PAK4-LIMK1-Cofilin信号通路对非小细胞肺癌迁移及侵袭能力的影响*
2016-01-31李冬霞王永玲蔡松旺
李冬霞, 王永玲, 蔡松旺
(1新乡医学院基础医学院,河南 新乡 453003;2中山大学附属第三医院胸外科,广东 广州 510630)
PAK4-LIMK1-Cofilin信号通路对非小细胞肺癌迁移及侵袭能力的影响*
李冬霞1,王永玲1,蔡松旺2△
(1新乡医学院基础医学院,河南 新乡 453003;2中山大学附属第三医院胸外科,广东 广州 510630)
[摘要]目的: 探讨p21活化激酶4(PAK4)对非小细胞肺癌(NSCLC)侵袭和迁移能力的影响及相关机制。方法: PAK4-siRNA或阴性对照转染A549和NCI-p20细胞株,实时荧光定量PCR和Western blot分别检测细胞PAK4 mRNA和蛋白表达水平,Transwell小室法检测其对细胞侵袭和迁移能力的影响;Western blot检测其LIMK1、cofilin及其磷酸化水平;免疫共沉淀检测PAK4和LIMK1蛋白是否直接绑定;体外激酶分析实验检测LIMK1是否是PAK4的激酶底物;Western blot检测10例非小细胞肺癌组织中PAK4和磷酸化LIMK1的相关性;PAK4-siRNA和LIMK1质粒共转染A549和NCI-p20细胞后观察细胞迁移和侵袭能力变化。结果: 沉默PAK4后A549和NCI-p20细胞迁移和侵袭能力明显减弱(P<0.05);LIMK1和cofilin蛋白水平无显著变化,而磷酸化LIMK1和磷酸化cofilin水平显著下调;免疫共沉淀结果示PAK4与LIMK1相互绑定;激酶分析实验结果显示,激酶缺陷型PAK4(K350M)使LIMK1磷酸化的作用显著低于野生型PAK4或活化型PAK4(S445N)(P<0.05)。Western blot检测结果示非小细胞肺癌组织中PAK4表达上调的程度与磷酸化LIMK1含量呈正相关(P<0.05);PAK4-siRNA和LIMK1质粒共转染A549和NCI-p20细胞后,迁移和侵袭细胞数均高于PAK4-siRNA转染组(P<0.05)。结论: PAK4通过直接磷酸化LIMK1而促进非小细胞肺癌的迁移及侵袭能力。
[关键词]p21活化激酶4; LIM激酶1; 非小细胞肺癌; 细胞迁移; 细胞侵袭
p21活化激酶4(p21-activated kinase 4,PAK4)属于丝氨酸/苏氨酸蛋白激酶家族中的成员,是Rho家族Rac、Cdc42等下游的靶蛋白,具有调节细胞骨架、细胞增殖、凋亡和迁移等功能[1-3]。目前已发现人体多种恶性肿瘤的发生发展与PAK4异常有关[4-5],而PAK4在人非小细胞肺癌(non-small-cell lung cancer,NSCLC)中的生物学作用及其机制未见相关报道。我们前期的研究发现PAK4与NSCLC的分化程度、淋巴结转移、远处转移和临床分期相关,预后分析提示PAK4高表达与预后差相关。本研究旨在通过RNA干扰技术,探讨PAK4在非小细胞肺癌侵袭及迁移中的作用及相关机制。
材料和方法
1材料
A549和NCI-p20细胞株购自中国医学科学院上海生科院细胞资源中心;选用中山大学第三附属医院2012~2013年10例原发性非小细胞肺癌手术新鲜切除标本(切除后标本迅速放入液氮中,标本均经病理医生检查验证和归类);RPMI-1640培养基和胎牛血清(Gibco);siPORTTMNeoFXTM转染剂(Am-bion);PrimeScript RT试剂盒(Promega);醋酸纤维膜(Bio-Rad);PAK4-siRNA(si-PAK4; 圣克鲁斯生物技术公司);LIMK1质粒(上海吉玛生物有限公司);TRIzol试剂和LIMK2纯化蛋白(Life Technologies);兔抗人PAK4抗体、LIMK1抗体、p-LIMK1、cofilin 抗体、p-cofilin 抗体和GAPDH内参照抗体(CST);ECL显色试剂盒(Pierce)。
2方法
2.1细胞培养与质粒转染A549和NCI-p20细胞于含10%胎牛血清的RPMI-1640培养基中培养,置于37 ℃、5% CO2培养箱培养,隔天传代1次。转染前24 h将A549和NCI-p20 细胞分别接种于6孔板中,细胞密度大约为5×108/L,待细胞汇合80%左右时,按照siPORTTMNeoFXTM转染试剂操作手册将si-PAK4、si-PAK4+LIMK1质粒、LIMK1质粒或阴性对照转染细胞,继续在37 ℃、5% CO2培养箱中培养。
参考文献2.2Western blot实验参照[3]步骤进行。转染48 h后提取蛋白,利用10%的SDS-PAGE分离蛋白,转膜,封闭,Ⅰ抗(1∶1 000)、Ⅱ抗(1∶1 000)孵育,ECL发光检测,GAPDH作为内参照。使用Quantity One 软件进行定量分析。
2.3实时荧光定量PCR实验按照一步法使用Invitrogen的TRIzol试剂来提取总RNA。使用PrimeScript RT Reagent Kit试剂盒逆转录成单链cDNA。使用ABI 7900HT Fast Real-Time PCR体系来进行实时荧光定量PCR。PAK4的上游引物为5’-ATGTGGTGGAGATGTACAACAGCTA-3’,下游引物为5’-GTTCATCCTGGTGTGGGTGAC-3’;内参照U6的上游引物为5’-TGCGGGTGCTCGCTTCGGCAGC-3’,下游引物为 5’-CCAGTGCAGGGTCCGAGGT-3’。
2.4Matrigel侵袭及Transwell迁移实验Matrigel侵袭实验:每个Transwell 小室铺50 μL Matrigel基质蛋白,5×104个细胞加入不含血清的Matrigel侵袭小室(BD),下室加含10%胎牛血清的RPMI-1640培养液,培养24 h后用棉签擦去小室上层未迁移的细胞,迁移至小室下层的细胞用4%多聚甲醛固定、结晶紫染色。各取5个400倍视野,显微镜下观察拍照。Transwell迁移实验不需铺胶,其余操作同Matrigel侵袭实验。
2.5免疫共沉淀实验将A549或NCI-p20细胞(6×106)溶解在400 μL细胞裂解液(1% Triton X-100,150 mmol/L NaCl,20 mmol/L Tris-HCl,1 mmol/L EDTA,1 mmol/L Na3VO4,2.5 mmol/L焦磷酸,1 mmol/L磷酸甘油和蛋白酶抑制剂混合物)中4 ℃裂解10 min,于4 ℃、14 000×g离心15 min并迅速转移上清至另一离心管中。上清液中加入4 μg PAK4或LIMK1抗体和60 μL Protein G Plus/Protein A-Agarose 4 ℃摇床振荡孵育3 h。裂解液清洗3次,每次10 mim,沉淀复合物加入等体积的2×SDS上样缓冲液,其余步骤同Western blot实验。
2.6体外激酶分析实验PAK4 (WT) cDNA克隆入大肠杆菌表达载体pET30a,突变载体PAK4 (S445N)和PAK4 (K350M)通过定点突变构建并纯化相应蛋白。将上述等量蛋白及LIMK1纯化蛋白与含有100 mmol/L NaCl、10 mmol/L MgCl2、50 mmol/L HEPES(pH 7.5)、1 mmol/L DTT和50 μmol/L ATP的缓冲液30 ℃孵育30 min,3×SDS样品缓冲液终止。其余步骤同Western blot实验。
3统计学处理
计量资料数据以均数±标准差(mean±SD)表示,数据比较应用SPSS 17.0 统计软件处理,采用单因素方差分析比较组间均数差异;采用Spearman相关分析确定PAK4与p-LIMK1的相关性。以P<0.05为差异有统计学意义。
结果
1siRNA抑制转染细胞PAK4蛋白和mRNA表达
A549和NCI-p20细胞分别转染si-PAK4 48 h后,Western blot和实时荧光定量PCR方法检测PAK4表达水平。与对照组比较,转染siPAK4的A549和NCI-p20细胞PAK4蛋白和mRNA表达明显下调(P<0.05),见图1。这表明构建的siRNA质粒在蛋白和mRNA水平上抑制PAK4表达。

Figure 1.The protein (A) and mRNA (B) expression of PAK4 in A549 cells and NCI-p20 cells transfected with PAK4-siRNA (si-PAK4) or control siRNA (NC). Mean±SD.n=5.*P<0.05vsNC.
图1转染siPAK4对A549细胞和NCI-p20细胞PAK4蛋白及mRNA表达的影响
2沉默PAK4基因对细胞迁移及侵袭能力的影响
转染si-PAK4的A549和NCI-p20细胞侵袭和迁移出的细胞数明显少于对照组,差异有统计学意义(P<0.05),表明转染si-PAK4后,A549和NCI-p20细胞的侵袭及迁移能力下降,见图2。
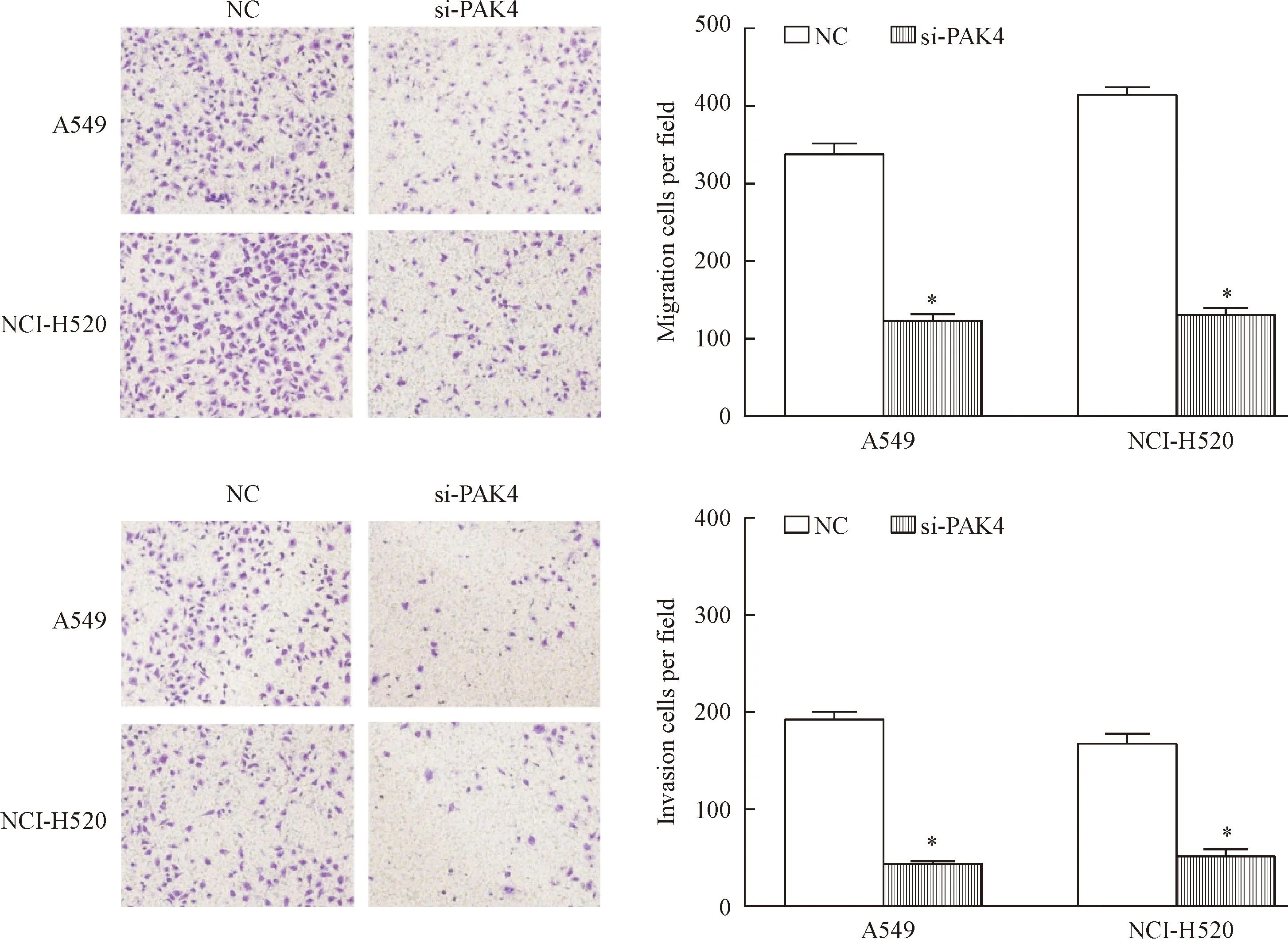
Figure 2.PAK4 knockdown suppressed NSCLC cell migration and invasion (×40). Mean±SD.n=5.*P<0.05vsNC.
图2转染si-PAK4对A549细胞和NCI-p20细胞迁移及侵袭能力的影响
3LIMK1是PAK4的激酶底物
Western blot结果显示,si-PAK4转染A549和NCI-p20细胞后,转染si-PAK4组p-LIMK1和p-cofilin均低于对照组(P< 0.05),LIMK1和cofilin的蛋白水平无变化(图3A)。免疫共沉淀实验结果显示,LIMK1蛋白出现在含有抗PAK4抗体的复杂免疫沉淀中;PAK4也出现在含有抗LIMK1抗体的免疫沉淀物中,而在与对照组免疫球蛋白IgG相联系的免疫复合物中未检测到PAK4和LIMK1(图3B)。这表明PAK4与LIMK1在A549和NCI-p20细胞中相互绑定。
体外激酶分析实验结果显示,激酶缺陷型PAK4(K350M)使LIMK1磷酸化的作用显著低于野生型PAK4或活化型PAK4(S445N)(P<0.05),见图3C。进一步在10例NSCLC组织中行Western blot检测,发现PAK4表达上调的程度与p-LIMK1含量呈正相关(P<0.05),见图3D。以上结果提示LIMK1是PAK4的激酶底物。
Figure 3.LIMK1 was the kinase substrate of PAK4. A: the protein levels of LIMK1, p-LIMK1, cofilin, and p-cofilin in the A549 cells and NCI-p20 cells transfected with si-PAK4 or control determined by Western blot. NC: negative control. B: A549 cell and NCI-p20 cell lysates were immunoprecipitated with PAK4 antibody (top panels) or LIMK1 antibody (bottom panels), and subjected to Western blot to ascertain LIMK1 and PAK4 interaction. C:invitrokinase assay using purified activated PAK4 (S445N), kinase-defective PAK4 (K350M), PAK4 (WT), and LIMK1 protein. D: correlation between the protein levels of PAK4 and p-LIMK1 in human NSCLC tissues (n=10).
图3LIMK1是PAK4的激酶底物
4LIMK1对沉默PAK4抑制非小细胞肺癌细胞迁移和侵袭能力的恢复性作用
体外迁移及侵袭实验结果显示si-PAK4和LIMK1质粒共转染组细胞迁移和侵袭细胞数均显著高于si-PAK4转染组(P<0.05);同时行Western blot检测结果显示si-PAK4和LIMK1质粒共转染组p-LIMK1蛋白表达水平高于转染si-PAK4组(P<0.05),见图4,表明LIMK1对沉默PAK4抑制NSCLC细胞迁移和侵袭能力的恢复性作用。进一步在功能学上说明PAK4通过磷酸化LIMK1而促进非小细胞肺癌细胞的迁移及侵袭能力。
讨论
作为癌基因,PAK4参与调节正常细胞基因转录、迁移、生长和凋亡等过程,其异常高表达促进肿瘤细胞增殖、侵袭、转移。研究表明,PAK4上调在多种癌症中具有促进肿瘤细胞迁移的作用[6-11]。PAK4在不同肿瘤中可通过不同的途径促进肿瘤细胞增殖、迁移和侵袭,如在绒毛膜癌中是通过下游的膜型基质金属蛋白1(membrane-type 1 matrix metalloproteinase, MT1 MMP)实现的[6];在卵巢癌中是通过c-Src/丝裂原活化蛋白激酶激酶1(mitogen-activated protein kinase kinase-1, MEK1)/细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)1/2和MMP-2介导的[7];在前列腺癌中是通过激活LIMK1/cofilin信号通路完成的[8],在脑胶质瘤中与MMP-2[9]及胃癌中与SCG10或LIMK1/cofilin信号通路有关[10-11]。我们前期研究结果证实PAK4与NSCLC的恶性特征及预后有关,然而,PAK4在NSCLC中的生物学作用及具体机制尚不清楚。本研究通过沉默PAK4基因,体外细胞迁移及侵袭实验结果表明PAK4具有促进非小细胞肺癌的迁移及侵袭作用。
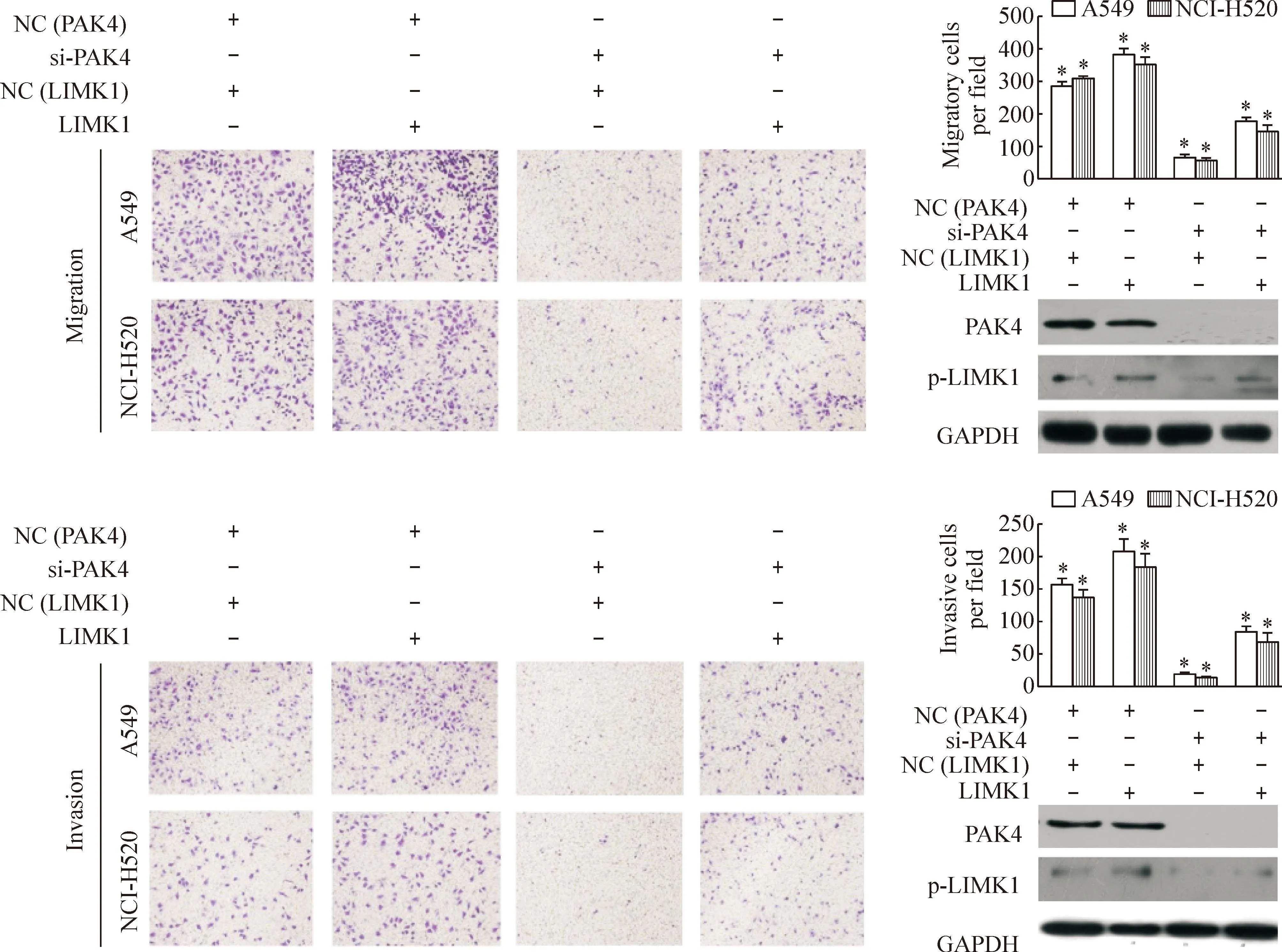
Figure 4.LIMK1 overexpression rescued the effects of si-PAK4 on the migration and invasion of A549 cells and NCI-p20 cells (×40). Mean±SD.n=5.*P<0.05vsother groups.
图4LIMK1对沉默PAK4抑制非小细胞肺癌细胞迁移和侵袭能力的恢复性作用
目前关于PAK4上游信号调控机制的研究不多,蛋白激酶D(protein kinase D, PKD)被报道可以通过色氨酸474位点直接磷酸化PAK4[12];肝细胞生长因子 (hepatocyte growth factor, HGF) 和促卵泡激素 (follicle stimulating hormone, FSH) 被报道在卵巢癌中可以调节PAK4磷酸化从而影响肿瘤的侵袭及转移[13]。Mak等[14]报道CDK5RAP3可以通过绑定PAK4从而使其活化,促进肝癌的转移。
LIMK1是PAK4下游的靶蛋白,通过使cofilin磷酸化和失活调节激动蛋白细胞骨架的重组,参与肿瘤血管、细胞迁移和和迁移等[15]。PAK4-LIMK1-cofilin信号通路促进前列腺癌和胃癌细胞迁移的作用已被证实[8-11]。PAK4促进NSCLC细胞迁移和侵袭是否通过LIMK1介导的未见报道。本研究显示siRNA介导的PAK4表达下调后,LIMK1和cofilin的磷酸化水平均降低,则LIMK1和cofilin的蛋白表达水平无变化,提示NSCLC中PAK4可以调节LIMK1及cofilin的磷酸化。同时我们通过免疫沉淀发现PAK4与LIMK1蛋白分别出现在抗对方抗体的免疫沉淀物中,进一步证实了PAK4可特异地与LIMK1相互作用,最后通过体外激酶分析实验证实LIMK1是PAK4的激酶底物。
综上所述, PAK4通过直接磷酸化LIMK1进而促进非小细胞肺癌细胞的迁移和侵袭,有望为非小细胞肺癌治疗提供新的靶点,PAK4高表达的上游信号通路将在下一步研究中探讨。
[1]李翔,张晓艳,赵继敏,等.下调p21活化蛋白激酶2表达对人乳腺癌细胞增殖和凋亡的影响[J]. 中国病理生理杂志,2014,30(6):975-981.
[2]蔡松旺,谢迭来,翁毅敏,等.p21活化激酶6对人非小细胞肺癌A549细胞侵袭及迁移能力的影响[J]. 中国病理生理杂志,2013,29(9):1637-1640.
[3]蔡松旺,李冬霞,安军,等.p21活化激酶6削弱非小细胞肺癌A549细胞侵袭及迁移能力的分子机制[J]. 中国病理生理杂志,2014,30(1):67-71.
[4]Radu M, Semenova G, Kosoff R, et al.PAK signalling during the development and progression of cancer[J]. Nat Rev Cancer, 2014, 14(1): 13-25.
[5]Abo A, Qu J, Cammarano MS, et al.PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia[J]. EMBO J, 1998, 17(22): 6527-6540.
[6]Zhang HJ, Siu MK, Yeung MC, et al. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma[J]. Carcinogenesis, 2011, 32(5): 765- 771.
[7]Siu MK, Chan HY, Kong DS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients[J]. Proc Natl Acad Sci U S A, 2010, 107(43):18622-18627.
[8]Ahmed T, Shea K, Masters JR, et al. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF[J].Cell Signal, 2008, 20(7):1320-1328.
[9]Kesanakurti D, Chetty C, Rajasekhar Maddirela D, et al.Functional coo-perativity by direct interaction between PAK4 and MMP-2 in the regulation of anoikis resistance, migration and invasion in glioma[J]. Cell Death Dis,2012, 3:e445.
[10]Guo Q, Su N, Zhang J, et al. PAK4 kinase-mediated SCG10 phosphorylation involved in gastric cancer metastasis[J]. Oncogene, 2014, 33(25):3277-3287.
[11]Li X, Ke Q, Li Y, et al. DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1[J]. Int J Biochem Cell Biol, 2010, 42(1):70-79.
[12]Spratley SJ, Bastea LI, Doppler H, et al. Protein kinase D regulates cofilin activity through p21-activated kinase 4[J]. J Biol Chem, 2011, 286(39): 34254-34261.
[13]Siu MK, Chan HY, Kong DS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients[J]. Proc Natl Acad Sci U S A, 2010, 107(43): 18622-18627.
[14]Mak GW, Chan MM, Leong VY, et al. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis[J]. Cancer Res, 2011, 71(8): 2949-2958.
[15]Dan C, Kelly A, Bernard O, et al.Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin[J]. J Biol Chem, 2001, 276(34):32115-32121.
(责任编辑: 卢萍, 罗森)

*[基金项目]深圳市科技计划(No. JCYJ20140414160300592)
Effect of PAK4-LIMK1-cofilin signaling on non-small-cell lung cancer migration and invasionLI Dong-xia1, WANG Yong-ling1, CAI Song-wang2
(1SchoolofBasicMedicalScience,XinxiangMedicalUniversity,Xinxiang453003,China;2DepartmentofCardiothoracicSurgery,TheThirdAffiliatedHospital,SunYat-senUniversity,Guangzhou510630,China.E-mail:songwangcai@yahoo.com)
[ABSTRACT]AIM: To explore the mechanism of p21-activated kinase 4 (PAK4) on non-small-cell lung cancer (NSCLC) migration and invasion.METHODS: After A549 and NCI-p20 cell lines were transfected with PAK4-siRNA or negative control, the expression of PAK4 at mRNA and protein levels was detected by real-time PCR and Western blot, respectively. The invasion and migration of A549 cells and NCI-p20 cells were measured by Matrigel invasion assay and Transwell migration assay. LIMK1, cofilin, and their respective phosphorylation were examined by Western blot. The interaction of PAK4 and LIMK1 was investigated by co-immunoprecipitation assay. The relationship between PAK4 and LIMK1 phosphorylation was examined by a protein kinase assay in the A549 cells and NCI-p20 cells. The expression of PAK4 and p-LIMK1 in 10 human NSCLC tissues was examined by Western blot. A549 cells and NCI-p20 cells were individually or commonly transfected with PAK4-siRNA or LIMK1 plasmid in order to observe the cell migration and invasion. RESULTS: After A549 cells and NCI-p20 cells were transfected with PAK4-siRNA for 48 h, the expression of PAK4 at mRNA and protein levels, and the numbers of invasion and migration cells in PAK4-siRNA group were lower than those in control group. Compared with control group, the phosphorylation of LIMK1 and cofilin was lower in PAK4-siRNA group, whereas the total expression levels of LIMK1 and cofilin did not change. The results of co-immunoprecipitation assays showed that PAK4 specifically interacted with LIMK1 in A549 and NCI-p20 cells. LIMK1 phosphorylation in the presence of PAK4 (K350M) was significantly lower than that in the presence of PAK4 (WT) or PAK4 (S445N) in the protein kinase assay. The PAK4 upregulation was positively correlated with the level of p-LIMK1 (P<0.05). After A549 cells and NCI-p20 cells were co-transfected with PAK4-siRNA and LIMK1 plasmid, the migration and invasion cell numbers in co-transfection group were higher than those in PAK4-siRNA transfection group. CONCLUSION: PAK4 promotes the invasive and migratory abilities of NSCLC, which is mediated by LIMK1 phosphorylation.
[KEY WORDS]p21-activated kinase 4; LIM kinase 1; Non-small-cell lung cancer; Cell migration; Cell invasion
通讯作者△Tel: 020-38688039; E-mail: tangshaohui205@163.com
[收稿日期]2015- 04- 29[修回日期] 2015- 09- 10
[文章编号]1000- 4718(2015)12- 2136- 08
doi:10.3969/j.issn.1000- 4718.2015.12.004
[中图分类号]R363
[文献标志码]A
