2型猪链球菌中国强毒株及其covR基因突变株的蛋白质组学研究
2016-07-29李先富王长军潘秀珍
倪 华,张 剑,郑 峰,胡 丹,李先富,王长军,潘秀珍
2型猪链球菌中国强毒株及其covR基因突变株的蛋白质组学研究
倪华1,2,张剑1,2,郑峰2,胡丹2,李先富2,王长军2,潘秀珍1,2
1.南京师范大学生命科学学院,南京210023;2.南京军区军事医学研究所,南京210002
摘要:目的通过比较2型猪链球菌中国强毒株05ZYh13及其covR基因突变株△covR蛋白表达谱差异,质谱鉴定差异表达蛋白,分析CovR在蛋白表达调控中的作用。方法首先将05ZYh13和△covR在THB培养基中培养至对数期,然后裂解细菌制备蛋白样品。第一向等电聚焦电泳在ph1~10的IPG胶条上完成后进行SDS-PAGE电泳,电泳胶经扫描分析后选取蛋白点进行质谱鉴定。结果05ZYh13和△covR全菌蛋白裂解液经二维电泳分别得到559和491个蛋白点,经比对发现两菌株蛋白表达量差异3倍以上蛋白点40个,经质谱鉴定出15个蛋白,主要涉及细胞代谢的酶类如谷氨酸脱氢酶、腺苷酸激酶、PTS系统成分等,以及分子伴侣蛋白如GroEL和Dnak等;电泳分离得到△covR特异蛋白点124个,质谱鉴定出15个,主要为参与细胞糖代谢过程中的酶,如磷酸甘油酸激酶、甘油醛-3-磷酸脱氢酶、丙酮酸激酶等;质谱鉴定05ZYh13特异表达蛋白5个。结论鉴定05ZYh13和△covR差异表达蛋白35个,部分蛋白涉及细菌毒力、宿主细胞粘附、细胞分裂等生命过程,同时蛋白分子伴侣在△covR中的表达变化说明CovR的调控可能发生在蛋白修饰水平,为研究CovR在调控细菌毒力中的作用和分子机制奠定基础。
摘要:目的通过比较2型猪链球菌中国强毒株05ZYh13及其covR基因突变株△covR蛋白表达谱差异,质谱鉴定差异表达蛋白,分析CovR在蛋白表达调控中的作用。方法首先将05ZYh13和△covR在THB培养基中培养至对数期,然后裂解细菌制备蛋白样品。第一向等电聚焦电泳在ph1~10的IPG胶条上完成后进行SDS-PAGE电泳,电泳胶经扫描分析后选取蛋白点进行质谱鉴定。结果05ZYh13和△covR全菌蛋白裂解液经二维电泳分别得到559和491个蛋白点,经比对发现两菌株蛋白表达量差异3倍以上蛋白点40个,经质谱鉴定出15个蛋白,主要涉及细胞代谢的酶类如谷氨酸脱氢酶、腺苷酸激酶、PTS系统成分等,以及分子伴侣蛋白如GroEL和Dnak等;电泳分离得到△covR特异蛋白点124个,质谱鉴定出15个,主要为参与细胞糖代谢过程中的酶,如磷酸甘油酸激酶、甘油醛-3-磷酸脱氢酶、丙酮酸激酶等;质谱鉴定05ZYh13特异表达蛋白5个。结论鉴定05ZYh13和△covR差异表达蛋白35个,部分蛋白涉及细菌毒力、宿主细胞粘附、细胞分裂等生命过程,同时蛋白分子伴侣在△covR中的表达变化说明CovR的调控可能发生在蛋白修饰水平,为研究CovR在调控细菌毒力中的作用和分子机制奠定基础。
关键词:2型猪链球菌;毒力调控因子CovR;二维电泳;蛋白质组
2型猪链球菌(Streptococcussuisserotype 2,S.suis2)是一种世界性广泛分布的人兽共患病病原菌[1-3]。
细菌致病过程是与宿主细胞相互作用的复杂过程,依靠复杂而精密的信号系统感受、传导和响应外界环境变化,进而调节相应的基因表达以作出适应性反应[4]。二元信号转导系统(two-component signal transduction system,TCS),是细菌中普遍存在的一种跨膜信号转导系统[5-6]。
1材料与方法
1.1材料
1.1.1菌株与培养条件05ZYh13本实验室保存[12];△covR本实验室构建[11]。将05ZYh13和△covR菌株冻存菌分别接种于5%羊血哥伦比亚血平板,挑单菌落于THB(Todd-Hewitt Broth)液体培养基在37 ℃摇床震荡培养至平台期,1%比例转接至THB后培养至对数晚期;△covR菌株THB培养基中加入100 μg/mL盐酸壮观霉素。
1.1.2仪器和试剂水化缓冲液:8 mol/L脲,2% CHAPS,0.2% Bio-Lyte 4/7,50 mMDTT,0.01%溴酚蓝;平衡缓冲液:6 mol/L脲,2% SDS,20%甘油,375 mM Tris-HCl pH 8.8,含2% DTT或者2.5%碘乙酸铵;IPG干胶条(Ready Strip TMIPG Strip, ph1~10,17 cm)、蛋白定量试剂盒、CHAPS(Bio-Rad);等电聚焦仪、垂直板电泳仪、蛋白转印系统(Bio-Rad);扫描仪Powerlook 2100XL;质谱仪4700型MALDI-TOF/MS。
1.2实验方法
1.2.1全菌蛋白的制备05ZYh13和△covR培养至稳定生长期,6 000 g离心15 min收集菌体。菌体在液氮中研磨后加入蛋白裂解液1 mL/100 mg,蛋白样品制成匀浆液后超声破碎5 min,10 ℃下40 000g离心30 min,取上清,分装后于-80 ℃冻存。改良Bradford 法测定蛋白含量。
1.2.2二维电泳及凝胶染色将05ZYh13和△covR蛋白样品各取500 μg溶解于400 μL水化缓冲液中,蛋白样载入17 cm IPG胶条后进行等电聚焦电泳,50 V,20 ℃主动水化12 h,S1 250 V 30 min,S2 1 000 V 1 h,S3 10 000 V 5 h,S4 10 000 V 60 000伏时聚焦,S5 500 V 任意时间。聚焦完成后的胶条分别用DTT和碘乙酸铵平衡缓冲液平衡15 min,10%的聚丙烯酰胺凝胶分离蛋白。每个蛋白样品均平行进行3次。
1.2.3差异点分析及质谱鉴定TYPHOON SCANNE扫胶后用Image Master4.01进行图像分析,获取蛋白质点位置坐标和表达量等信息。以05ZYh13作为对照,分析△covR蛋白表达谱,统计有或无差异点以及蛋白表达Ratio≥3 的蛋白点,SPSS12.0 进行统计学分析。MALDI-TOF/MS获得候选蛋白的肽质量指纹图谱(Peptide Mass Fingerprint, PMF),Mascot软件将PMF数据在NCBI非冗余蛋白数据库进行比对搜索,搜索参数(搜索类型:PMF;消化酶:胰蛋白酶;固定化修饰:Carbamidomethyl;可变修饰:Oxidation;质量值:单一同位素;肽质量偏差:±100 ppm;Max missed cleavages: 1)。Mowse分值的概率P评价搜索结果的质量,分值大于51表明差异有统计学意义(P<0.05)。
2结果
2.1二维电泳结果05ZYh13和△covR菌株全菌蛋白经二维电泳分离后蛋白点多集中于pp~7,分子量多集中于30~70 kD,等电点(pI)多在3~6 之间,结果见图1。

图1 05ZYh13与突变株△covR全菌蛋白二维电泳图谱Fig.1 Two-dimensional gel map of proteins from 05ZYh13 and △covR
2.2凝胶图像分析结果对二维凝胶电泳结果进行扫胶比较分析,结果发现05ZYh13分离得到559个蛋白点,△covR菌株分离得到491个蛋白点,其共有的匹配蛋白点数367个。对367个匹配蛋白点表达量变化分析发现,3倍以上差异蛋白点共40个(图2),其中15个蛋白点表达下调,25个蛋白点表达上调。对未匹配蛋白点分析发现,△covR特异表达蛋白点124个(图3),05ZYh13特异蛋白点192个(图4)。

注:绿色圈表示ΔcovR较05ZYh13菌株3倍以上差异蛋白点,红色箭头表示质谱鉴定蛋白点
Note: The green circles represent more than 3 ratios changed proteins of 05ZYh13 and △covRstrains. The red arrow represent identified proteins by mass spectrometry
图205ZYh13与△covR菌株差异表达蛋白点分析图
Fig.2Differentially expressed proteins analysis of 05ZYh13 and △covR

注:绿色圈表示△covR特异蛋白点,蓝色箭头表示质谱鉴定蛋白点
Note: The green circles represent specific proteins of △covRstrain. The blue arrow represent identified proteins by mass spectrometry
图3△covR菌株特异蛋白点分析图
Fig.3Specific expressed proteins of △covRmutant strain
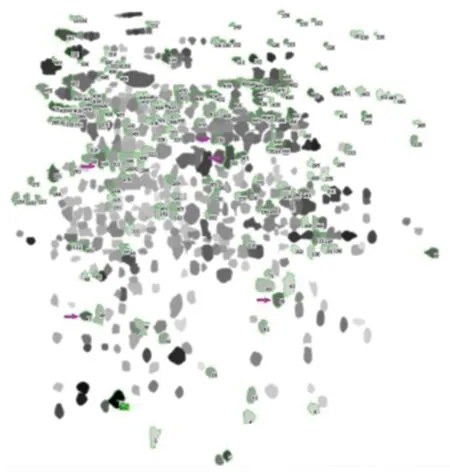
注:绿色圈表示05ZYh13菌株特异蛋白点,粉色箭头指示质谱鉴定蛋白点Note: The green circles represent specific proteins of 05ZYh13 strain. The pink arrow represent identified proteins by mass spectrometry 图4 05ZYh13菌株特异蛋白点分析图Fig.4 Specific expressed proteins of 05ZYh13 strain
2.3质谱鉴定结果
05ZYh13和△covR差异表达蛋白MALDI-TOF/MS质谱鉴定35个蛋白质,其中3倍以上差异表达蛋白15个,△covR菌株7个蛋白下调表达,8个蛋白上调表达。其中NADPH依赖的谷氨酸脱氢酶上调表达9.29倍,蛋白点Spot43是一未知功能蛋白,上调9倍;另外分子伴侣GroEL和DnaK分别上调表达5.59倍和6.78倍,在核苷酸代谢过程中具有重要作用的腺苷酸激酶上调表达3.13倍。在下调表达蛋白中50S核糖体蛋白L10下调7.79倍,下调变化最大;糖代谢相关酶6-磷酸果糖激酶和乳酸脱氢酶分别下调4.17、4.65倍;细胞壁合成密切相关的N-乙酰葡萄糖胺磷酸转移酶下调表达3.36倍。
质谱鉴定△covR菌株特异蛋白15个,主要是涉及代谢过程中的酶和物质转运过程。如在糖代谢过程中的甘油醛-3-磷酸脱氢酶、磷酸甘油酸激酶、磷酸甘油酸变位酶、丙酮酸激酶都是糖酵解途径中重要的催化酶;Spot99蛋白点是3-酮乙酰-ACP还原酶,该酶是脂肪酸合酶的重要成分之一,催化脂肪酸合成;Spot5蛋白点是尿嘧啶DNA糖基化酶催化含尿嘧啶的单链和双链DNA释放游离尿嘧啶,Spot36蛋白点是脱氧核糖醛缩酶,能够催化5-磷酸-2-脱氧-D-核糖分解成3-磷酸-D-甘油醛和乙醛,并可催化其逆反应,这两个酶都是参与核苷酸代谢过程的重要酶类;Spot36蛋白点是二氢吡啶二羧酸还原酶,Spot312蛋白点是二氢碟酸合酶,二者都与氨基酸代谢过程紧密联系;另外支链氨基酸转运ATP酶和ABC型精氨酸转运子都是物质转运相关蛋白;鉴定的蛋白中还发现了一些结构蛋白如30S核糖体蛋白,膜锚定脂蛋白等。对05ZYh13特异蛋白点进行质谱鉴定共得到5个对应蛋白,分别是跨膜蛋白、核糖体蛋白、翻译延伸因子、葡萄糖-1-磷酸胸腺嘧啶转移酶以及丙酮酸脱氢酶。结果见表1。
表105ZYh13与△covR菌株差异蛋白点质谱鉴定结果
Tab.1Mass spectrum identification results of differentiated proteins from 05ZYh13 and △covR strains
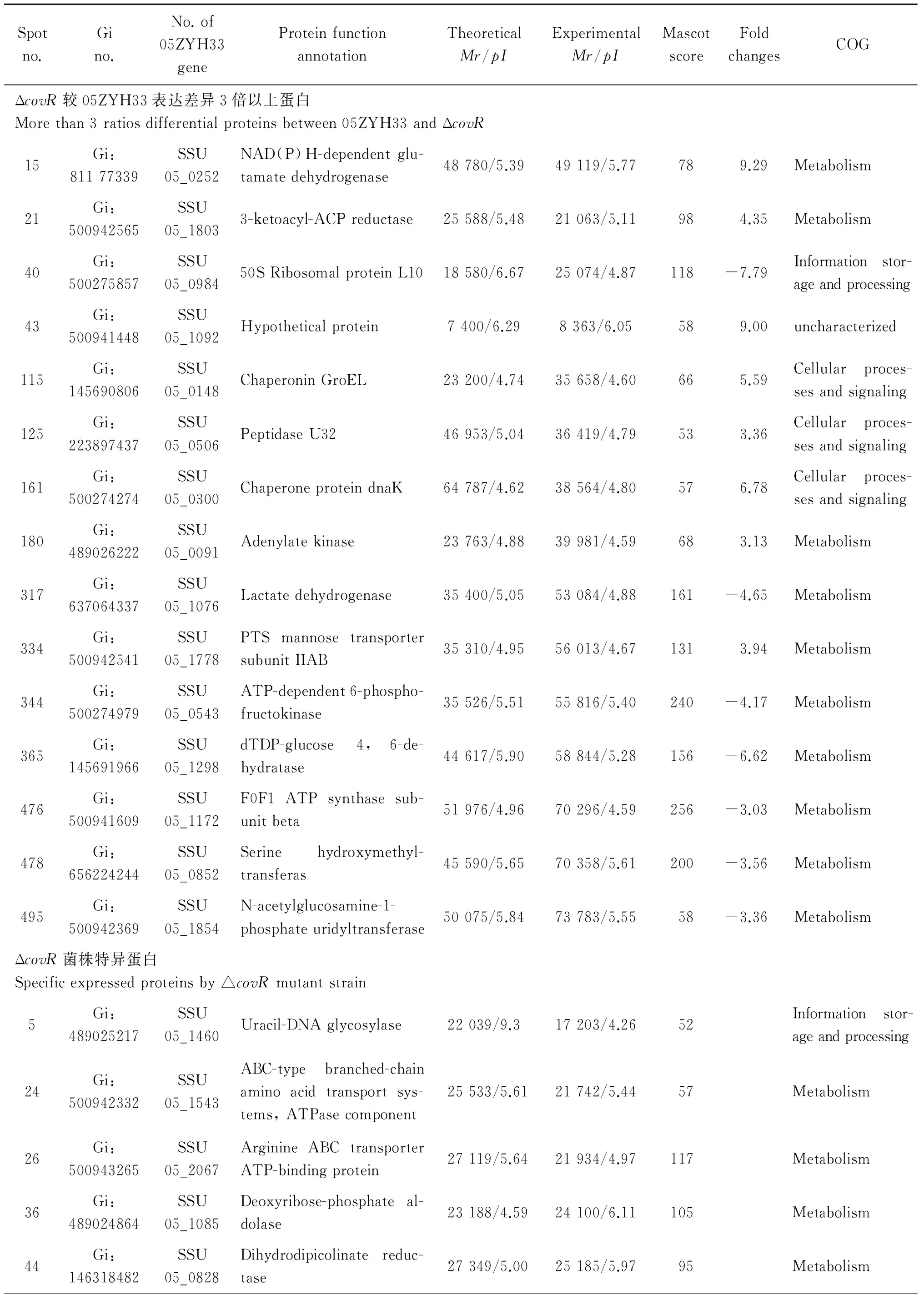
Spotno.Gino.No.of05ZYH33geneProteinfunctionannotationTheoreticalMr/pIExperimentalMr/pIMascotscoreFoldchangesCOGΔcovR较05ZYH33表达差异3倍以上蛋白Morethan3ratiosdifferentialproteinsbetween05ZYH33andΔcovR15Gi:81177339SSU05_0252NAD(P)H-dependentglu-tamatedehydrogenase48780/5.3949119/5.77789.29Metabolism21Gi:500942565SSU05_18033-ketoacyl-ACPreductase25588/5.4821063/5.11984.35Metabolism40Gi:500275857SSU05_098450SRibosomalproteinL1018580/6.6725074/4.87118-7.79Informationstor-ageandprocessing43Gi:500941448SSU05_1092Hypotheticalprotein7400/6.298363/6.05589.00uncharacterized115Gi:145690806SSU05_0148ChaperoninGroEL23200/4.7435658/4.60665.59Cellularproces-sesandsignaling125Gi:223897437SSU05_0506PeptidaseU3246953/5.0436419/4.79533.36Cellularproces-sesandsignaling161Gi:500274274SSU05_0300ChaperoneproteindnaK64787/4.6238564/4.80576.78Cellularproces-sesandsignaling180Gi:489026222SSU05_0091Adenylatekinase23763/4.8839981/4.59683.13Metabolism317Gi:637064337SSU05_1076Lactatedehydrogenase35400/5.0553084/4.88161-4.65Metabolism334Gi:500942541SSU05_1778PTSmannosetransportersubunitIIAB35310/4.9556013/4.671313.94Metabolism344Gi:500274979SSU05_0543ATP-dependent6-phospho-fructokinase35526/5.5155816/5.40240-4.17Metabolism365Gi:145691966SSU05_1298dTDP-glucose4,6-de-hydratase44617/5.9058844/5.28156-6.62Metabolism476Gi:500941609SSU05_1172F0F1ATPsynthasesub-unitbeta51976/4.9670296/4.59256-3.03Metabolism478Gi:656224244SSU05_0852Serinehydroxymethyl-transferas45590/5.6570358/5.61200-3.56Metabolism495Gi:500942369SSU05_1854N-acetylglucosamine-1-phosphateuridyltransferase50075/5.8473783/5.5558-3.36MetabolismΔcovR菌株特异蛋白Specificexpressedproteinsby△covRmutantstrain5Gi:489025217SSU05_1460Uracil-DNAglycosylase22039/9.317203/4.2652Informationstor-ageandprocessing24Gi:500942332SSU05_1543ABC-typebranched-chainaminoacidtransportsys-tems,ATPasecomponent25533/5.6121742/5.4457Metabolism26Gi:500943265SSU05_2067ArginineABCtransporterATP-bindingprotein27119/5.6421934/4.97117Metabolism36Gi:489024864SSU05_1085Deoxyribose-phosphateal-dolase23188/4.5924100/6.11105Metabolism44Gi:146318482SSU05_0828Dihydrodipicolinatereduc-tase27349/5.0025185/5.9795Metabolism
注:Score分数大于51具有统计学意义,P<0.05
Note: More than 51 scores showed the data has significant differences.P<0.05
3讨论
病原菌在致病过程由多种致病因子共同参与,如荚膜多糖、溶血素、胞外因子、溶菌酶释放蛋白等,也包括一些生长及代谢相关的相互作用因子[4]。本文对CovR基因缺失株与中国强毒株05ZYh13蛋白质谱的比较分析后,用MADLI-TOF-MS方法鉴定出35个差异表达蛋白。为进一步研究毒力负调控因子CovR在调控蛋白表达的分子机制奠定基础。
糖酵解是生物代谢的重要部分,能够为细胞提供能量,参与该过程的很多酶类定位于链球菌或其它细菌表面[13-14]。其中甘油醛-3-磷酸脱氢酶(GAPDH)、磷酸甘油酸激酶、磷酸甘油醛变位酶、丙糖磷酸异构酶和烯醇化酶(Enolase)除参与糖代谢过程外,还具有纤溶酶/纤溶酶原蛋白结合能力,能够增加细菌对组织的侵入能力[15]。Brassard等[16]克隆并纯化获得S.suis2的GAPDH重组蛋白,并证明其与宿主细胞的粘附相关。Quessy等[17]构建S.suis2的gapdh基因缺陷株,发现GAPDH表达缺失株对宿主气管表皮细胞的粘附能力降低。另外Tsugaw等[18]对类志贺氏菌的研究发现GroEL能增强细菌对宿主细胞的粘附。Singh等[19]对金黄色葡萄球菌的研究发现DnaK表达缺陷导致,细菌合成生物被膜减少,对宿主细胞的粘附减弱。本研究发现GAPDH(Spot63)、磷酸甘油酸激酶(Spot57)、磷酸甘油醛变位酶(Spot208)、GroEL(Spot115)和DnaK(Spot161)在△covR中表达均明显增加,此类与粘附作用相关蛋白表达的增加可能与△covR菌株对宿主细胞的粘附增强有关。
丙酮酸激酶(Spot59),其同源蛋白在肺炎链球菌中与毒力密切相关[20],Burall等[21]在绵羊病原菌Chlamydiaabortus的研究中发现,当丙酮酸激酶表达缺陷时该菌毒力减弱。Wang等[22]在S.suis2二元信号系统VirR/VirS的蛋白质组研究发现,△virRS菌株丙酮酸激酶表达下调1.7倍。蛋白点Spot15是SSU05_0252基因编码的NADPH依赖的谷氨酸脱氢酶(NAD(P)H-dependent glutamate dehydrogenase, GDH),该蛋白位于S.suis2的细胞表面,能够刺激机体产生高滴度的抗体,作为一个毒力蛋白已经引起广泛关注[23]。GDH在△covR菌株中上调9.29倍,丙酮酸激酶为特异性表达蛋白,提示CovR可能通过调节GDH和丙酮酸激酶的表达而影响细菌毒力,然而CovR与GDH和丙酮酸激酶的调控关系仍需要进一步研究证实。
PTS是一个多蛋白系统,在许多细菌的糖代谢过程中起关键作用,参与细菌代谢和翻译过程的调节。在S.mutans[24]和L.monocytogenes[25]病原菌中PTS甘露糖转运子亚单位IIAB在碳代谢抑制和毒力基因下调中承担重要作用。另外腺苷酸激酶(Adk)可能也参与细菌致病过程,在肺炎链球菌中SpAdk为肺炎链球菌生长必须基因,其细胞内ATP水平随SpAdk增加成比例增加,SpAdk影响细胞内能荷稳态,其突变体导致细胞分裂发生缺陷[26]。在鼠疫耶尔森氏菌的研究中,AKyp的突变导致细菌毒力显著下降[27]。在本研究中PTS甘露糖转运子亚单位IIAB(Spot334)和Adk(Spot180)在△covR菌株中的表达分别上调表达3.94和3.13倍,但CovR是否对这两个蛋白具有调控作用仍需进一步研究。
本文对S.suis2中国强毒株05ZYh13与△covR菌株蛋白质谱比较鉴定了35个差异表达蛋白,结果表明CovR可能通过对细胞代谢过程中关键酶如GAPDH、磷酸甘油酸激酶、磷酸甘油醛变位酶、GDH、丙酮酸激酶以及分子伴侣蛋白GroEL和DnaK等多方面的表达调控,从而影响细菌的毒力及致病性。但是由于本研究采用全菌蛋白裂解法制备样品,所制样品中多为胞浆蛋白,而胞膜蛋白含量低、缺乏分泌蛋白。因此未鉴定出荚膜多糖、溶血素(Sylisin)、溶菌酶释放蛋白(MRP)、胞外因子(EF)以及血清浑浊因子(OFS)等分泌蛋白或胞膜蛋白类的S.suis2毒力因子;另外由于质谱鉴定蛋白点偏少,也可能造成一些差异表达的毒力因子未被发现。后续课题组将分别制备细菌分泌蛋白、胞膜蛋白和胞浆蛋白样品,从而获得△covR菌株更全面的蛋白表达信息,为进一步研究CovR调控S.suis2毒力的分子机制奠定基础。
参考文献:
[1] Feng Y, Zhang H, Wu Z, et al.StreptococcussuisInfection: an emerging/reemerging challenge of bacterial infectious diseases?[J]. Virulence, 2014, 5(4): 477-497.DOI:10.4161/yiru.28595.
[2] Pan Z, Ma J, Dong W, et al. Novel variant serotype ofStreptococcussuisisolated from piglets with meningitis[J]. Appl Environm Microbiol, 2014, 81(3): 976-985. DOI: 10.1128/AEM.02962-14.
[3] Gottschalk M, Xu J, Calzas C, et al.Streptococcussuis: a new emerging or an old neglected zoonotic pathogen?[J]. Future Microbiol, 2010, 5(3): 371-391. DOI: 10.2217/fmb.10.2.
[4] Calva E, Oropeza R. Two-component signal transduction systems, environmental signals, and virulence[J]. Microbial Ecol, 2006, 51(2): 166-176.
[5] Han H, Liu C, Wang Q, et al. The two-component system Ihk/irr contributes to the virulence ofStreptococcussuisserotype 2 strain 05ZYh13 through alteration of the bacterial cell metabolism[J]. Microbiology, 2012, 158(7): 1852-1866. DOI: 10.1099/mic.0.057448-0.
[6] Li J, Chen T, Yang Z, et al. The two-component regulatory system ciarh contributes to the virulence ofStreptococcussuis2[J]. Vet Microbiol, 2011, 148(1): 99-104. DOI: 10.1016/j.vetmic.2010.08.005.
[7] Mitrophanov AY, Churchward G, Borodovsky M. Control ofStreptococcuspyogenesvirulence: modeling of the CovR/S signal transduction system[J]. J Theoret Biol, 2007, 246(1): 113-128.
[8] Jiang SM, Cieslewicz MJ, Kasper DL, et al. Regulation of virulence by a two-component system in Group BStreptococcus[J]. J Bacteriol, 2005, 187(3): 13-1105.
[9] Dmitriev A, Mohapatra SS, Chong P, et al. CovR-controlled global regulation of gene expression inStreptococcusmutans[J]. PLoS One, 2011, 6(5): e20127. DOI: 10.1371/journal.pone.0020127.
[10] Trihn M, Ge X, Dobson A, et al. Two-component system response regulators involved in virulence ofStreptococcuspneumoniaeTIGR4 in infective endocarditis[J]. PLoS One, 2013, 8(1): e54320. DOI: 10.1371/journal.pone.0054320.
[11] Pan XZ, Ge JC, Li M, et al. The orphan response regulator CovR: a globally negative modulator of virulence inStreptococcussuisserotype2[J]. J Bacteriol, 2009, 191(8): 2601-2612. DOI: 10.1128/JB.01309-08.
[12] Chen C, Tang J, Dong W, et al. A glimpse of Streptococcal toxic shock syndrome from comparative genomics ofS.suis2 Chinese isolates[J]. PLoS One 2007,2(3): e315.
[13] Jones MN, Holt RG. Cloning and characterization of an α-enolase of the oral pathogen streptococcus mutans that binds human plasminogen[J]. Biochem Biophysic Res Communicat, 2007, 364(4): 924-929.
[14] Hughes MJ, Moore JC, Lane JD, et al. Identification of major outer surface proteins ofStreptococcusagalactiae[J]. Infect Immun, 2002, 70(3): 1254-1259.
[15] Kinnby B, Booth NA, Svensater G. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions[J]. Microbiology, 2008, 154(Pt 3): 924-931. DOI: 10.1099/mic.0.2007/013235-0.
[16] Brassard J, Gottschalk M, QuessyS.Cloningand purification of theStreptococcussuisserotype 2 glyceraldehyde-3-phosphate dehydrogenase and its involvement as an adhesin[J]. Vet Microbiol, 2004, 102(1/2): 87-94.
[17] Brassard J, Gottschalk M, QuessyS.Decreaseof the adhesion ofStreptococcussuisserotype 2 mutants to embryonic bovine tracheal cells and porcine tracheal rings[J]. Canad J Vet Research, 2001, 65(3): 156-160.
[18] Tsugawa H, Ito H, Ohshima M, et al. Cell adherence-promoted activity of Plesiomonas shigelloides groEL[J]. J Med Microbiol, 2007, 56(1): 23-29.
[19] Singh VK, Syring M, Singh A, et al. An insight into the significance of the Dnak heat shock system inStaphylococcusaureus[J]. Int J Med Microbiol, 2012, 302(6): 242-252. DOI: 10.1016/j.ijmm.2012.05.001.
[20] Yesilkaya H, Spissu F, Carvalho SM, et al. Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence[J]. Infect Immun, 2009, 77(12): 5418-5427. DOI: 10.1128/IAI.00178-09.
[21] Burall LS, Rodolakis A, Rekiki A, et al. Genomic analysis of an attenuatedChlamydiaabortuslive vaccine strain reveals defects in central metabolism and surface proteins[J]. Infect Immun, 2009, 77(9): 4161-4167. DOI: 10.1128/IAI.00189-09.
[22] Wang H, Shen X, Zhao Y, et al. Identification and proteome analysis of the two-component VirR/virS system in epidemicStreptococcussuisserotype 2[J]. FEMS Microbiol Lett, 2012, 333(2): 160-168. DOI: 10.1111/j.1574-6968.2012.02611.x.
[23] Silva LM, Baums CG, Rehm T, et al. Virulence-associated gene profiling ofStreptococcussuisisolates by PCR[J]. Vet Microbiol, 2006, 115(1/3): 117-127.
[24] Abranches J, Candella MM, Wen ZT, et al. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence inStreptococcusmutans[J]. J Bacteriol, 2006, 188(11): 3748-3756.
[25] Vu-Khac H, Miller KW. Regulation of mannose phosphotransferase system permease and virulence gene expression inListeriamonocytogenesby the EII(t)Man transporter[J]. Appl Environm Microbiol, 2009, 75(21): 6671-6678. DOI: 10.1128/AEM.01104-09.
[26] Thach TT, Luong TT, Lee S, et al. Adenylate kinase fromStreptococcuspneumoniaeis essential for growth through its catalytic activity[J]. FEBS Open Bio, 2014, 4(1): 672-682. DOI: 10.1016/j.fob.2014.07.002.
[27] Munier-Lehmann H, Chenal-Francisque V, Ionescu M, et al. Relationship between bacterial virulence and nucleotide metabolism: a mutation in the adenylate kinase gene rendersYersiniapestisavirulent[J]. Biochem J, 2003, 373(2): 515-522.
DOI:10.3969/j.issn.1002-2694.2016.05.001
通讯作者:潘秀珍,Email:panxiuzhen_2004@163.com
中图分类号:R378
文献标识码:A
文章编号:1002-2694(2016)05-417-07
Corresponding author:Pan xiu-zhen, Email: panxiuzhen_2004@163.com
收稿日期:2015-12-16修回日期:2016-03-18
Comparative proteomic research between theStreptococcussuisserotype 2 Chinese highly virulent strain and thecovRisogenic mutant
NI Hua1,2,ZHANG Jian1,2, ZHENG Feng2, HU Dan2,LI Xian-fu2, WANG Chang-jun2, PAN Xiu-zhen1,2
(1.CollegeofLifeSciences,NanjingNormalUniversity,Nanjing210023,China;2.InstituteofMilitaryMedicalSciences,NanjingCommand,Nanjing210002,China)
Abstract:In order to search for the virulence factor regulated by CovR, the proteomics of the whole-cell protein were compared between Streptococcus suis serotype 2 wild strain 05ZYh13 and an isogenic mutant strain △covR by two-dimensional gel electrophoresis. The 05ZYh13 and △covR were cultured in Todd-Hewitt Broth medium then the whole-cell proteins sample were extracted. The 2-DE gel was conducted using the ph1-10 IPG strip for the first dimension IEF and followed by SDS-PAGE. After electrophoresis, the gels were stained and analyzed. Results showed that the 05ZYh13 and △covR had 559 and 491 protein spots respectively. Compared with the 05ZYh13, the mutant strain had 40 proteins more than 3 folds changed which identified 15 proteins by mass spectrum. Those proteins major involved in cell metabolic enzymes, such as adenylate kinase, glutamate dehydrogenase and PTS system components, etc., as well as molecular chaperone proteins GroEL and Dnak. The △covR had about 124 specific protein spots in which 15 proteins were identified by mass spectrum. Those proteins were participate in the cell process of sugar metabolism, such as phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase and pyruvate kinase, etc. Beside of those 5 specific proteins of 05ZYh13 was identified by mass spectrum. Appraisement of 35 differentially expressed proteins involved in bacterial virulence, host cell adhesion and cell division, etc., and the molecular chaperone up-regulated expression showed that the regulation may be occurred in the profile of protein modification. These results provided better understanding on pathogenic mechanisms of Streptococcus suis type 2 at the level of protein expression.
Key words:Streptococcus suis serotype 2; CovR; two-dimensional gel electrophoresis; proteome Funded by the National Natural Science Foundation of China (Nos. 81571965 and 81471920), the Natural Science Foundation of Jiangsu Province (No. BK20151091) and the 333 Engineering Science Foundation of Jiangsu Province (No. BRA2014363)
国家自然科学基金(Nos. 81571965, 81471920)、江苏省自然科学基金(No. BK20151091)和江苏省333工程科研资助项目(BRA2014363)联合资助
