Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
2016-06-05MiaoYUZhihuiHELiyingXUANXiaotongSHANJieLONGJingyiFENGYuWANGMingZHAO
Miao YU, Zhi-hui HE, Li-ying XUAN, Xiao-tong SHAN, Jie LONG, Jing-yi FENG, Yu WANG, Ming ZHAO
Effect of creatine phosphate sodium on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured cardiomyocytes
Miao YU1, Zhi-hui HE1, Li-ying XUAN1, Xiao-tong SHAN1, Jie LONG1, Jing-yi FENG1, Yu WANG1, Ming ZHAO2
1. Inner Mongolia University for the Nationalities, Tongliao, Inner Mongolia, China;
2. First Clinical Medical of Inner Mongolia University for Nationalities, Tongliao, Inner Mongolia, China
Objecti ve:To investi gate the eff ect of creati ne phosphate sodium (sodium phosphocreati ne) on miRNA378, miRNA378* and calumenin mRNA in adriamycin-injured suckling mouse myocardium.Methods:The suckling mouse myocardium of primary culture were randomly divided into control group, adriamycin group and treatment group. To identi fy the suckling mouse myocardium, Smooth muscle acti n-α (α-SMA) was monitored by immunohistochemical method. Cardiac function was evaluated by transthoracic echocardiography. The mRNA change of miRNA378, miRNA378* and calumenin mRNA were detected by quantitative real-time PCR. The expression of calumenin and GRP78 were identified by western blot.Results:Mitochondrial damage and vacuolization were found in adriamycin-induced suckling mouse myocardium compared with control group, while creati ne phosphate sodium could reduce this phenomenon. Compared with the control group, the mRNA of miRNA378, miRNA378* and calumenin in adriamycin group was reduced, while creati ne phosphate sodium could increase this phenomenon. The expression of calumenin and GRP78 were decreased aft er adriamycin treatment in suckling mouse myocardiums, creati ne phosphate sodium increased the expression of calumenin and GRP78.Conclusion: The results of this experiment might be closely related to the eff ects of that creati ne phosphate sodium reduced the pathological mechanism of suckling mouse myocardium with myocarditi s caused by adriamycin.
adriamycin; creati ne phosphate sodium; miRNA; calumenin; endoplasmic reti culum stress (ERS)
Introduction
As an anthracycline antibiotic, adriamycin is one of the most effective anti-neoplastic agent in recent years. High concentrations of adriamycin tend to cause severe toxicity to myocardium of which can turn into inrreversible myocardial damage, even congestive heart-failure in the end [1]. The pathogenesis of adriamycin induced myocardiopathy is not clear, it’s been considered that it has intimate connection with myocardial apoptosis [2-4].
Apoptosis plays an important role in development and in some physiological processes. For the past few years, we have learned that endoplasmic reticulum stress (ERS) pathway-mediated myocardial apoptosis is a new signal transduction pathway [5]. ER stress is a condition that is accelerated by the accumulation of unfolded or misfolded protein aft er a disturbance in the ER quality control system because of kinds of pathological and physiological occurrences [6]. Through researching ERS, it has been found the calumenin protein played an essential role in the alleviation of ERS in neonatal rat cardiomyocytes [7]. According to recent reports, the down regulation of calumenin in H9C2 cardiomycytes might be associated with miRNA378 and miRNA378* [8].
Sodium phosphocreatine is a high effi cient energy, which could provide energy in cardiomycytes. The mechanism of sodium phosphocreatine has been reported, such as inhibit the permeability of cellmembrane, change pore of myocardial to protect the mitochondria, even reduce apoptosis [9].
In this study, we investigated the potential mechanism of the protective eff ect of sodium phosphocreatine on adriamycin-induced cardiotoxicity injury. We suggested sodium phosphocreatine relieved adriamycin-induced cardiotoxicity by reduction ER-initiated apoptisis. This effect related with calumenin protein and miRNA378 and miRNA378*.
Materials and Methods
Materials
The 1-3d suckling mice were obtained from the Ji Lin university, license key: SCXK (Ji) 2011-0004; Super M-MLV reverse transcriptase from BioTeke Company, RNA simple Total RNA Kit from TIANGEN Company and Primers made from Sangon Biotech (Shanghai); Creatine phosphate sodium was purchased from the medicine of Ying Lian (Ji Lin, China), adramycin was purchased from the medicine of Wan Dong (ShenZhen, China).
Isolation and culture of myocardial cells of suckling mouse
The suckling mouse myocardium was put into the petridish and cleaned out several times with PBS. After the cleaning, it was cut into 1-3mm3pieces, then digested with 0.1% collagenase II solution and 0.1% trypsin at 37°C for 25 minutes. Th en transferred to a 15 ml-centrifuge tube before centrifuged 1 500 g×7 minutes, the supernatant was abandoned. Suspended the precipitate in PBS and homogenized by pipetting, centrifuged it for 5 minutes at 1 000 r/ min, suspended the cells in medium, removed the residue with 200 screen mesh. Washed the cells with PBS twice and centrifuged for 10 minutes at 1 000 r/min, removed the supernatant fluid and obtained the precipitate. Added 1 ml suspended cells and transfused into the culture plate aft er cell-counting. Th ough the speed of fi broblasts adhering to the wall was really fast, a mass of fi broblasts could be cleaned out by the 2-hour differential velocity adherent technique, leaving the pure cardiomycytes in the supernatant. The cells were placed in the culture plate over 24 hours. Adjusted the cell concentration to 5×104/ml, plated in 6-well tissue culture plates, incubated them in 5% CO2incubator for one night at 37°C. The cell density usually maintained at 70% appropriately.
Identify myocardial cells by immunohistochemical method
Immunohistochemical method was used to identify the myocardial cells. Th e cells were incubated in 0.1% Triton-100 and 3%Hydrogen peroxide. Then they were incubated with primary antibody and secondary antibody. Last using microscopic to exam.
Cardiomyocytes injury model by adriamycin inducedThe suckling mouse myocardium were plated in 6-well tissue culture plates as the cell concentration of 5×104/ml and were incubated in 5% CO2incubator for one night at 37°C. Next day, the adriamycin infection group was added 3mg/L adriamycin and the creatine phosphate sodium group was added both 3 mg/L adriamycin and creatine phosphate sodium (10 mmol/L) each for 3 days, both groups were maintained at the temperature of 37°C [10].Transmission electron microscopy analysis
Th e Cardiomyocytes were immersed in 4 % formalin in three groups. After dehydration in a graded concentration of alcohol, ultrathin sections of tissues were mounted on formvar-coated slot grids, stained with uranyl acetate, lead citrate, then examined with a JEOL 1200 electron microscope (JEOL Co., Tokyo, Japan).
Detected the expression of miRNA378, miRNA378* and calumenin mRNA of each group by Quantitative Real-time PCR
Informations of primers. CalumeninF: 5’GGT GAA GAC AGA GCG AGA AC 3’; CalumeninR: 5’ ATC TCC TCC TTG GTG AGC TTG 3’; RP78F: 5’ CAG CCA ACT GTA ACA ATC AAG 3’; RP78R: 5’ CTG TCA CTC GGA GAA TAC CA 3’; β-actinF: 5’ CTG TGC CCA TCT ACG AGG GCT AT 3’; β-actinR: 5’TTT GAT GTC ACG CAC GAT TTC C 3’.
Total RNA was extracted from the samples. RNA concentration Detection, reverse transcription and fl uorescence quantitative PCR. A parallel experiment was made for each gene of every sample in 4 parallel holes and was repeated for 3 times. Test data with a wide deviation from the estimated value should be rejected, and the remaining test data should be averaged and taken as the required fi nally results. And the results should be analyzed by 2-△△CTmethod.
Statistical analysis
All data were expressed as means±SE. Two-groupcomparisons of the means were carried out by matched t-test using SPSS 11.5.
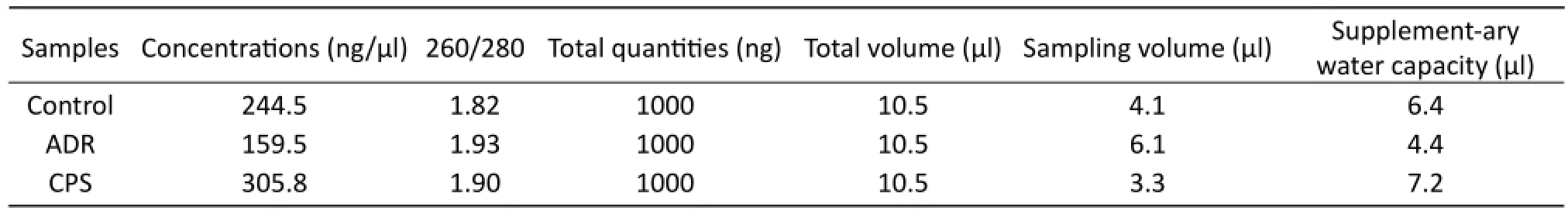
Tab. 1 Adriamycin-injured cardiomyocytes model preparation.
Results
The suckling mouse myocardium cells were isolated successfully by immunohistechemical
The suckling mouse myocardium cells were isolated by previous approaches. The morphology of isolated cells was nearly round at first. After 24 hour, the number of adherent cells was signifi cantly increased and the morphology of adherent cells showed multiple angular or spindle (Fig.1A). Using alpha-smooth muscle actin (a-SMA), the suckling mouse myocardium cells were identifi ed by immunohistechemical (Fig.1B).
Ultrastructural remodelling of nucleus tractus solitariiCompared with control group, myocardial fiber dissolution, flake disappeared, part of cell nuclear membrane disappeared, cytoplasm concentration, mitochondrial damage and vacuolization were frequently found in adriamycin-injured suckling mouse myocardiocytes but minimal ultrastructural changes in similarly treated creatine phosphate sodium treated in adriamycin-injured suckling mouse myocardium. Th e structures of cardiac muscle fibers, mitochondria, nuclei and intercalated discs were not obviously abnormal in creatine phosphate sodium group (Fig.2).
Th e expression of GRP78 was analyzed by quantitative real-time PCR
Compared with the control group, the expression of GRP78 mRNA in creatine phosphate sodium was overtly increased. Compared with the adriamycin infection group, the expression of which in creatine phosphate sodium group was decreased (Fig.3).
The expression of miRNA378 and miRNA378* were analyzed by quantitative real-time PCR
Compared with the control group, the expression of miRNA378 and miRNA378*in adriamycin infection group was reduced. On the contrary, compared with the adriamycin infection group, the expression of which in creatine phosphate sodium group was increased. No changes were observed in the creatine phosphate sodium group (Fig.4).
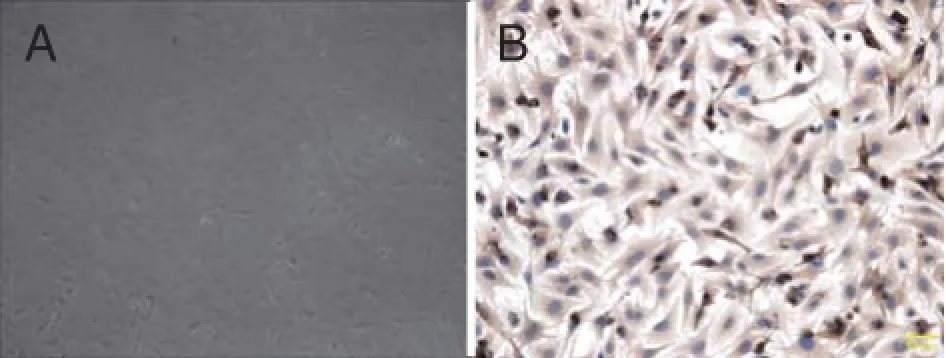
Fig. 1 The suckling mouse myocardium cells were analyzed. A. Suckling mouse myocardium cells cultured for 24 hours; B. Suckling mouse myocardium cells were identified by immunohistechemical.

Fig. 2 Transmisson electron microscopy images of hearts. Original magnification: ×1,500. A: CON; B: CPS; C: ADR; D: ADR+CPS.
Th e expression of calumenin was analyzed by quantitative real-time PCR
Compared with the control group, the expression of calumenin mRNA in adriamycin infection group was distinctly decreased. Compared with the adriamycininfection group, the expression of which in creatine phosphate sodium group was visibly increased (Fig.5).

Fig. 3 Effects of ADR and CPS on expression of GRP78. All data are shown as means±SE. **P<0.01 vs CON;##P<0.01 vs ADR.

Fig. 4 Effects of ADR and CPS on expression of miRNA378 and miRNA378*. A/B: Real-time PCR analyzed the expression of miRNA378 and miRNA378*. All data are shown as mean ±SE.**P<0.01 vs CON;##P<0.01 vs ADR.
Discussion
In this present research, we prove the underlying mechanisms of sodium phosphocreatine against adriamycin induced cardiotoxicity. Th is mechanism related with ER stress by calumenin protein and miRNA378 and miRNA378*.

Fig. 5 Effects of ADR and CPS on expression of calumenin. All data are shown as means±SE.**P<0.01 vs CON;##P<0.01 vs ADR.
Adriamycin induced cardiotoxicity was involved with myocardial apoptosis has been reported [11]. However, the mechanism of sodium phosphocreatine related with apoptosis [9]. ER stress induced apoptosis has been studied [12]. Endoplasmic reticulum (ER) has been linked to many diseases, including cardiotoxicity. The accumulation of misfolded protein disrupts ER and leads to the activation of the classic coping mechanism termed the unfolded protein response [13]. ER alleviated and relieved itself by several ways such as upregulating the expression of GRP78 inhibiting protein synthesis, accelerating the misfolding protein and degrading the unfolding protein. To investigate whether sodium phosphocreatine would relieve ER damage, we examined grp78 gene by real-time PCR. Form the result, we demonstrated a dose-related decrease in the mRNA expression of grp78 after treating with sodium phosphocreatine. The result suggested that ER stress can be relieved in adriamycininduced cardiotoxicity after treating with sodium phosphocreatin.
Recent studies demonstrated that miRNA378 has the ability to regulate the activation of cardiac fi broblasts by indirect modulation of TGFβ1 synthesis and release from cardiomyocytes [14]. Th e experimental evidence showed that the inhibition of miRNA378 induced the cardiac fi broblasts of the suckling mouse [15]. Th e higher the degree of heart failure, the lower the expression of miRNA378 and miRNA378* in the severe heart failure patients. Luckly, the expression ofmiRNA378 and miRNA378* was increased significantly with the improvement of the patients’ cardiac function [16]. Th e down regulation of calumenin in h9c2 cardiomyocytes might be associated with miRNA378 and miRNA378*[17]. Calumenin is a CREC family Ca2+-binding protein with multiple EF-hand motifs which is located in the endo/sarcoplasmic reticulum of mammalian cardiomyocytes [18]. Calumenin plays an essential role in the alleviation of ERS in neonatal rat cardiomyocytes. At the same time, calumenin reduced ER-initiated apoptosis in neonatal rat ventricular cardiomyocytes [19]. In our study, we tested the expression of miRNA378, miRNA378* and calumenin by real-time PCR. The expression of miRNA378, miRNA378* and calumenin were down-regulated aft er treating with adriamycin in cardiomyocytes. Sodium phosphocreatine up-regulated this phenomenon.
The cardiomyocytes injury caused by adriamycin reduced the expression of miRNA378, miRNA378* and calumenin mRNA and increased the expression of GRP78 mRNA. Sodium phosphocreatine actived the phenomenon.
Th e experimental results comfi rmed that parts of our scientific hypothesis was established. Sodium phosphocreatine did relieve the ERS by increasing the expression of mRNA of miRNA378, miRNA378* and calumenin.
1. Zhao WY, Chen DY, Qi Q. Effect of creatine phosphate sodium on adriamycin-induced cardiotoxicity[J]. Chin J Pathophysio, 2011, 27(5): 939–943.
2. Yang XD, Shu WQ, YangSB. Protective effect of Shengmai injection on adriamycin-induced myocardium injury in rats and its mechanism[J].Cent South Pharm, 2008, 6(2): 162–165.
3. Yao LM, Chen YM, Yin RX. Effects of grouth hormone on cardiomyocyte apoptosis in adriamycin-induced cardiomyopathy in rats[J]. South Chin J Cardiovascular Diseases, 2005, 11(2): 137–139.
4. Zhao JY, WangYL, ZhaoM, et al. The calumenin changes in the apoptosis induced adriamycin injured cardiomyocytes[J]. J Clinic.cardiol, 2015, 31(8): 895–898.
5. Niu XF, Zhao YJ, Zhao M, et al. The protective effects of total fl avonoids of astragalus on inhibition of endoplasmic reticulum stress in murine model with coxsackie virus B3-induced acute viral myocarditis[J]. J Clinic Cardiol, 2015, 31(2): 129–132.
6. Chiang CK, Wang CC, Lu TF, et al. Involvement of Endoplasmic Reticulum Stress, Autophagy, and Apoptosis in Advanced Glycation End Products-Induced Glomerular Mesangial Cell Injury[J]. Sci Rep, 2016, 6: 34167.
7. Shen M, Wang L, Guo X, et al. A novel endoplasmic reticulum stress-induced apoptosis model using tunicamycin in primary cultured neonatal rat cardiomyocytes[J]. Mol Med Rep, 2015, 12(4): 5149-5154.
8. Malla Y, Tritsch E, Ladouce R, et al. Proteome modulation in H9c2 cardiac cells by microRNAs miR-378 and miR-378*[J]. Mol Cell Proteomics, 2014, 13(1): 18-29.
9. Xu Y, Jiang L, Huang Y, et al. Solid-state characterization and transformation of various creatine phosphate sodium hydrates[J]. J Pharm Sci, 2014, 103(11): 3688-3695.
10. Ming Z, XiaoTong S, YaHong Z, et al. The relationship research on miRNA378﹡, calumenin, GRP78, bax and bcl-2 in suckling mouse myocardium with myocarditis caused by adriamycin[J]. Chin J Clin Cardiol, 2016, 32: 603-606.
11. Park JH, Choi SH, Kim H, et al. Doxorubicin Regulates Autophagy Signals via Accumulation of Cytosolic Ca2+in Human Cardiac Progenitor Cells[J]. Int J Mol Sci, 2016, 17(10): E1680.
12. Choi SK, Lim M, Byeon SH, et al. Inhibition of endoplasmic reticulum stress improves coronary artery function in the spontaneously hypertensive rats[J]. Sci Rep, 2016, 6: 31925.
13. Boß M, Newbatt Y, Gupta S, et al. AMPK-independent inhibition of human macrophage ER stress response by AICAR[J]. Sci Rep, 2016, 6: 32111.
14. Nagalingam RS, Sundaresan NR, Noor M, et al. Defi ciency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor β (TGFβ1)-dependent paracrine mechanism[J]. J Biol Chem, 2014, 289(39): 27199–27214.
15. Nagalingam RS, Sundaresan NR, Gupta MP, et al. A cardiacenriched microRNA, miR-378, blocks cardiac hypertrophy by targeting Ras signaling[J]. J Biol Chem, 2013, 288(16): 11216-11232.
16. Ooi JY, Bernardo BC, McMullen JR. the therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy[J]. Future Med Chem, 2014, 6(2): 205-222.
17. Fang J, Song XW, Tian J, et al. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes[J]. Apoptosis, 2012, 17(4): 410-423.
18. Sahoo SK, Kim DH. Characterization of calumenin in mouse heart[J]. BMB Rep, 2010, 43(3): 158-163.
19. Lee JH, Kwon EJ, Kim DH. Calumenin has a role in the alleviation of ER stress in neonatal rat cardiomyocytes[J]. Biochem Biophys Res Commun, 2013, 439(3): 327-332.
doi 10.13459/j.cnki.cjap.2016.06.007
Yu WANG, PhD, Professor, Inner Mongolia University for the Nationalities, 22 Holin He Street, Tongliao 028002, China. Tel/Fax:86-475-8134245; E-mail : shen348@126.com. Ming Zhao, PhD, Professor, First Clinical Medical of Inner Mongolia University for Nationalities, 1472 Holin He Street, Tongliao 028002, China. Tel/Fax: 86-475-8267852; E-mail address: langzhe73@163.com.
Miao YU and Zhi-hui HE contributed equally to this work.
2016-07-14; accepted 2016-10-18
杂志排行
中国应用生理学杂志的其它文章
- 双受体激动剂CI-1206拮抗Aβ1-42所致小鼠空间学习记忆损伤的作用*
- 当归黄芪提取物对慢性腹膜功能衰竭大鼠腹膜功能、结构及TGF-β1表达的影响*
- 阿霉素损伤心肌细胞miRNA378与网腔钙结合蛋白、内质网应激的关系*
- 5-HT1B受体亚型对小脑顶核介导的运动行为的影响*
- Changes of microcirculation in healthy volunteers and patients with septic shock in Xining
- The infl uence of heterogeneity on the analysis of sleep-wake architecture in the single-prolonged stress rats
