PhotoluminescencepropertiesofBa2+-dopedSr2.4Y0.2Eu0.2V2O8:Ba2+phosphors
2016-09-20,,,,
, , , ,
(Anhui Key Laboratory of Information Materials and Devices, School of Physics and Materials Science,Anhui University, Hefei 230039, China)
PhotoluminescencepropertiesofBa2+-dopedSr2.4Y0.2Eu0.2V2O8:Ba2+phosphors
DAIZhenxiang,WANGWeiwei,PENGZhangwan,ZHUYanan,ZHENGGanhong
(AnhuiKeyLaboratoryofInformationMaterialsandDevices,SchoolofPhysicsandMaterialsScience,AnhuiUniversity,Hefei230039,China)
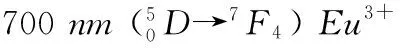
TheBa2+-dopedSr2.4Y0.2Eu0.2V2O8phosphorsweresynthesizedbytheconventionalsolid-statereaction.Uponexcitationwith416nmirradiation,theemissionspectrafortheBa2+-dopedSr2.4Y0.2Eu0.2V2O8sampleswerecomposedofthewell-knownlinelocatingat595nm(5D0→7F1), 619nm(5D0→7F2), 652nm(5D0→7F3),and700nm(5D0→7F4)resultingfromtheemissionofEu3+duetotransitionof5D0→7FJ.TheemissionintensityincreasedandthendecreasedwithincreasingBacontent,andthemaximumemissionintensitywasachievedwhenxwas0.15.TheseresultsindicatedthattheBadopantactsasonesensitizerandenhancedthephotoluminescenceintensity.
solid-statereaction;Ba2+-doped;luminescence
0 Introduction
Alargenumberofvanadateshavebeenfound,suchas(Y,Gd,Lu)VO4[1],Sr3V2O8[2],Ca9Dy(VO4)7[3],whichcanbeeffectivelyexcitedbyultraviolet(UV)andconvertUVradiationtobroadbandemissioninthevisiblelightregion.Asinterestingcandidatesforredphosphors,materialscontainingEu3+,whichhave5D0→7F2transitionat619nmasionsarelocatedinanon-centrosymmetricsite,showbettercolorpuritythanthosephosphorswithbroademissionband.GreatattentionshavebeenpaidtoEu3+-dopedmanganites,borosilicate,andtitanate,whichshowtheeffectivef-ftransitionofEu3+underexcitationintheUVspectralregion,suchasBaGa2(MnO4)4:Eu3+[4],Eu0.14Mg0.18Ca0.07Ba0.12-B0.17Si0.32Oδ[5],(K,Li,Na)x(Y,Gd,La,Eu)yTizOδ[6].
Asweknow,theopticalpropertiesofphosphorscanbetunedwiththelocalenvironmentsaroundluminescenceions.Recently,Wangetal.[7]usedahydrothermalmethodtoprepareYVO4:Ln3+(Ln=Eu,Dy,Sm,Ce)nano-crystalsco-dopedwithBa2+,andreportedalargeenhancementintheirluminescence.Similarly,Zhangetal.foundthatthephotoluminescenceofSr3Al2O6:Eu3+phosphorscanbegreatlyincreasedbydopingBa2+ions[8].Jiaetal.usingasolvothermalmethodtosynthesizeYVO4:Ln3+(Ln=Eu,Dy)andreportedthatthereisalargeenhancementintheluminescencebyco-dopingBa2+[9].
Inthispaper,wesynthesizedBa2+-dopedSr2.4Y0.2Eu0.2V2O8phosphorsbytheconventionalanddescribedthephotoluminescentpropertieswithBaindetail.
1 Experimental
ThephosphorsSr2.4Y0.2Eu0.2V2O8:xBa2+(0≤x≤0.20)werepreparedbyconventionalsolid-satereaction.ThereactantsincludeSrCO3(99.99%),Y2O3(99.9%),Eu2O3(99.99%),NH4VO3(99.0%),andBaCO3(99%).Thestartingmaterialsweremixedtogetherinanagatemortarandfinelyground.Theobtainedmixturewasheatedinanaluminacrucible(inair)at680 ℃for10h.Afternaturalcooling,grindcarefullyandpressitintotablets.Thentheobtainedmixturewasheatedinanaluminacrucible(inair)at850 ℃for10h.Afternaturalcooling,grindcarefullyandpressitintotablets.Finallytheobtainedmixturewasheatedinanaluminacrucible(inair)at1 000 ℃for12h.
ThecrystalstructureofthephosphorpowderswascharacterizedbyX-raydiffraction(XRD)analysis.IntheprocessofXRDanalysis,oneX-raydiffractometerDX-2000SSCwithCuKαirradiation(λ=0.154 06nm)isused,withoperatingvoltagebeing36kVandtheoperatingcurrentbeing25mA.TheelementcontentsarealsodetectedusingX-rayspectrometry(PHI5702).TheactivationandemissionspectraweremeasuredonanFLfluorescencespectrophotometer(F-4500).Theweightofeverysamplewasequalto0.8g.Alltheseoperationswerecarriedoutatroomtemperature.
2 Results and Discussion
2.1The crystal structure and Ba content of Sr2.4Y0.2Eu0.2V2O8:xBa2+
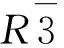
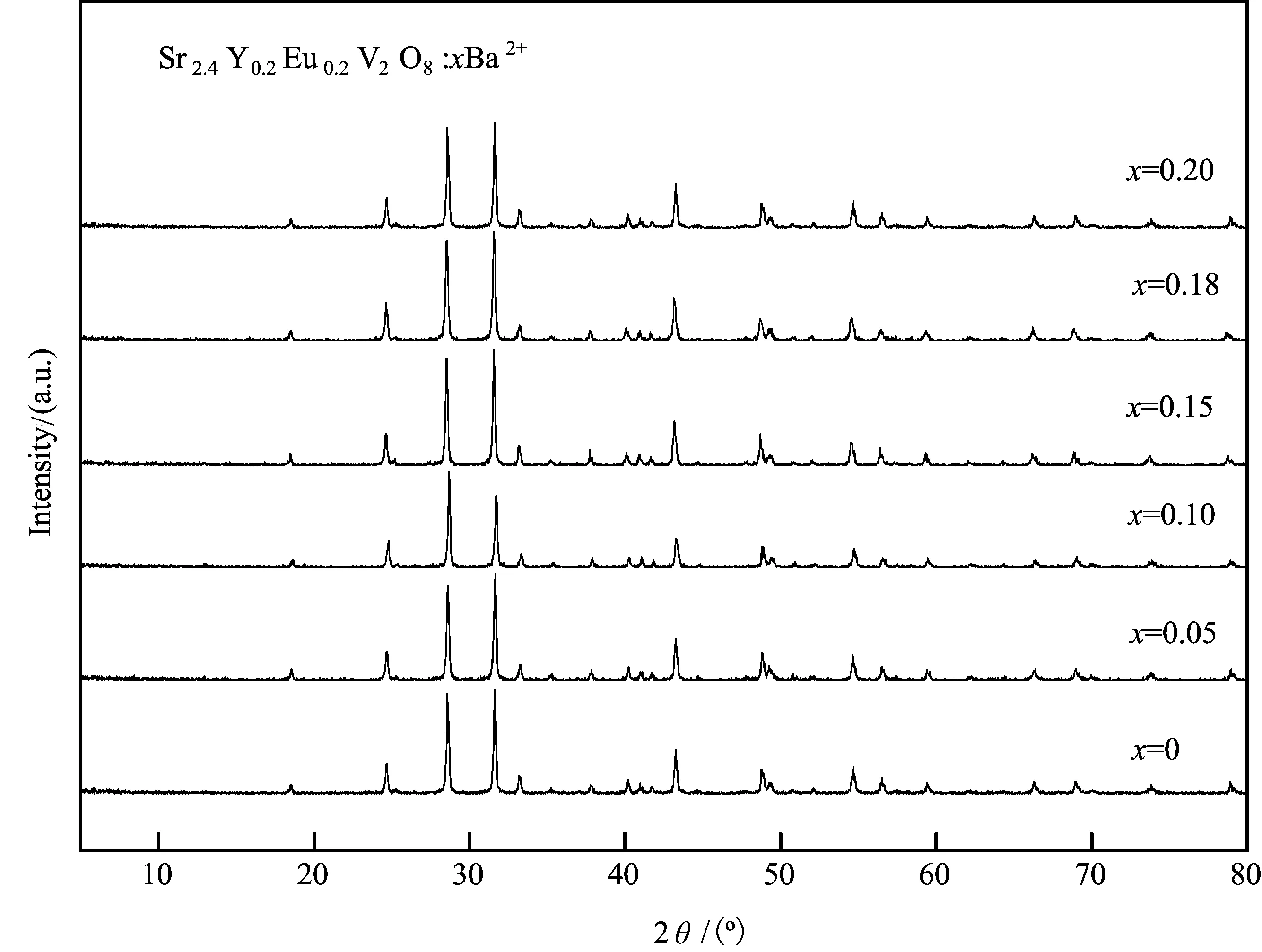
Fig.1 XRD patterns of Sr2.4Y0.2Eu0.2V2O8:xBa (0≤x≤0.20)
2.2Emission spectra of Sr2.4Y0.2Eu0.2V2O8:xBa+(0≤x≤0.20) phosphor
TheemissionspectraofSr2.4Y0.2Eu0.2V2O8:xBa2+(x=0, 0.05, 0.10, 0.15, 0.18, 0.20)phosphor(λex=416nm)areshowninFig.2.Uponexcitationwith416nmirradiation,theemissionspectraconsistofthewell-known5D0→7FJ(J=0, 1, 2,etc.)ofthe4f6configurationforEu3+ions.Theemissionlineat619nm,correspondingtotheforcedelectricdipole5D0→7F2transitionofEu3+,ismuchstrongerthantheemissionlineat595nm.The595nmemissionlineisknowntobeassignedtothemagneticdipole5D0→7F1transitionofEu3+.Accordingly,wecanconcludethatEu3+occupiedtheasymmetrylatticesites.Theothertwopeaksfromthe5D0→7F3and5D0→7F4,whicharelocatedat652nmand700nm,arealsoobserved.
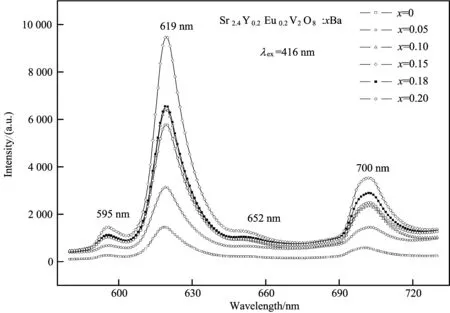
Fig.2 The emission spectra under excitation at λex=416 nm excitation of theSr2.4Y0.2Eu0.2V2O8:xBa (0≤x≤0.20) phosphors
Despitedifferentcompositions,allthesesamplesSr2.4Y0.2Eu0.2V2O8:xBa2+(x=0, 0.05, 0.10, 0.15, 0.18, 0.20)showsimilaremissionspectra.TheemissionwavelengthsarealsofoundtobeindependentofBaaddition,indicatingthattheBa2+-dopingdoesnotchangethehoststructure.However,therelativeemissionintensityatthehighest5D0→7F2transitionofEu3+ischangedwiththedopedBa2+content,asshowninFig.3.
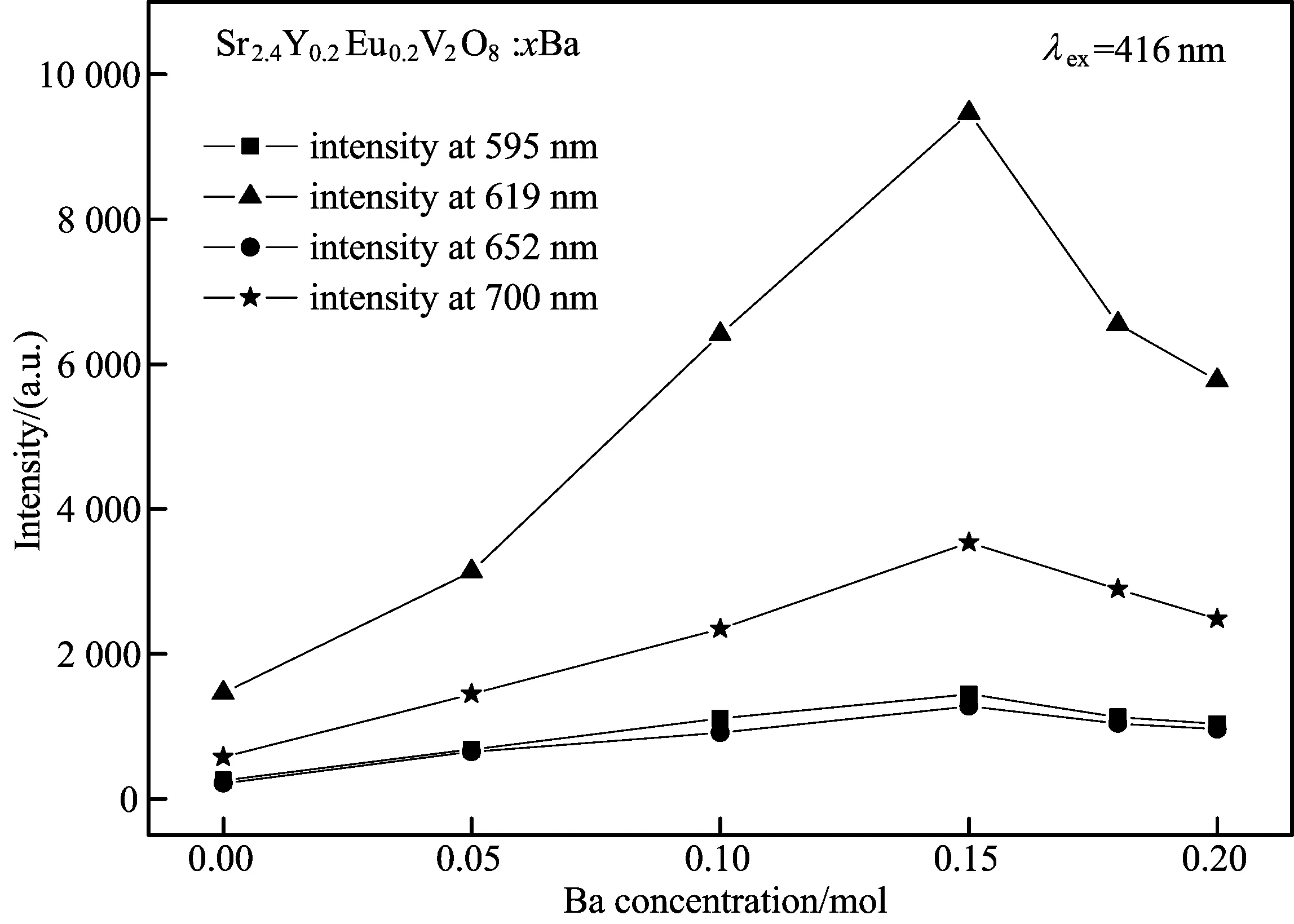
Fig.3 The emission intensity at 595, 619, 652 and 700 nm ofSr2.4Y0.2Eu0.2V2O8:xBa (0≤x≤0.20) versus the Ba concentration
Thephotoluminescenceintensityat595, 619, 652,700nmisalsofoundtochangewithincreasingBacontent,andthehighestvalueisobservedatx=0.15.WithfurtherincreaseofBaconcentration,thephotoluminescenceintensitygraduallydecreases.Thus,theoptimalBa2+dopedcontentinSr2.4Y0.2Eu0.2V2O8:xBa2+phosphoris0.15.Inthiscase,theemissionintensityat619nmofSr2.4Y0.2-Eu0.2V2O8:xBa2+isincreasedbyfivetimesduetotheBadopant.ThissuggeststhattheefficientenergytransferprocessbetweenBa2+andEu3+ionoccurs[10].TheemissionintensityincreaseswithincreasingBadopant.Thesimilarphenomenonisalsoreportedinotheralkalimetal[11-12].Ontheotherhand,consideringtheionicradiiBa2+being0.135nmandSr2+being0.112nm,whenSr2+ionissubstitutedbyBa2+,theionicradiidifferenceisnoticeablewithBadopant.Certainly,thecrystalfieldsurroundingEu3+ionsishighlychangedcorrespondingly.Asdiscussedabove,5D0→7F2isanelectricdipoletransitionandhypersensitivetothelocalsymmetry.Therefore,incaseofx≤0.15,theemissionintensityincreaseswiththeincreaseofBadopantcontent.However,theexcessdistortioncausedbytheadditionofBa2+inthecaseofx≥0.15maybeanotherreasonfordecreasingtheemissionintensity,asreportedinthereference[13].WhenBacontentismorethan0.15,moreandmoreBaionsareintroducedintotheSr2.4Y0.2Eu0.2V2O8samples.Subsequently,thedistancebetweentwoBaionswouldbedecreasedgraduallyandbecloseenough.ThiswillresultinoneefficientanddirectenergytransferprocessbetweenBaions,whichreducestheprobabilityofenergytransferfromBatoSr.ThesensitizedeffectivenessofBa2+ontheSr2+emissionintensityisreduced.Andeventually,theemissionintensityofSr2.4Y0.2Eu0.2V2O8:xBa2+systemislimitedasshowninFig.3.
2.3Excitation spectra of Sr2.4Y0.2Eu0.2V2O8:xBa2+(0≤x≤0.20) phosphor
TheexcitationspectraofSr2.4Y0.2Eu0.2V2O8:xBa2+(0≤x≤0.20)formonitoringthe5D0→7F2transitionofEu3+(λem=619nm)areshowninFig.4.Theintensityofactivationbandistoostrongtoexceedtherangeofmeasurement,therefore,weonlypresenttheresultsoftherepresentativesamples(x=0, 0.05)here.Inaddition,itexhibitssomebandsinthereignof400-550nm,resultingfromthef-ftransitionswithinEu3+4f6electronconfiguration,andthepeaksat416, 468,539nmarecorrespondingtotheelectrontransitionsfromthe7F0,1groundstateto5D3,5D2and5D1,consistentwithspectralcharacterizationsobservedinpreviouswork[14-15].
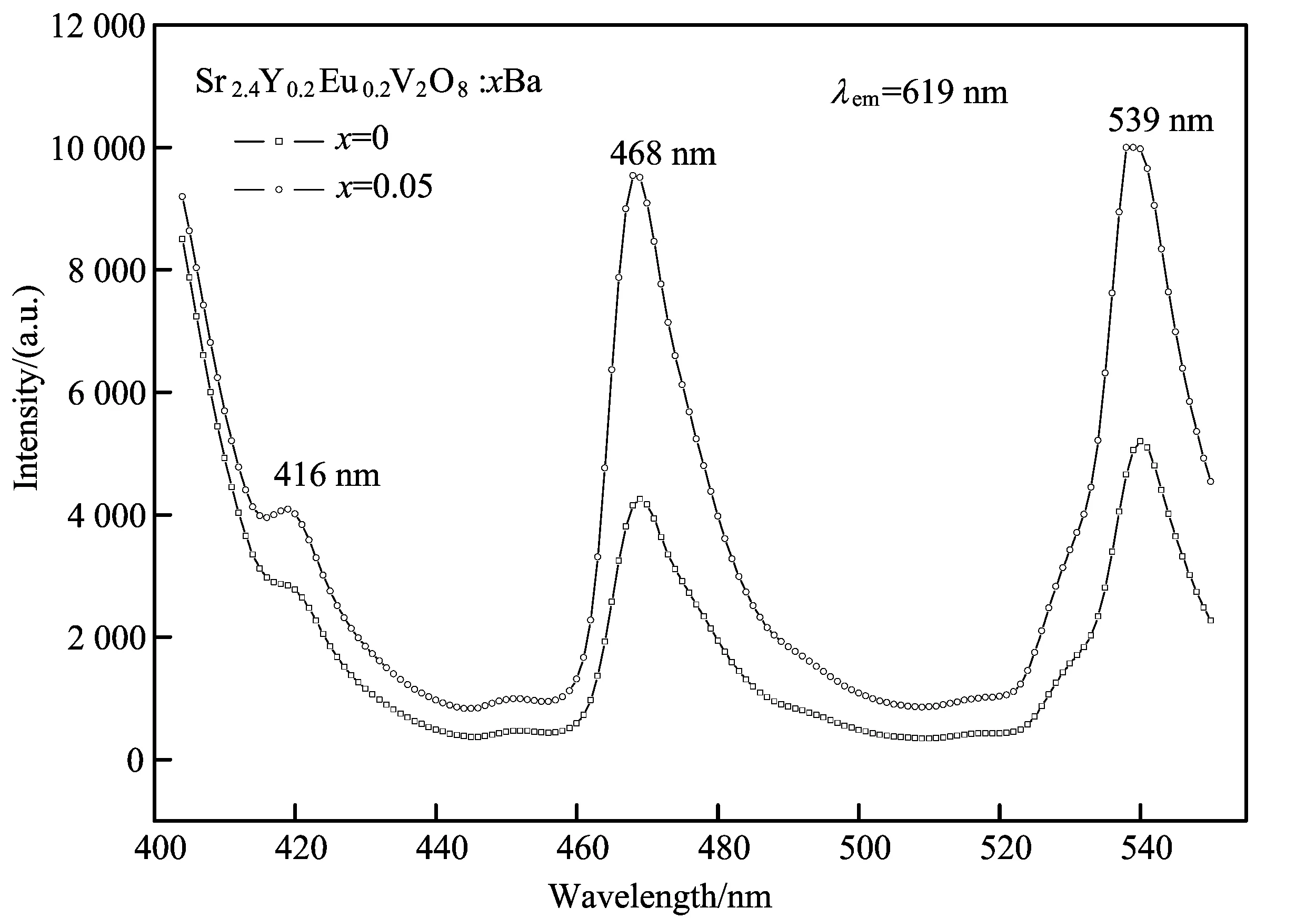
Fig.4 The excitation spectra for monitoring the emission at λex=619 nm of theSr2.4Y0.2Eu0.2V2O8:xBa (x=0, 0.05)
3 Conclusions
TheSr2.4Y0.2Eu0.2V2O8:xBa2+(0≤x≤0.20)phosphorshavebeensynthesizedbysolid-statereactionmethod.Thecorrespondingphotoluminescepropertiesarealsoprobed.TheemissionintensityisenhancedfirstlywithincreasingtheBaconcentration,andthendecreasesgradually.Thestrongestemissionintensityisachievedatx=0.15.Thistendencyisascribedtothefollowingtwofactors.OneistheenergytransferprocessbetweenBa+ionsandEu3+ions,theotherisrelatedtothedistortionofthecrystalfieldsurroundingEu3+ions.SuchadistortionisenhancedbysubstitutingBa2+.Asforthefirstfactor,theenergyisabsorbedbyBa2+ionsandthentransferredtoEu3+,consequentlytheemissionintensityisenhanced.However,whenBacontentisabove0.15,thedistancebetweenBaionsisshortenoughtocauseoneefficientBa-Baenergytransferprocess,theprobabilityofenergytransferfromBatoEuiscorrespondinglydecreased.Asforthesecondfactor,thecrystalfieldsurroundingEu3+ionsislocallydistorted,withsubstitutingBa2+,thentheasymmetryofsuchacrystalfieldisincreased.Thisdistortionleadstotheenhancementoftheemissionintensity.However,theexcessdistortioncausedbytheadditionofBa2+over0.15mayresultintheemissionintensitydecreases.
References:
[1]LIJF,QIUKH,LIJF,etal.AnovelbroadbandemissionphosphorCa2KMg2V4O12forwhitelight-emittingdiodes[J].MaterResBull, 2010, 45 (5): 598-602.
[2]NAKAJIMAT,ISOBEM,TSUCHIYAT,etal.Directfabricationofmetavanadatephosphorfilmsonorganicsubstratesforwhite-light-emittingdevices[J].NatureMater, 2008, 7: 735-740.
[3]WUX,HUANGYL,SHIL,etal.SpectroscopycharacteristicsofvanadateCa9Dy(VO4)7forapplicationofwhite-light-emittingdiodes[J].MaterChemPhys, 2009, 116: 449-452.
[4]GUOCF,YANGHK,FUZL,etal.Apotentialred-EmittingphosphorBaGd2(MoO4)4:Eu3+fornear-UVwhiteLED[J].JAmCeramSoc, 2009, 92: 1713-1718.
[5]SOHNKS,LEEJM,SHINN.Asearchfornewredphosphorsusingacomputationalevolutionaryoptimizationprocess[J].AdvMater, 2003, 15: 2081-2084.
[6]SOHNKS,PARKDH,CHOSH,etal.ComputationalevolutionaryoptimizationofredphosphorforuseintricolorwhiteLEDs[J].ChemMater, 2006, 18: 1768-1772.
[7]WANGGF,WANGQW,ZHANGDS,etal.EnhancedphotoluminescenceofwatersolubleYVO4:Ln3+(Ln=Eu,Dy,Sm,andCe)nanocrystalsbyBa2+doping[J].JPhysChemC, 2008, 112 (44): 17042-17045.
[8]ZHANGL,QIUKH,LUXG,etal.EffectofdopingBa2+ionsonluminescencepropertiesofSr3Al2O6:Eu2+redphosphor[J].ChinJLumin, 2012, 33 (11): 1219-1223.
[9]JIAG,ZHANGC,DINGS,etal.Synthesisandenhancedluminescenceofuniformandwell-dispersedquasisphericalYVO4:Ln3+(Ln=Eu,Dy)nanoparticlesbyasolvothermalmethod[J].CrystEngComm, 2012, 14: 573-577.
[10]WANGZJ,LIUHY,ZHANGK,etal.LuminescencecharacteristicsofLiCaBO3:Sm3+phosphor[J].ChinJLumin, 2010, 31 (1): 49-53.
[11]WUJ,ZHANGP,JIANGCD,etal.Preparationandluminescencepropertiesofreddish-orangephosphorsCa3Y2Si3O12:Sm3+[J].ChinJLumin, 2014, 35 (7): 772-776.
[12]WANG,QINW,ZHANGD,etal.EnhancedphotoluminescenceofwatersolubleYVO4:Ln3+(Ln=Eu,Dy,Sm,andCe)nanocrystalsbyBa2+doping[J].JPhysChemC, 2008, 112: 17042-17045.
[13]XIAOXZ,YANB.HybridprecursorssynthesisandopticalpropertiesofLnNbO4:Bi3+bluephosphorsandBi3+sensitizingofonDy3+luminescenceinYNbO4matrix[J].JAlloyCompd, 2006, 421: 252-257.
[14]LIYH,HONGGY.SynthesisandluminescencepropertiesofnanocrystallineYVO4:Eu3+[J].JSolidStateChem, 2005, 178: 645-649.
[15]RIWOTZKIK,HAASEM.Wet-chemicalsynthesisofdopedcolloidalnanoparticles:YVO4:Ln(Ln=Eu,Sm,Dy)[J].JPhysChemB, 1998, 102: 10129-10135.
(责任编辑郑小虎)
10.3969/j.issn.1000-2162.2016.02.008
Sr2.4Y.Eu.V2O8:Ba2+发光性能的研究
戴振翔,汪伟伟,彭张皖,朱亚男,郑赣鸿
(安徽大学 物理与材料科学学院,安徽省信息材料与器件重点实验室, 安徽 合肥 230039)
固相反应;Ba2+掺杂;发光性质
date:2015-10-15
SupportedbytheNationalNaturalScienceFoundationofChina(11204001,11174004),AnhuiProvincialNaturalScienceFoundation(1208085QA07,1308085MA04),theHigherEducationalNaturalScienceFoundationofAnhuiProvince(KJ2013A031),AnhuiUniversityScientificResearchFund(201410357005,KYXL2013009), “211Project”ofAnhuiUniversity(SZJYKC2013020,XJGXKC1401)
Author’sbrief:DAIZhenxiang(1975-),male,borninShaoyangofHunanProvince,associateprofessorofAnhuiUniversity,E-mail:physdai@ahu.edu.cn.
O433.2Documentcode:AArticleID:1000-2162(2016)02-0043-06
