposit[22]. The DTN and NH4-N concentrations (Figs.7(b), 7(c)) are insignificantly higher than those under the control condition(p-, regardless of the rotational speed. Averaged across the 21 measurement days, the DTN and NH4-N concentrations increase with the disturbance intensity and the elapse time. The TN and PN concentrations increase with the disturbance intensity and the elapse time as long as the rotational speed, whereas they decrease at the rotational speed is 400 rad/min. Overally, a low to moderate disturbance is judged to prompt the release of nitrogen nutrients from the bed sediments in the Zhusan Bay of the Taihu Lake, while such promotion would be more uncertain and thus less consistent than that for phosphate nutrients (Fig.7 versus Fig.6).
2.3 Laboratory experiment results versus field observations
The relation between the flow velocity, the shear stress and the rotational speed (Table 1) indicates that the shear stress increases with the rotational speed or the bottom flow velocity. The rotational speed of 300 rad/min or the bottom flow velocity of 0.06 m/sproduces a shear stress of 0.069 N/m2through the above computation, which is the predominant hydrodynamic condition of the Taihu Lake. A comparison analysis is conducted utilizing the Chl-a data from the laboratory experiment and the field observation. The Chl-a concentration under the disturbance condition of the rotational speedis found to be significantly higher than that at the rotational speed is 400 rad/min () in the laboratory. A similar relation is also observed in the field, the Chl-a concentration whenis significantly higher than that when(Fig.8). As in the laboratory experiment, the field observation has also revealed a positive re lation tha t a mode rate hydrodynamic disturbance isgoodforthealgaegrowth.However, the negative relation indicates that a strong hydrodynamic disturbance would be bad for the algae growth.
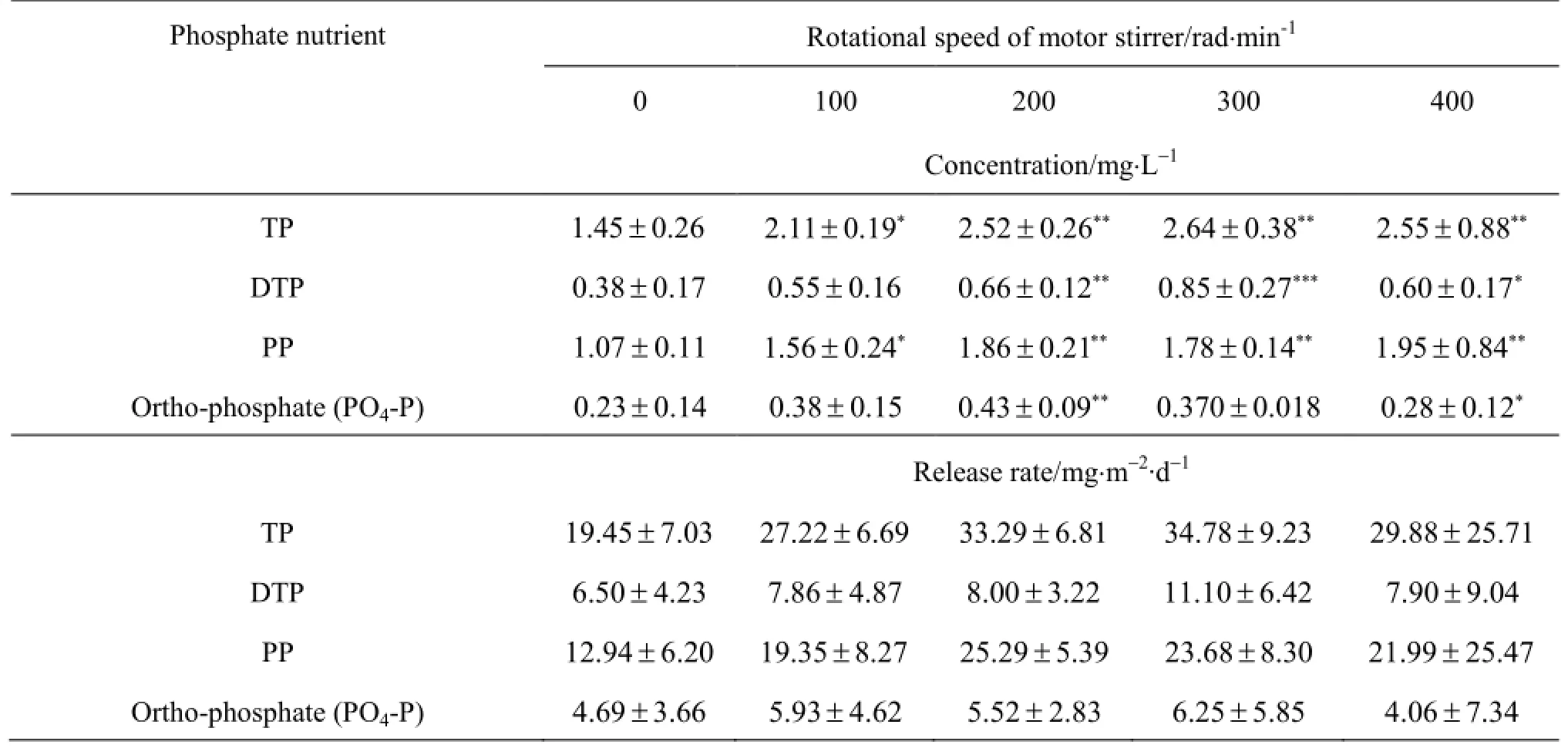
Table 3 The mean ± standard deviation of phosphate nutrient concentration or release rate
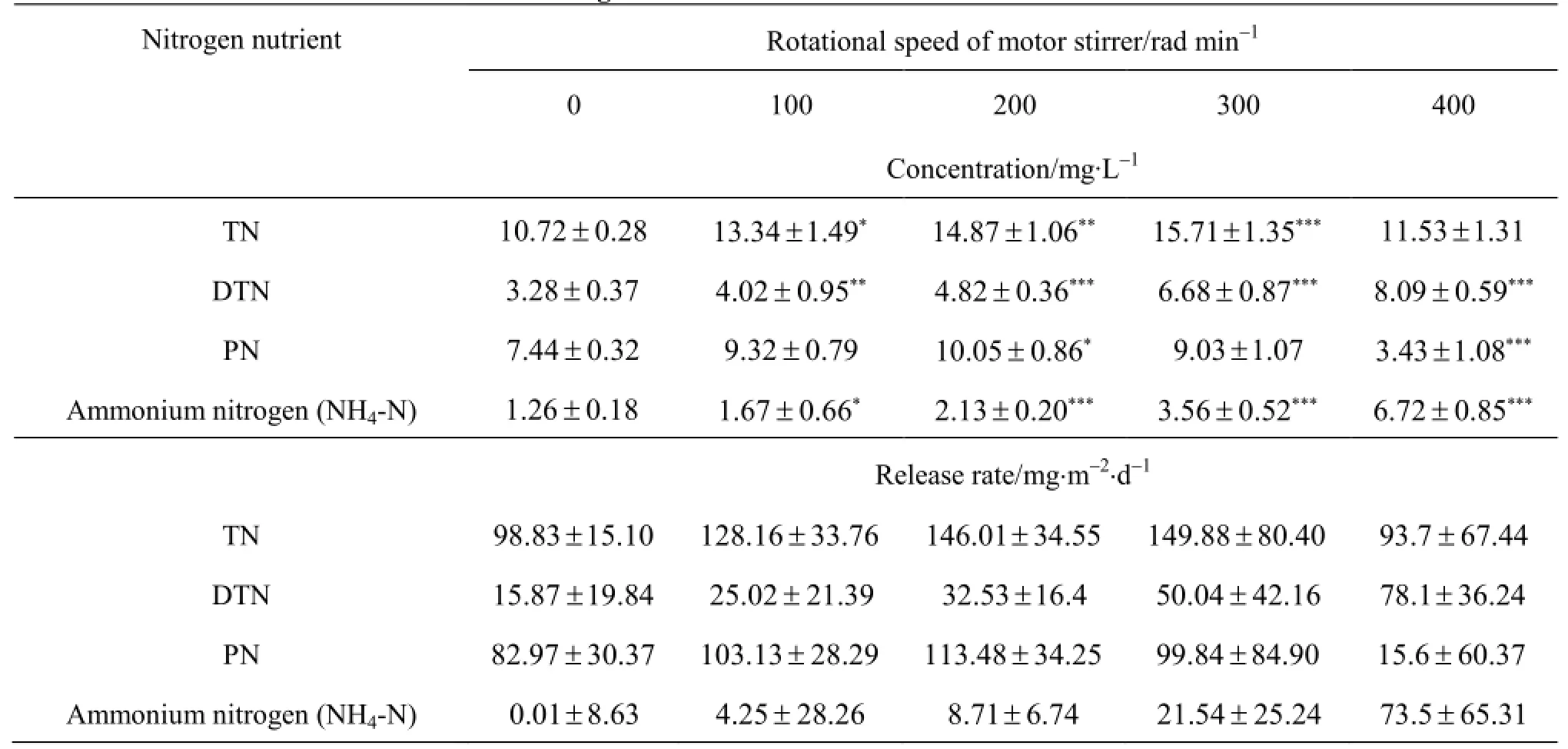
Table 4 The mean ± standard deviation of nitrogen nutrient concentration or release rate

Fig.7 Plots showing mean (solid line) and range (vertical bar)of trial concentrations versus measurement/elapse time at rotational speeds (rad/min) of 0 (CK), 100 (C1), 200 (C2),300 (C3), and 400 (C4)
However, Chl-a concentrations in the laboratory are found to be higher than those in the field. One possible explanation is that the laboratory experiment provides more favorite conditions for the algae growth where the hydrodynamic stress dominates, whereas the algae growth in the real lake is likely affected by more factors, such as the dynamic water currents, the complex flow regimes, and the temperature and light conditions that could not be reflected in the laboratory setting. Also, in the real lake, the algae could be consumed by other aquatic species[49]. Another possible explanation is that different spatial scales of the experiment apparatus and the real lake need to be considered to interpret the results. For example, the mean water depth of the Taihu Lake is about 1.9 m but the water depth of the simulator is only 0.16 m. The average field Chl-a concentration is 25 µg/L while the average experiment Chl-a concentration is 1 200 µg/L. The ratio of the concentrations is at the similar magnitude with the reciprocal of the ratio of the water depths. This indicates that the experiment results might better reflect the real lake situations after the scaling effect is taken into account.

Fig.8 Chlorophyll-a (Chl-a) concentrations from the laboratory experiment versus field observation. Chl-a concentrations in the experiment classified by rotational speed400rad/min or400rad/min. Chl-a concentrations in the field classified by flow velocity<0.05m/sor0.05m/sand wind speed)<4m/sor4m/s
3. Discussion
3.1 Hydrodynamic mechanism for algae growth
Despite some similarities among various lake systems, the effects of hydrodynamic disturbances on the algae growth can vary depending on the characteristics of the disturbance-induced flows. The possible effects include the shear action on the algae community and the water-sediment-algae mixture. These effects can be either positive or negative on the algae growth.
This experiment-field-integrated study has clearly elucidated the mechanism of the algae growth as affected by hydrodynamic disturbances. This study reveals that the average growth rate of the M. aeruginosa under the disturbance condition of the rotational speed≤300rad/minin the experiment (corresponding to the condition that the bottom flow velocity≤0.06 m/s,the shear stress ≤0.069N/m2, or the wind speed≤4m/sin field) is higher than that when the rotational speed is 400 rad/min (correspondingly the bottom flow velocity of 0.08 m/s, the shear stress of 0.124 N/m2). Our results show that low to mediatelevel (i.e., appropriate) disturbances can be beneficial for the M. aeruginosa growth while high-level (i.e.,severe) disturbances are harmful. This is consistent with the findings of Cao[20]and Yan et al.[50]. The possible reasons for the positive effects can be attributed to the fact that an appropriate hydrodynamic disturbance: (1) tends to prompt the release of the endogenous nitrogen and the phosphate from the bed sediments to continuously supply nutrients[51], providing favorable conditions for the algae growth, (2) can increase productivity and photosynthesis of algae[21]because it can reduce light fluctuations, increase the access of the algae cells to nutrients in the surrounding medium, and prompt the metabolization, and (3) can greatly increase the circulation of the nutrients within the algae cells.
In addition, this study indicates that a severe disturbance adversely impacts the M. aeruginosa growth. This negative effect can be attributed to the shear stress resulting from the severe disturbance. Firstly, the algae gathering can become very difficult due to the turbulent shear stress. Secondly, the transport and the redistribution of nutrients tend to be interrupted by strong turbulent currents. The release rates of some nutrients (e.g., TN, PN, and PO4-P) under a severe disturbance condition become lower than those under the static condition, while the release rates of other nutrients (e.g.,TP, DTP, PP, PO4-P, TN, and PN)start to decline after a peak at the rotational speed of 300 rad/min. This is probably because the level of the inorganic particulate materials with a hydroxyalkyl,such as iron, manganese oxide colloid, within the resuspended sediment suspension is high enough to absorb nutrients. Another possible reason is that the increased collision and contact between the suspended sediment and the nutrients particles in the water facilitate the deposition-favorite processes of flocculation,adsorption, sedimentation[52]. Finally, the light penetration rate is influenced by the hydrodynamic disturbances, resulting in changes in the primary productivity. The reduced light penetration due to the resuspended solids has a negative effect on the M. aeruginosa growth.
3.2 The importance of sediment-water interface
A sediment-water-algae concept is proposed in our experiment, that can be used to better understand the algae growth and the nutrient release at the sediment-water interface as affected by hydrodynamics. In previous studies, the cultured algae is used with or without sediments. Firstly, the M. aeruginosa growth cycle at the sediment-water interface in this experiment study is longer than that in those studies (21 versus 14 d). This is probably because the M. aeruginosa can accommodate for longer time at the sediment-water interface than within the medium. Secondly, the M. aeruginosa density in this experiment study is larger than that in those studies. Our experiment shows a maximum M.aeruginosa density of 3.26×107cells m/L,which is almost three times higher than that found by Yan et al.[21]. The possible reason is the longer growth cycle of the M. aeruginosa at the sediment-water interface because the nutrients are continuously released from the sediment-water interface to provide an optimal growth condition for the algae. Finally, this experimental study indicates that the nutrient concentrations increase with time in the process of the M. aeruginosa growth, which is opposite to the finding of Cao[20]. This may be because the algae in those authors’ studies was cultivated within the medium and thus the nutrients were consumed in the substrate,leading to the decline of the nutrients. In contrast, in our study, the nutrients are released into the overlying water by continuous disturbances from the sedimentwater interface to persistently supply the nutrients into the water.
This experimental study demonstrates that the sediment-water interface plays an important role in the process of the M. aeruginosa growth. This is also true for actual aquatic systems including the Taihu Lake,where the sediment-water interfaces can subject to varying degrees of hydrodynamic disturbances. Further, this experiment study reveals the important dependences of the algae bloom on the dynamics at the sediment-water interface. In actual lakes including the Taihu Lake, the dependences are usually confounded by other factors (e.g., water depth) and thus were poorly documented in the existing literature[53]. Our proposed sediment-water-algae concept can be an costeffective tool for simulating the mechanistic relations between the hydrodynamics and the algae growth in real shallow lakes.
4. Conclusion
By combining the laboratory experiment and the field observation, this study proposes a sedimentwater-algae concept to simulate the sophisticated effects of hydrodynamic disturbances on the algae growth in the Taihu Lake. The experiment result indicates that under the disturbance condition of a rotational speed equal to or slower than 300 rad/min, the M. aeruginosa populations are not only positively related to the nutrient release but also to the algae movement and growth. However, the disturbance condition of a rotational speed 400 rad/min is found to negatively affectthe algae growth. Similarly, the field observation reveals a positive relation between the Chl-a concentrations and the hydrodynamics when the flow velocity is less than 0.05 m/s, but a negative relation otherwise. Also, the Chl-a concentrations under the disturbance condition of a wind speed less than 4 m/s are higher than those under the disturbance condition of a wind speed greater than 4 m/s. Therefore, a low to moderate hydrodynamic disturbance is judged to likely prompt the release of the nitrogen as well as the phosphate nutrients from the bed sediments in the Taihu Lake,amplifying the eutrophication of the lake. Nevertheless, in order to simulate these processes in the Taihu Lake, the hydrodynamic models, either physical or mathematical, will be indispensible.
Acknowledgements
This work was supported by the Water Pollution Control and Management of Major Projects(WPCMMP) committee of China (Grant No. 2012ZX07101-002), the Integrated Technology for Water Pollution Control and Remediation at Watershed Scale and its Benefit Assessment (Grant No. 2014ZX07510-001). Also, the China Scholarship Council (CSC) provided the primary author (JH) with a scholarship to support a joint Ph. D. studentship at Old Dominion University in the United States. We would like to thank our colleagues at the Groundwater and Environmental Systems Engineering Innovation Base of the Chinese Research Academy of Environment Sciences (CRAES). We would like to thank Najing Institute of Geography and Limnology Chinese Academy of Sciences (NIGLCAS) for providing field data for this study. We are also grateful for the thoughtful comments provided by the anonymous reviewers.
References
[1] ZHAI S. J., HU W. and ZHU Z. Ecological impacts of water transfers on lake Taihu from the Yangtze River,China[J]. Ecological Engineering, 2010, 36(4): 406-420.
[2] PADISÁK J., REYNOLDS C. S. Shallow lakes: The absolute, the relative, the functional and the pragmatic[J]. Hydrobiologia, 2003, 506-509(1-3): 1-11.
[3] READ J. S., HAMILTON D. P. and JONES I. D. et al. Derivation of lake mixing and stratification indices from high-resolution lake buoy data[J]. Environmental Model and Software, 2011, 26(11): 1325-1336.
[4] VICENTE I. D., LOPEZ R. and POZO I. et al. Nutrient and sediment dynamics in a Mediterranean shallow lake in southwest Spain[J]. Limnetica, 2012, 31(2): 231-250.
[5] SØNDERGAARD M., BJERRING R. and JEPPESEN E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes[J]. Hydrobiologia, 2013, 710(1): 95-107.
[6] HUANG Z. H., XUE B. and PANG Y. Simulation on stream flow and nutrient loading in Gucheng Lake, Low Yangtze River Basin, based on SWAT model[J]. Quaternary International, 2009, 208(1-2): 109-115.
[7] PAERL H. W., XU H. and MACARTHY M. J. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient(N&P) management strategy[J]. Water Research, 2011,45(5): 1973-1983.
[8] ZHANG E., LIU E. and JONES R. et al. A 150-year record of recent changes in human activity and eutrophication of Lake Wushan from the middle reach of the Yangze River, China[J]. Journal of Limnology, 2010, 69(2): 235-241.
[9] RICHARD W. B., BENNION H. Palaeolimnology and its developing role assessing the history and extent of human impact on lake ecosystems[J]. Journal of Paleolimnology,2011, 45(4): 399-404.
[10] MOOIJ W. M., JANSE J. H. and De SENERPONT DOMIS L. S. et al. Predicting the effect of climate change on temperate shallow lakes with the ecosystem model PC Lake[J]. Hydrobiologia, 2007, 584(5): 443-454.
[11] PAERL H. W., PAUL V. J. Climate change: Links to global expansion of harmful cyanobacteria[J]. Water Research, 2012, 46(5): 1349-1363.
[12] XIE L., XIE P. and TANG H. Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms-an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake[J]. Environment Pollution,2003, 122(3): 391-399.
[13] VIJVERBERG T., WINTERWERP J. C. and AARNINKHOF S. G. J. et al. Fine sediment dynamics in a shallow lake and implication for design of hydraulic works[J]. Ocean Dynamics, 2011, 61(6): 187-202.
[14] COUCEIRO F., FONES G. R. and THOMPSON C. E. L. et al. Impact of resuspension of cohesive sediments at the Oyster Grounds (North Sea) on nutrient exchange across the sediment-water interface[J]. Biogeochemistry, 2013,113(1): 37-52.
[15] LIU H., BAO L. and ZENG E. Y. Recent advances in the field measurement of the diffusion flux of hydrophobic organic chemicals at the sediment-water interface[J]. Trends in Analytical Chemistry, 2014, 54(6): 56-64.
[16] FAN Jing-yu, WANG Dao-zeng. Experimental investigation on diffusive contaminant release from permeable sediment layer under unidirectional unsteady flow[J]. Journal of Hydrodynamics, 2014, 26(6): 965-970.
[17] HIGASHINO M., STEFAN H. Non-linear effects on solute transfer between flowing water and a sediment bed[J]. Water Research, 2011, 45(18): 6074-6086.
[18] WANG Li-li. Research on the relevant factors of the algal growth in hydrodynamic condition[D]. Doctoral Thesis, Chongqing, China: Chongqing University, 2006,98-112(in Chinese).
[19] ZHOU Wei. The effects of disturbance on the growth of microcystisa aeruginosa[D]. Doctoral Thesis, Shanghai,China: Shanghai Jiao Tong University, 2013, 33-42(in Chinese).
[20] CAO Qiao-li. Study on the effect of hydrodynamic conditions on the occurrence and disappearance of cyanobacteria bloom[J]. Disaster Prevention and Control Engineering, 2008, 1: 67-71(in Chinese).
[21] YAN Run-run, PANG Yong and ZHAO Wei et al. Influence of circumfluent type waters hydrodynamic on growth of algae[J]. China Environmental Sciences, 2008, 28(9): 813-817(in Chinese).
[22] GAO Y., SUN X. and ZHANG Z. et al. Simulated studyon concentration change of different form phosphorus in shallow lakes caused by wind-wave disturbance[J]. Advances in Water Science, 2007, 18(5): 668-673.
[23] ZHANG Yi-min, ZHANG Yong-chun and ZHANG Longjiang et al. The influence of lake hydrodynamics on blue algal growth[J]. China Environmental Science, 2007,27(5): 707-711(in Chinese).
[24] YANG Zai-fu, SHI Wei-gang and CHEN Yi-qiao. Ecological environment succession and countermeasure of East Taihu[J]. China Environment Science, 2003, 23(1): 64-68(in Chinese).
[25] LI Y., TANG C. and WANG C. et al. Improved Yangtze River diversions: Are they helping to solve algal bloom problems in Lake Taihu, China?[J]. Ecological Engineering, 2013, 51(1): 104-116.
[26] HU W., JORGENSEN S. E. and ZHANG F. A verticalcompressed three-dimensional ecological model in Lake Taihu, China[J]. Ecological Modeling, 2006, 190(3-4): 367-398.
[27] ZHAO Q., SUN J. and ZHU G. Simulation and exploration of the mechanisms underlying the spatiotemporal distribution of surface mixed layer depth in a large shallow lake[J]. Advances in Atmospheric Sciences, 2012,29(6): 1360-1373.
[28] XIA J., SU G. and ZHANG X. et al. Dioxin-like activity in sediments from Tai Lake, China determined by use of the H4IIE-luc bioassay and quantification of individual AhR agonists[J]. Environmental Science and Pollution Research, 2014, 21(2): 1480-1488.
[29] MAO J., CHEN Q. and CHEN Y. Three-dimensional eutrophication model and application to Taihu Lake,China[J]. Journal of Environmental Sciences, 2008,20(3): 278-284.
[30] MEIS S., SPEARS B. M. and MABERLY S. C. et al. Assessing the mode of action of phoslock in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK)[J]. Water Research, 2013,47(13): 4460-4473.
[31] CHEN Y., QIN B. and TEUBNER K. et al. Long-term dynamics of phytoplankton assemblages: Microcystisdomination in Lake Taihu, a large shallow lake in China[J]. Plankton Research, 2003, 25(1): 445-453.
[32] LIU X., LU X. and CHEN Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu,China: an 11-year investigation[J]. Harmful Aglae, 2011,10(3): 337-343.
[33] NEMOTO F., FUKUHARA H. The antagonistic relationship between chlorophyll a concentrations and the growth areas of Trapa during summer in a shallow eutrophic lake[J]. Limnology, 2012, 13(3): 289-299.
[34] WILHELM S. W., FARNSLEY S. E. and LECLEIR G. R. et al. The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai),China[J]. Harmful Algae, 2011, 12(10): 207-215.
[35] JIN X., CHU Z. and YAN F. et al. Effects of lanthanum(III) and EDTA on the growth and competition of Microcystis aeruginosa and Scendesmus quadricauda[J]. Limnologica, 2009, 39(6): 86-93.
[36] WANG X., HAO C. and ZHANG F. et al. Inhibition of the growth of two blue-green algae species (Microsystis aruginosa and Anabaena spiroides) by acidification treatments using carbon dioxide[J]. Bioresource Technology, 2011,102(10): 5742-5748.
[37] WU X., EADAOIN M. J. and TIMOTHY J. M. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies[J]. Water Research, 2012, 46(9): 2851-2858.
[38] ZHOU Q., CHEN W. and ZHANG H. et al. A flow cytometer based protocol for quantitative analysis of bloomforming cyanobacteria (Microcystis) in lake sediments[J]. Journal of Environmental Sciences, 2012, 24(9): 1709-1716.
[39] JIANG X., JIN X. and YAO Y. et al. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China[J]. Water Research, 2008, 42(8): 2251-2259.
[40] SUN Xiao-jing, QIN Bo-qiang and ZHU Guan-wei. Release of colloidal N and P from sediment of lake caused by continuing hydrodynamic disturbance[J]. Environmental Science, 2007, 28(6): 1223-1229(in Chinese).
[41] CHEN Y., FAN C. and TEUBNER K. et al. Changes of nutrients and phytoplankton chlorophyll-a in a large shallow Lake Taihu, China: an 8-year investigation[J]. Hydrobiologia, 2003, 25(6): 506-509(1): 273-279.
[42] EPA. In vitro determination of chlorophylls a, b, c1+c2 and pheopigments in marine and freshwater algae by visible spectrophotometry[R]. EPA Method 446.0,Washington DC, USA: Environmental Protection Agency,1997.
[43] HAWLEY N. Sediment resuspension near the Keweenaw Peninsula, Lake Superior during the fall and winter 1990-1991[J]. Great Lake Resources, 2000, 26(4): 495-505.
[44] LAENEN A., LETOURNEAU A. P. Upper Klamath Basin nutrient-loading study: Estimate of wind-induced resuspension of bed sediment during periods of low lake elevation[R]. Open-File Report, Washington DC, USA: Geological Survey, 1996, 95-414.
[45] WÜEST A., LORKE A. Small-scale hydrodynamics in lakes[J]. Annual Review Fluid Mechanics, 2003, 35(6): 373-412.
[46] FINNEMORE E. J., FRANZINI J. B. Fluid mechanics with engineering application[M]. 10th Edition, New York, USA: McGraw Hall, 2002.
[47] ONO E., CUELLO J. L. Carbon dioxide mitigation using thermophilic cyanobacteria[J]. Biosystems Engineering,2007, 96(1): 129-134.
[48] FAN Cheng-xin. Sediment physical and chemical characteristics and phosphorus release[J]. Journal of Lake Sciences, 1995, 7(4): 341-350(in Chinese).
[49] ZHANG Y., TANG C. Y. and LI G. The role of hydrodynamic conditions and pH on algal-rich water fouling of ultrafiltration[J]. Water Research, 2012, 46(15): 4783-4789.
[50] YAN Run-run, PANG Yong. Disturbance effect on growth of two species of algae under different culture conditions[J]. Environmental Science and Technology, 2007,30(3): 10-13(in Chinese).
[51] HU K., PANG Y. and WANG H. et al. Simulation study on water quality based on sediment release flume experiment in Lake Taihu, China[J]. Ecological Engineering,2011, 37(4): 607-615.
[52] SUN Xiao-jing, ZHU Guang-wei and LUO Lian-cong et al. The study on sediment phosphorus release with wave flume experiment in shallow lake[J]. Science in China Series D Earth Sciences, 2005, 35(1): 81-89.
[53] AMORES V., CRUZ-PIZARRO L. and De VICENTE I. Instability of shallow lakes: A matter of the complexity of factors involved in sediment and water interaction?[J]. Limnetica, 2006, 25(1-2): 253-270.
September 4, 2014, Revised May 6, 2016)
* Project supported by the National Natural Science Foundation of China (Grant No. 41473110).
Biograpgy: Jian HUANG (1985-), Female, Ph. D. Candidate
Correspondind author: Bei-dou XI,
E-mail: xibeidou@263.net
猜你喜欢
杂志排行
水动力学研究与进展 B辑的其它文章
- Scattering of gravity waves by a porous rectangular barrier on a seabed*
- Numerical simulations of viscous flow around the obliquely towed KVLCC2M model in deep and shallow water*
- A simple method for estimating bed shear stress in smooth and vegetated compound channels*
- Theoretical analysis and numerical simulation of mechanical energy loss and wall resistance of steady open channel flow*
- A robust WENO scheme for nonlinear waves in a moving reference frame*
- Oscillating-grid turbulence at large strokes: Revisiting the equation of Hopfinger and Toly*
