Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
2016-06-05JingZHAOLinLUYongnianLIUZhanhaiSUYingzhongYANG
Jing ZHAO, Lin LU, Yong-nian LIU, Zhan-hai SU, Ying-zhong YANG
Association study between the angiotensin converting enzyme gene insertion/deletion polymorphism and Qinghai Han Chinese with congenital heart disease
Jing ZHAO1,3,4,5, Lin LU2, Yong-nian LIU1, Zhan-hai SU1, Ying-zhong YANG3,4,5
1. Qinghai University School of Medicine, Xining 810001, China; 2. Department of Cardiac Surgery, Qinghai Cardiovascular Diseases Vocational Hospital, Xining 810012, China; 3. Research Center for High Altitude Medical Sciences, Qinghai University School of Medicine, Xining 810001, China; 4. Basic and Applied Key Laboratory for High Altitude Medical Science and Technology of Qinghai, Xining 810001, China; 5. Qinghai-Utah United Key Laboratory for High Altitude Medical Science, Xining 810001, China
Objecti ve:The aim of this work is to determine whether the angiotensin converti ng enzyme (ACE) I/D (inserti on/deleti on) polymorphism is associated with the suscepti bility to congenital heart disease (CHD) in the Qinghai Han Chinese.Methods:This study enrolled 59 CHD pati ents and 193 CHD controls from Qinghai Cardiovascular Diseases Vocati onal Hospital. Blood samples were collected from each of the pati ent and control groups. The ACE-I/D polymorphism was detected by polymerase chain reacti on (PCR).Results:The genotype frequencies of ACE-I/D for II, ID, DD in pati ents and controls were 0.475, 0.441, 0.085 and 0.430, 0.446, 0.124, respecti vely. The allelic frequencies of I and D were 0.650, 0.350 and 0.695, 0.305, respecti vely. The OR of ID, DD and D alleles relati ve to II for CHD was 1.116 (0.604-2.060), 1.619 (0.564-4.648) and 1.211 (0.777-1.889). There was no signifi cant diff erence of the genotypic and the allelic frequencies in ACE-I/D polymorphism between the pati ent and control groups.Conclusion:There is no relati on between ACE-I/D polymorphism and CHD in current Qinghai Han Chinese.
congenital heart disease; ACE gene; inserti on/deleti on; polymorphism
Introduction
Congenital heart disease (CHD) is the most common type of birth defect, which refers to the structural, functional or metabolic abnormalities of heart or major blood vessels that develop in the process of embryonic development, affecting approximately 0.8% of live births worldwide [1, 2]. Improved treatment strategies and interventions have increased survival of aff ected children are expected to live well into adulthood [3, 4]. Over the last three decades, adults with congenital heart disease have become one of the fastest growing populations of adults with chronic heart disease [5]. The pathogenesis of CHD still has not been clearly identifi ed. Nowadays, the well recognized causes of CHD are partitioned into genetic factors and environmental factors [6, 7]. Many agents included in environmental factors, viral infection, drugs, maternal condition, physical and chemical factors [6]. In terms of genetic factors, with the advances of molecular techniques, reports showed that the high incidence of CHD was associated with the existence of an abnormal number of chromosomes and genes [8]. Although we have made some progress, the genetic factors underlying CHD remain elusive. Several candidates have been proposed as CHD susceptibility genes including angiotensin I-converting enzyme (ACE) gene [9], because of the ACE is one of the rate-limiting enzymes in the renin-angiotensin-aldosterone system (RAAS) that maintains vascular tone and regulates blood pressure [10]. The ACE gene islocated on chromosome 17 at q23 and encoded by a 21-kilobase, 26-exon gene. An insertion/deletion polymorphism in intron 16 of human ACE gene was firstly reported by Rigat [11]. Although I/D polymorphism is located in the intron of the ACE gene, it has been confi rmed to be associated with the increase in serum ACE activity [12]. The ACE-I/D polymorphism is associated with serum ACE levels which are higher for the DD genotype than those for the ID/II genotypes [13, 14].
In order to examine whether the ACE-I/D polymorphism is associated with the susceptibility to CHD in Qinghai Han Chinese, we detected the ACE-I/D polymorphisms in CHD patients and healthy controls in Qinghai Han Chinese.
Patients and Methods
Subjects
Fifty-nine CHD patients had been hospitalized in Qinghai Cardiovascular Diseases Vocational Hospital between March 2016 and September 2016. Th e diagnosis of CHD was based on ECG, Doppler echocardiography, and some other items. A total of 193 healthy controls were collected from the Health Check Centre matched with age, gender, ethnicity, living altitude. Th e CHD patients and CHD healthy controls, whose family had lived at Qinghai over three generations, were unrelated Chinese. Th e Ethics Committee of Qinghai University School of Medicine (Xining, China) approved the current study. All patients and controls sampled in this study signed their informed consents.
Sample collection
With informed consents from all the subjects, 5 mL venous blood samples were collected from each of CHD patients and healthy controls. The whole blood samples were separated into blood cells and plasma by centrifuging at 2 500 g for 10 minutes, and then stored for the following genetic polymorphism analysis.
DNA extraction and genotyping assays
Genomic DNA (gDNA) was extracted from leukocytes by Gentra Puregene Blood Kit (Qiagen, 158389, Germany) according to the standard procedures. The I/D polymorphism was examined by the polymerase chain reaction (PCR) as the Rigat’s method [11]. The PCR mixture was made up of 12.5 μl 2×Taq PCR MasterMix (TIANGEN Biotech (Beijing) Co., Ltd.), 1 μL for each primers and 100 ng of gDNA, adjust the final volume to 25 μl with ddH2O. The PCR cycles were comprised of predenaturation at 95°C for 1 minute, 30 cycles of 95°C for 10 seconds, 60.5°C for 30 seconds, and 72°C for 20 seconds, followed by a fi nal elongation at 72 °C for 10 minutes. Amplified fragments were resolved on 1.5% agarose gels stained with GeneGreen (RT210, TIANGEN Biotech (Beijing) Co., Ltd.).
Statistical analysis
Statistical analyses were performed using the SPSS soft-ware (version17.0, SPSS, Inc,Chicago, IL). The unpaired Student’s t-test was used to compare the mean ages of the groups. Deviation of genotype frequency from Hardy-Weinberg equilibrium was assessed by chi-square test. Allele frequencies were calculated based on genotype frequencies in CHD patient and Healthy control groups, and the intergroup diff erence was estimated with chi-square test. Moreover, odds ratios (OR) and 95% confidence intervals (CI) were calculated, the signifi cant level is α=0.05.
Results
Phenotype characteristics
A total of 252 subjects were recruited in this study. Th ese subjects comprised 59 CHD patients and 193 healthy controls. A statistical analysis regarding the age and gender of the population under study is described in table 1. There was no significant difference between control and CHD subjects regarding gender (P = 0.382) and age (P = 0.069). Th e clinical diagnosis of the patient group is described in table 2. Th e high incidences of CHDs are atrial septal defect (ASD) and patent ductus arteriosus (PDA).
Assessment of ACE gene I/D polymorphism
In the present study we investigated I/D polymorphism of the ACE gene. The presence or absence of a 287 bp Alu-type sequence in the 16th intron leads to ACE gene D/I polymorphism. In the case of homozygous genotypes I/I and D/D, one band was observed at respectively 480 or 192 bp. In heterozygous samples (I/D) both bands occurred simultaneously (Fig.1).
Genotype and allele distribution
Th e statistical analysis regarding the age and gender of the population under study is described in Table 1. Th ere was no signifi cant diff erence between CHD patients and CHD controls in gender (P=0.382)and age (P=0.069). As shown in table 3, the ACE-I/ D genotype of the two groups was in the Hardy-Weinberg equilibrium. The genotype frequencies of ACE-I/D for II, ID, DD in patients and controls groups were 47.5%, 44.1%, 8.5% and 43.0%, 44.6%, 12.4%, respectively. Th e allelic frequencies of I and D were 0.695, 0.305 and 0.650, 0.350, respectively. Th e OR of ID, DD and D alleles relative to II for patients were 1.116 (0.604-2.060), 1.619 (0.564-4.648) and 1.211 (0.777-1.889), respectively. The prevalence of the D allele was 30.5% in patients group and 35.0% in controls group. Th ere was no signifi cant diff erence of the genotypic and the allelic frequencies in the ACE-I/D polymorphism between the patients and controls groups. Furthermore, the effects assuming the D allele of the ACE-I/D polymorphism in the additive, recessive, and dominant modes on the phenotype of CHD patients showed no significant association of the ACE-I/D polymorphism with the patients phenotype (Tab.4, P>0.05). There was no significant association between the ACE-I/D genotype and the risk of CHD.
Discussion
In our present study, the genotype frequencies of ACE-I/D for II, ID, DD in both patient and control groups were 47.5%, 44.1%, 8.5% and 43.0%, 44.6%, 12.4%, respectively. Th e allelic frequencies of I and D in both patient and control groups were 0.695, 0.305 and 0.650, 0.350, respectively. Th e prevalence of the D allele was 30.5% in patient subjects and 35.0% in control subjects. Th ere was no signifi cant diff erence of the genotypic and the allelic frequencies in the ACE-I/D polymorphism between the two groups. The effects assuming the D allele of the ACE-I/ D polymorphism in the additive, recessive, and dominant modes showed no significant association of the ACE-I/D polymorphism with the CHD. Based on our current study, we conclude that ACE-I/D gene polymorphism has no eff ect on susceptibility to CHD in Qinghai Han Chinese.
CHDs present at birth and the most common congenital disorders in newborns and the most common type of birth defect [1]. Worldwide, 1.35 million infants are born with CHD each year, with an estimated prevalence of 0.93% live births in Asia[15], and 0.82% on average in China [4], and Wu [16] showed the total incidence of CHD was 1.15% higher than lowland at Qinghai-Tibet Plateau, the incidence of PDA and ASD were 0.58% and 0.30% , respectively. Th e prevalence of ASD at the three high altitudes sites in the study at Qinghai Province 1988 was 2.4%, which is much higher than the Baltimore-Washington infant study (0.0317%) and the New England Regional Infant Cardiac Program (0.0073%). The prevalence of PDA was 1.2% at high altitude in Qinghai Province, which is also much higher than the prevalence in Baltimore-Washington (0.0089%) & New England Study (0.01381%) [17]. those studies showed that high altitude has been linked with high incidence of CHDs [18].
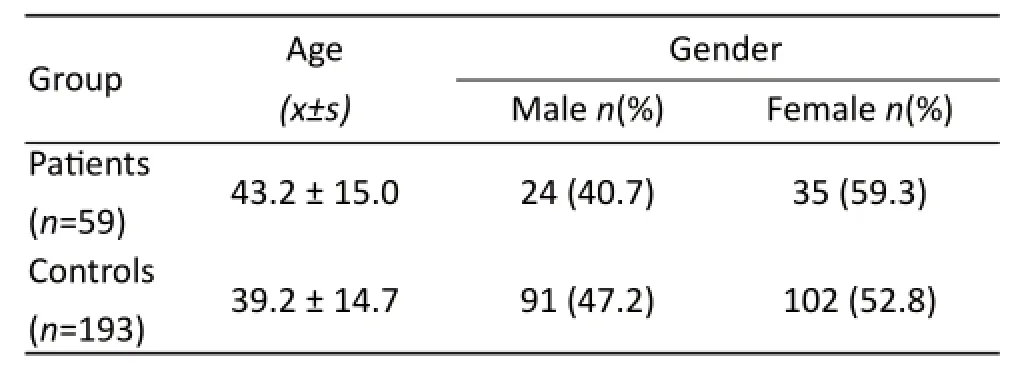
Tab. 1 Age and gender in the population under study.

Tab. 2 Clinical diagnosis of patients’ group.

Fig. 1 The result of amplified fragments.
Qinghai-Tibet Plateau, more than 4 000 m above sea level on average, is the highest and largest plateau in the world. Strong hypoxia is the most important factors, which have profoundly effected on the population [19]. Wu’s studies indicated failure of lower oxygen tension lead to CHDs happened; those anomalies are due to hypoxemia-induced failure of normal neonatal processes [16, 17]. Although hypoxia is a major trigger factor, the pathogenesis of CHDs remains unclear. Growing evidence suggests that a complex interplay between environmental exposures, genetic, and epigenetic factors play an important role in the pathogenetic mechanism of CHDs [15]. In the past few years, researchers have identified multiple genetic variants associated with CHDs [8,15], including ACE. ACE is one of the rate-limiting enzymes in the renin-angiotensin-aldosterone system (RAAS) that maintains vascular tone and regulates blood pressure [9, 10]. Rigat first reported an insertion/ deletion (I/D) polymorphism in itron 16 of human ACE gene [11]; The ACE-I/D polymorphism is as-sociated with serum ACE levels which are higher for the DD genotype than those for the ID/II genotypes [14]. Previous studies have showed ACE and other RAS-related genes have association with premature coronary heart disease and indicated ACE DD polymorphisms is the risk of premature coronary heart disease development [20]. Some studies reported ACE-I/D polymorphism is entangled with the development of diff erent cardiovascular disorders, such as cardiomyopathy, left ventricular hypertrophy [21]. Some human disease susceptibility in Chinese was also related to ACE gene polymorphism, the D allele frequency was related to Han-Chinese essential hypertension (EH) [22]. Some studies had also reported that ACE expression was gradually increased in hypoxic environment, the II genotype of ACE gene expression was much higher in mountain climbers and in the people who lived at the high plateau for a long time than the other genotypes of ACE gene [23, 24]. It was also showed that D allele of ACE gene was related to Tibetan hypertension women, but not to Tibetan men [25]. Meanwhile, it revealed that compared to allele D, the allele I of ACE gene was expressed much higher in Tibetan-Chinese healthy group, it could indicate that allele I was related to plateau environment adaptation in a long time [26, 27]. Droma found that the overrepresented I allele of the ACE gene in Sherpas probably had an advantageous physiological role in adapting to a high-altitude environment [28]. Zhang’s results conclude that ACE is directly regulated by HIF-1α, whereas ACE2 is regulated in a bidirectional way during hypoxia and may play a protective role during the development of hypoxic pulmonary hypertension (HPH) [29], it’s partly explain why Wu’s results showed the total incidence of CHD was 1.15% higher than lowland at Qinghai-Tibet Plateau. ACE is directly upregulated by HIF-1α under hypoxia situation in maternal uterine, Much more Ang II were produced by high level ACE lead to high pulmonary vascular resistance and right atrial pressure inhibits early closure of foremen ovale.

Tab. 3 Frequencies of the allele and genotype of the ACE-I/D polymorphism in CHD patients and CHD controls Subjects.

Tab. 4 Effects of the D allele assuming the additive, recessive, and dominant modes on CHD patients subjects.
Based on our current study, we conclude that ACE-I/D gene polymorphism has no effect on susceptibility to CHD in Qinghai Han Chinese. Liu’s results supported the notion that ACE D allele carriers were at signifi cantly increased risk of developing CHD [30], however Alazhary’s study showed ACE-I/ D gene polymorphism is not associated with CHD [31].
Several limitations of this study should be pointed out. First, due to the limitations of the equipments, we did not measure other useful clinic parameters in the participants, such as SBP (systolic blood pressure), DBP (diastolic blood pressure), MAP (mean arterial pressure), PASP (pulmonary artery systolic pressure), which will provide more insight into the implication of ACE in CHDs. Second, the CHD patients’ groups were small; we need more samples in the future. In addition, with the advances of new techniques, it is necessary to employ GWAS, Whole Exome sequencing and Whole genome re-sequenc-ing to understand molecular mechanisms of CHD diseases, which will help develop new approaches for the prevention and treatment of CHD.
Acknowledgements
This paper is supported by Qinghai Science & Technology Support Program (2015-SF-124) and Basic Applied Study Foundation of Qinghai (2016-ZJ-706).
REFERENCES
1. Hoff man JI, Kaplan S. Th e incidence of congenital heart disease [J]. J Am Coll Cardiol, 2002, 39(12): 1890-1900.
2. Zhou FJ, Zhou CY, Tian YJ, et al. Diagnostic value of analysis of H-FABP, NT-proBNP, and cTnI in heart function in children with congenital heart disease and pneumonia [J]. Eur Rev Med Pharmacol SCI, 2014, 18(10): 1513-1516.
3. Gurvitz M, Burns KM, Brindis R, et al. Emerging Research Directions in Adult Congenital Heart Disease: A Report From an NHLBI/ACHA Working Group [J]. J Am Coll Cardiol, 2016, 67(16): 1956-1964.
4. Huang GY. Screening index and clinical value of congenital heart disease [J]. Chin J Pract Pediatr, 2013, 28(7): 503-504.
本方案是由三列结构相同的双层轮对存放装置组成,用于标准轨距机车轮对的存放(如SS4、HXD1、HXD3等),也可用于各种标准轨距的车辆、地铁轮对的存放,非常适合机车检修单位现场使用。存放数量可以按用户需要进行定制,所存放的轮对可带有轴箱和抱轴箱,每条轮对重约4吨,每条轮对均可单独吊运取出。
5. Cedars AM. Invited Commentary: Th e specialty of adult congenital heart disease [J]. Proc (Bayl Univ Med Cent), 2016, 29(2): 174-175.
6. Zhang L. The pathogenesis of congenital heart disease [J]. Chin J Eugenics Hered, 2008, 16(3): 1-4.
7. Nora JJ, Nora AH. Th e evolution of specifi c genetic and environmental counseling in congenital heart disease [J]. Circulation, 1978, 57(2): 205-213.
8. Chaix MA, Andelfi nger G, Khairy P. Genetic testing in congenital heart disease: A clinical approach [J]. World J Cardiol, 2016, 8(2): 180-191.
9. Alazhary NM, Morsy MM, Al-Harbi KM. Angiotensin-converting enzyme gene insertion deletion (ACE I/D) polymorphism in Saudi children with congenital heart disease [J]. Eur Rev Med Pharmacol Sci, 2015, 19(11): 2026-2030.
10. Hibi S, Goto Y, Ando T, et al. No association between angiotensin I converting enzyme (ACE) I/D polymorphism and gastric cancer risk among Japanese [J]. Nagoya J Med Sci, 2011, 73(3-4): 169-175.
11. Rigat B, Hubert C, Alhenc-Gelas F, et al. An insertion/ deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels [J]. J Clin Invest, 1990, 86(4): 1343-1346.
13. Villard E, Tiret L, Visvikis S, et al. Identifi cation of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation-linkage analysis [J]. Am J Hum Genet, 1996, 58(6): 1268–1278.
14. Sakuma T, Hirata RD, Hirata MH. Five polymorphisms in gene candidates for cardiovascular disease in Afro-Brazilian individuals [J]. J Clin Lab Anal, 2004, 18(6): 309-316.
15. Li M, Cleves MA, Mallick H, et al. A genetic association study detects haplotypes associated with obstructive heart defects [J]. Hum Genet, 2014, 133(9): 1127-1138.
16. Wu TY, Ge RL, Xiao SJ, et al. Epidemiological study on children with congenital heart disease in Qinghai-Tibet Plateau [J]. J Cardiovasc Pulm Dis, 1990, 9(3): 131-135.
17. Hasan A. Relationship of high altitude and congenital heart disease [J]. Indian Heart J, 2016, 68(1): 9-12.
18. Born GV, Dawes GS, Mott JC, et al. The constriction of the ductus arteriosus caused by oxygen and by asphyxia in newborn lambs [J]. J Physiol, 1956, 132(2): 304–342.
19. Yang YZ, Cao Y, Jin GE, et al. Molecular cloning and characterization of hemoglobin alpha and beta chains from plateau pika (Ochotona curzoniae) living at high altitude [J]. Gene, 2007, 403(1-2): 118-124.
20. de Divitiis M, Pilla C, Kattenhorn M, et al. Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta [J]. J Am Coll Cardiol, 2003, 41(12): 2259-2265.
21. Sekuri C, Cam FS, Ercan E, et al. Renin-angiotensin system gene polymorphisms and premature coronary heart disease [J]. J Renin Angiotensin Aldosterone Syst, 2005, 6(1): 38-42. 22. Liu Y, Zhou WY, Hou SQ, et al. Association analysis of polymorphisms of ace gene and agt gene with essential hypertension in chinese han’s population [J]. Chin Med Sci J, 1998, 13(2): 71-76.
23. Patel S, Woods DR, Macleod NJ, et al. Angiotensinconverting enzyme genotype and the ventilatory response to exertional hypoxia [J]. Eur Respir J, 2003, 22(5): 755-760.
24. Bigham AW, Kiyamu M, León-Velarde F, et al. Angiotensin-converting enzyme genotype and arterial oxygen saturation at high altitude in Peruvian Quechua [J]. High Alt Med Biol, 2008, 9(2): 167-178.
25. Gesang L, Liu G, Cen W, et al. Angiotensin-converting enzyme gene polymorphism and its association with essential hypertension in a Tibetan population [J]. Hypertens Res, 2002, 25(3): 481-485.
26. Rupert JL, Hochachka PW. Genetic approaches to understanding human adaptation to altitude in the Andes [J]. J Exp Biol, 2001, 204(Pt 18): 3151-3160.
27. Raleigh SM. Last word on viewpoint: epigenetic regulation of the ACE gene might be more relevant to endurance physiology than the I / D polymorphism [J]. J Appl Physiol, 2012, 112(6): 1086-1088.
28. Droma Y, Hanaoka M, Basnyat B, et al. Adaptation to high altitude in Sherpas: association with the insertion/deletion polymorphism in the Angiotensin-converting enzyme gene [J]. Wilderness Environ Med, 2008, 19(1): 22-29.
29. Zhang R, Wu Y, Zhao M, et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells [J]. Am J Physiol Lung Cell Mol Physiol, 2009, 297(4): L631-640.
30. Liu SM, Hu L, Liu FY. The angiotensin-converting enzyme gene polymorphism in Qinghai Plateau children with congenital heart disease [J]. High Alt Med, 2012, 22(3): 7-9.
31. Alazhary NM, Morsy MM, Al-Harbi KM. Angiotensin-converting enzyme gene insertion deletion (ACE I/D) polymorphism in Saudi children with congenital heart disease [J]. Eur Rev Med Pharmacol Sci, 2015, 19(11): 2026-2030.
doi 10.13459/j.cnki.cjap.2016.06.004
Ying-zhong YANG, MD, PhD, Professor, Research Center for High Altitude Medical Sciences, Qinghai University School of Medicine, 16 kunlun Road, Xining 810001, China. Tel: 86-971-8175660; Fax: 86-971-6142063; E-mail: yingzhong-yang@hotmail.com.
2016-11-11; accepted 2016-11-17
猜你喜欢
杂志排行
中国应用生理学杂志的其它文章
- 双受体激动剂CI-1206拮抗Aβ1-42所致小鼠空间学习记忆损伤的作用*
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
- 阿霉素损伤心肌细胞miRNA378与网腔钙结合蛋白、内质网应激的关系*
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
