miRNA-181a/b通过血清反应因子调控血管平滑肌细胞表型 *
2016-01-12侯雪,魏晓星,张振明等
*国家自然科学基金(31260015)
miRNA-181a/b通过血清反应因子调控血管平滑肌细胞表型*
侯雪1,魏晓星1*,张振明2,蔺枭2,吴琼2
(1.青海大学医学院 基础医学部青海 西宁 810001;2.清华大学生命科学学院 北京 100084)
摘要目的研究miR-181a/b通过血清反应因子(SRF,serum response factor )对血管平滑肌细胞向合成型表型转化及细胞增殖迁移能力的调控作用。方法将miR-181a/b 瞬时转染至人主动脉平滑肌细胞(HAoSMCs)中,用实时定量PCR、CCK-8检测方法和transwell实验分别检测平滑肌细胞的表型标记基因的表达水平、细胞增殖能力和迁移能力的变化。生物信息学分析预测miR-181a/b直接靶向血清反应因子的3’UTR(非编码区),并通过实时定量PCR、western blot及双荧光素酶报告系统分别验证。结果实时定量PCR结果表示在HAoSMCs中过表达miR-181a/b能够抑制收缩表型标记基因的表达,促进合成表型标记基因的表达,CCK-8与transwell 实验结果表明,miR-181a/b可增强细胞的增殖和迁移能力。双荧光素酶报告系统与western blot结果表明,miR-181a/b能够直接作用SRF,抑制SRF的蛋白表达。结论miR-181a/b通过直接作用血清反应因子的3’UTR促进平滑肌细胞由收缩型表型向合成型表型转化。
关键词miRNA-181a/b血清反应因子血管平滑肌细胞表型转化
通讯作者侯雪(1991~),女,汉族,山东籍,2012级在读硕士研究生. *:,研究生导师,Email:flemingo@126.com
中图分类号R34
文献标识码A
DOI:10.13452/j.cnki.jqmc.2015.03.005
AbstractObjectiveTo investigate the roles of miR-181a/b in the smooth muscle cells(SMC)phenotypic switch from contractile phenotype to synthetic phenotype through serum response factor(SRF).Methods miR-181a/b mimics or inhibitors were transiently transfected into Human Aortic Smooth Muscle Cells(HAoSMCs),the expression of SMC phenotype marker gene were detected by quantitative real time PCR(qRT-PCR),the proliferation and migration ability of HAoSMCs were measured by CCK-8 assay and transwell assay,respectively.In addition,miR-181a/b was indicated as directly targeting at 3’UTR of SRF based on bioinformatic analysis and identified by qRT-PCR,western blot assay and dual-luciferase assay.Results The results of qRT-PCR showed that miR-181a/b could down-regulate the expression level of SMC contractile marker genes and up-regulate the expression level of SMC synthetic marker genes.The results of CCK-8 assay and transwell assay showed that miR-181a/b could enhance the proliferation and migration ability of HAoSMCs,respectively.The results of Dual-luciferase assay and western blot assay showed that miR-181a/b could directly target 3’UTR of SRF and inhibit the protein expression level of SRF.Conclusion miR-181a/b regulates the shift of SMCs from contractile phenotype to synthetic phenotype through targeting at 3’UTR of SRF.
KeywordsmiRNA-181a/bSerum response factorVessel smooth muscle cellsPhenotype modulation
收稿日期2015-03-03
miRNA-181a/b REGULATES PHENOTYPES OF VESSEL SMOOTH
MUSCLE CELLS THROUGH SERUM RESPONSE FACTOR*
Hou Xue1,Wei Xiaoxing1*,Zhang Zhenming2,Lin Xiao2,Wu Qiong2
(1.Department of Basic Medical sciences,Qinghai University Medical College,Xining 810016,China;
2.School of Life Sciences,Tsinghua University,Beijing 100084,China)
本研究通过生物信息学分析发现,SRF是miR-181a/b可能的靶基因,因此我们假定miR-181a/b可能能通过靶向SRF调控平滑肌细胞的表型转化(图 1A)。本研究结果表明,miR-181a/b使平滑肌细胞收缩表型标记基因表达下调,使平滑肌细胞合成型表型标记基因表达上调。进一步研究表明,miR-181a/b可以直接靶向SRF的3’UTR并且调控SRF的mRNA和蛋白表达水平。综上所述,我们首次证明了miR-181a/b可通过直接靶向SRF进而调控平滑肌细胞表型。
1材料与方法
1.1 细胞培养与转染
人主动脉平滑肌细胞(HAoSMCs)使用VascuLife SMC medium(Lifeline Cell Technology,美国)在培养箱(37℃、5%CO2)培养。
PDGF诱导培养时,在细胞培养液中加入浓度为10 ng/mL的PDGF,在培养箱(37℃、5%CO2)诱导培养24 h。
miR-181a/b类似物(mimics)/抑制剂(inhibitor)或阴性对照(吉玛,上海,中国)通过使用转染试剂Lipofectamine RNAiMAX(Invitrogen,美国)瞬时转染至HAoSMCs中。pGL3-SRF或pGL3质粒使用转染试剂Lipofectamine 2000 reagent(Invitrogen,美国)瞬时转染至HAoSMCs中。
1.2 RNA提取及实时定量PCR检测
HAoSMCs的总RNA使用miRcute miRNA分离提取试剂盒(天根,北京,中国)根据产品使用说明进行提取。mRNA的反转录反应使用FastQuant反转录试剂盒(天根)进行,miRNA的反转录反应使用miRcute miRNA cDNA第一链合成试剂盒(天根)进行。miR-181a/b表达水平的实时定量PCR检测以U6作内参,使用miRcute miRNA qPCR 检测试剂盒(天根)进行,平滑肌细胞表型标记基因表达水平的实时定量PCR检测以GAPDH作内参,使用SuperReal PreMix(SYBR Green)检测试剂盒(天根)。
1.3 蛋白质印迹检测
HAoSMCs的蛋白质样品制备使用RIPA裂解液(华兴博创,北京,中国)裂解,并加入蛋白酶抑制剂(华兴博创)。将蛋白质样品在12%的SDS-PAGE中进行电泳,获得的分离的蛋白条带转移至PDVF膜(Millipore,纽约,美国)上,并在5%的脱脂牛奶中封闭1 h。一抗使用 SRF兔单克隆抗体(CST,波士顿,美国),4 ℃过夜孵育,二抗使用HRP-羊抗兔第二抗体(Sigma-Aldrich,密苏里州,USA)室温孵育2 h,最后使用化学发光剂ECL观察。
1.4 双荧光素酶报告系统检测
将SRF的3’UTR克隆到pGL3荧光素酶报告质粒(SRF-pGL3)(青兰,苏州,中国)中。将pGL3报告质粒与miR-181a/b mimics共转染至HeLa细胞,转染24 h后使用Dual-Luciferase报告检测系统(Promega BioSciences,美国)检测荧光素酶活性。
1.5 细胞增殖及迁移检测
1.5.1CCK-8实验
HAoSMCs以每孔约2000个细胞接种至96孔板中,分别转染miR-181a/b mimics、miR-181a/b inhibitor和阴性对照物至细胞中,72 h后使用CCK-8试剂盒(碧云天,江苏,中国)检测细胞增殖能力。每孔培养基中加入10 μL CCK-8溶液,用加了相应量细胞培养液和CCK-8溶液但没有加入细胞的孔作为空白对照。培养箱(37℃、5%CO2)孵育1 h后,多功能读数仪检测450 nm处吸光值。
1.5.2Transwell实验
细胞增殖能力在含有8 μm孔径的聚碳酸酯膜的24孔transwell细胞培养体系(Corning,纽约,美国)中进行。分别转染miR-181a/b mimics、miR-181a/b inhibitor及阴性对照物至HAoSMCs中36~48 h后,将约15,000个细胞接种至transwell内室中,外室加入含20% FBS的培养基,37 ℃诱导12 h。迁移至transwell内室膜外侧的细胞经结晶紫染色后在倒置显微镜下观察。
1.6 统计学分析
统计学分析使用t检验的方法。P<0.05、P<0.01分别代表显著性差异及极显著性差异。数据用平均值±标准差表示。
2结果
2.1 miR-181a/b在增殖的HAoSMCs中表达上调(图1)

(A)miR-181a/b通过血清反应因子调控血管平滑肌表型示意图;(B)与对照细胞相比,倒置显微镜下(200×)观察的PDGF诱导的HAoSMCs增殖能力增强;(C)实时定量PCR结果显示在PDGF诱导的HAoSMCs中,miR-143/145、miR-21(收缩型平滑肌细胞的生物标记)的表达水平明显降低,miR-181a和miR-181b的表达水平明显升高.*:P<0.05,**:P<0.01.n=6,U6作为内参
图1miR-181a/b在PDGF诱导的HAoSMCs中表达上调图
Figure 1miR-181a/b expression level increased in PDGF-treated HAoSMCs
PDGF(Platelet-derived growth factor,血小板源生长因子)是一种抑制平滑肌收缩表型标记基因表达,促使平滑肌细胞向合成型转化并促进平滑肌细胞增殖和迁移的一种生长因子。体外培养的HAoSMCs使用PDGF诱导24 h后使用倒置显微镜观察,发现HAoSMCs的增殖能力明显增强(图1B)。
实时定量PCR检测结果显示,miR-181a和miR-181b的表达水平明显升高(图 1C),miR-143、miR-145和miR-21的表达水平明显降低(图1C)。其中miR-143/145簇对于维持平滑肌细胞收缩表型有着重要意义[1,2]。MiR-21在平滑肌细胞收缩表型标记基因的表达中起着关键作用[3,4]。
2.2 miR-181a/b调控HAoSMCs表型标记基因的表达(图2)
平滑肌细胞由收缩型表型向合成型表型转化会改变细胞的增殖迁移能力以及平滑肌表型标记基因的表达[5]。一些在平滑肌细胞特定表达的基因作为收缩表型的标记基因包括SM22α(smooth muscle 22 alpha)[6]、SM-MHC(smooth muscle myosin heavy chain,平滑肌肌球蛋白重链)[7]、α-SMA(α-smooth muscle actin,α-平滑肌肌动蛋白,ACTA2)[8]、calponin(钙调蛋白)[9]、smoothelin[10]。与之相对应的CRBP-1(cellular retinol binding protein,细胞视黄醇结合蛋白)[11]、moesin(膜突蛋白)[12]、smemb(smooth muscle embryonic myosin heavy chain,平滑肌胚胎型肌球蛋白重链)[13]、OPN(osteopontin,骨桥蛋白)[14]、MGP(matrix Gla protein,细胞基质Gla蛋白)[15]是平滑肌细胞合成型标记基因。
为了揭示miR-181a/b对平滑肌细胞表型标记基因表达的调控作用,将miR-181a/b或抑制剂分别瞬时转染至HAoSMCs内。72 h后检测平滑肌细胞表型标记基因的表达水平,实时定量PCR结果显示,在过表达miR-181a/b的HAoSMCs中收缩表型基因表达水平下调(图 2A),合成表型标记基因表达水平上调(图2B)。与之相反的是在HAoSMCs中抑制miR-181a/b的表达水平使细胞中的平滑肌收缩表型标记基因表达上调(图2C),合成型标记基因表达水平下调(图2D)。
SRF是一种平滑肌收缩基因表达的重要调控因子[16]。在HAoSMCs中利用siSRF沉默SRF的表达(图2E-F),实时定量PCR结果显示,平滑肌收缩表型基因表达水平降低(图 2G),而合成型基因表达水平升高(图 2H)。
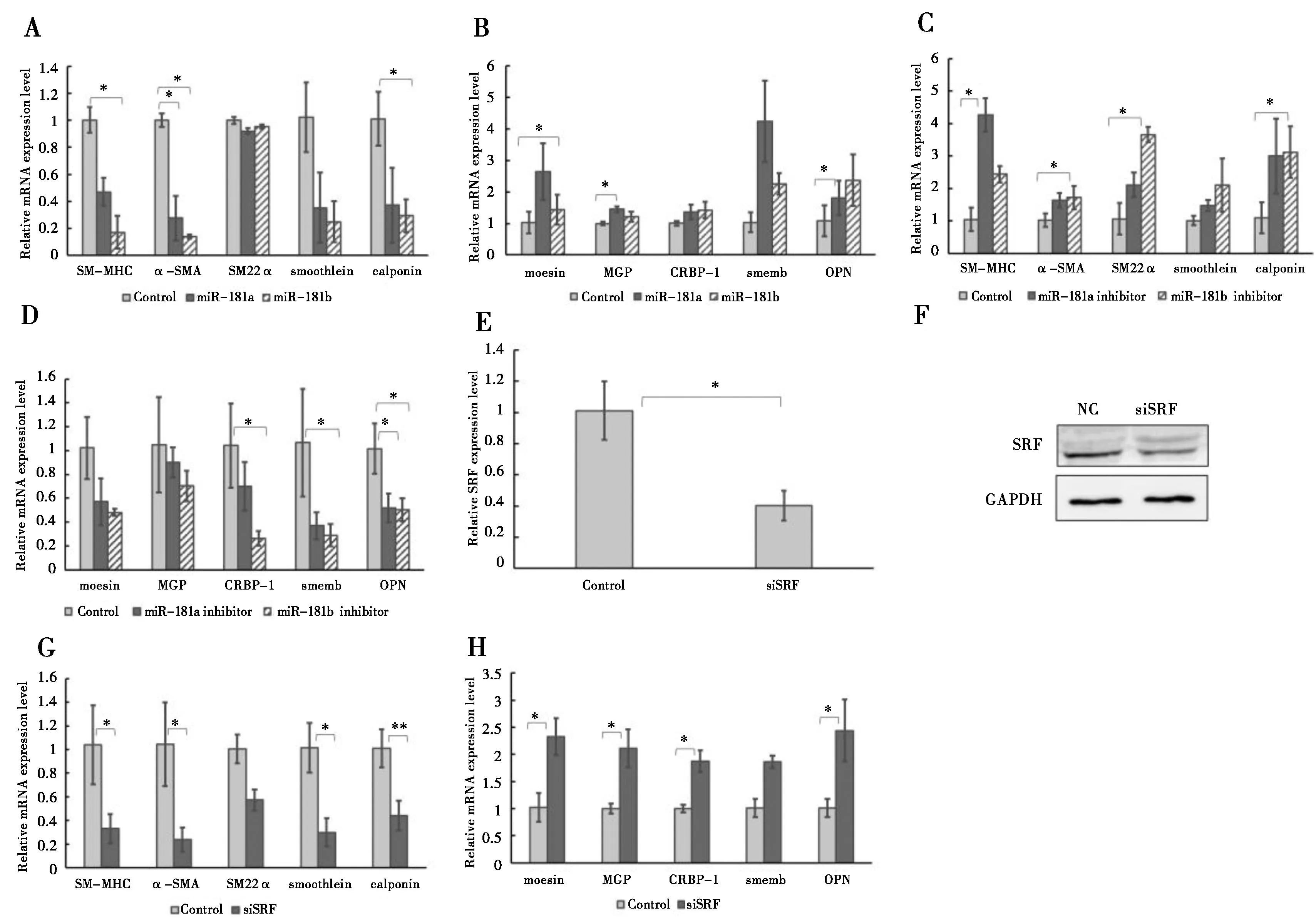
181a/b在HAoSMCs中过表达使收缩型标记基因(SM-MHC,α-SMA,SM22α,smoothelin,calponin)表达下调(A),合成型标记基因(moesin,MGP,CRBP-1,smemb,OPN)表达上调(B).抑制miR-181a/b的表达会促进收缩型标记基因的表达(C),抑制合成型标记基因的表达(D).使用siSRF敲除HAoSMCs的SRF后,SRF的mRNA(E)和蛋白表达(F)水平都明显下调,收缩型标记基因表达降低(G),合成型标记基因表达升高(H).*:P<0.05,**:P<0.01,n=6,GAPDH 作为内参
图2miR-181a/b 调控HAoSMCs表型标记基因的表达图
Figure 2miR-181a/b regulates expressions of HAoSMCs phenotype marker genes
2.3 miR-181a/b促进HAoSMCs的增殖及迁移能力(图3)


CCK-8检测结果表明miR-181a/b促进HAoSMCs的增殖(A),miR-181a/b抑制剂(inhibitor)抑制HAoSMCs 的增殖(B).*:P<0.05,**:P<0.01,n=6.(C)使用transwell检测法检测HAoSMCs的迁移能力变化,倒置显微镜下观察(200×),与对照组相比,过表达miR-181a/b细胞组迁移能力明显增强,而抑制miR-181a/b细胞组细胞迁移能力明显降低
图3miR-181a/b对HAoSMCs的增殖及迁移能力的作用图
Figure 3Effect of miR-181a/b on proliferation and migration of HAoSMCs
为了确定miR-181a/b是否对平滑肌细胞增殖和迁移能力有调控作用,miR-181a/b mimics或inhibitors分别被转染至HAoSMCs内72 h。HAoSMCs的增殖能力的检测使用CCK-8试剂盒进行,细胞迁移能力检测利用transwell系统进行。CCK-8检测结果显示,过表达miR-181a/b 能够促进HAoSMCs的增殖能力(图 3A),miR-181a/b inhibitor能够抑制HAoSMCs的增殖能力(图 3B)。transwell实验结果显示miR-181a/b明显地促进HAoSMCs的迁移能力,而miR-181a/b inhibitor能够减弱HAoSMCs的迁移能力(图 3C)。
2.4 miR-181a/b直接靶向HAoSMCs 中的SRF的3’UTR(图4)
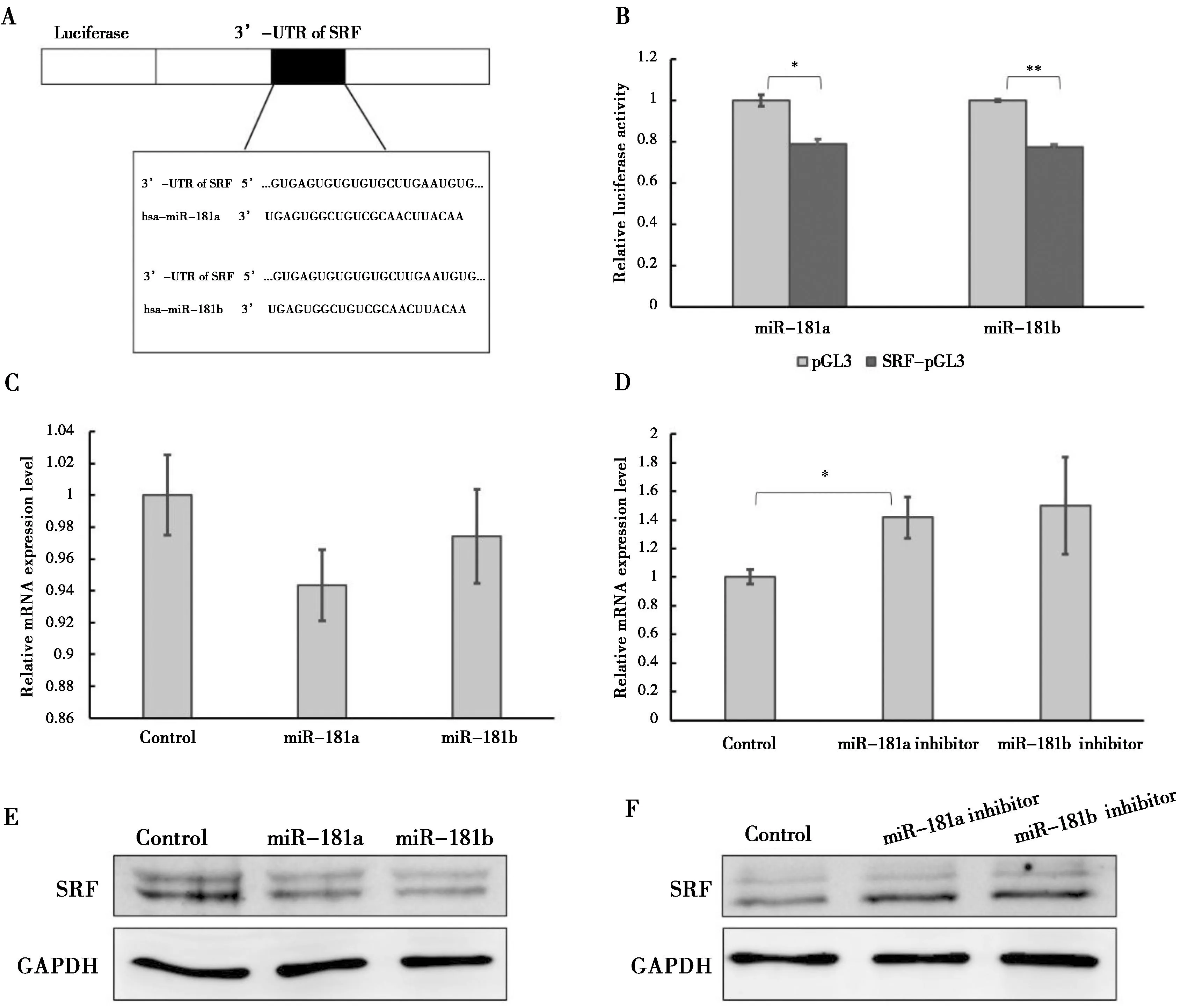
双荧光素酶报告系统结果表明miR-181a/b能够直接降低SRF的荧光活性(A,B).过表达miR-181a/b的细胞中SRF的mRNA表达水平(C)抑制miR-181a/b的细胞中的SRF表达水平(D).miR-181a/b抑制HAoSMCs中的SRF的蛋白表达水平(E),而抑制miR-181a/b的表达促进SRF的蛋白表达(F).*:P<0.05
图4miR-181a/b直接靶向SRF并降低SRF的表达图
Figure 4miR-181a/b directly binds to SRF and down-regulates the SRF level
双荧光素酶报告系统被用来确定miR-181a/b是否直接靶向HAoSMCs中的SRF的3’UTR。miR-181a或miR-181b与SRF-pGL3共转染至HAoSMCs中,24 h后检测荧光素酶的荧光活性。结果显示miR-181a/b能够直接抑制SRF-pGL3质粒的荧光活性(图 4A-B),表明miR-181a/b能够直接靶向HAoSMCs中的SRF的3’UTR。
为进一步确定miR-181a/b对SRF的表达的调控作用,将miR-181a/b类似物或抑制剂分别转染入HAoSMCs内,72 h后分别利用实时定量PCR和western blot检测SRF的基因及蛋白的表达水平。实时定量PCR结果显示,在过表达miR-181a/b的HAoSMCs中SRF的表达水平下调,而在抑制miR-181a/b的HAoSMCs中SRF的表达水平上调(图4C-D)。western blot结果显示,SRF的蛋白表达水平能够被miR-181a/b抑制(图 4E),被miR-181a/b inhibitor促进(图 4F)。
3讨论
血管平滑肌细胞的表型异常转变引发的过度增殖和迁移是导致动脉粥样硬化、术后再狭窄等血管疾病的细胞学基础[17-19]。影响平滑肌表型转化的因素多种多样,包括细胞外基质、血管受损、细胞因子、转录因子[20-22]。
SRF在平滑肌细胞的分化过程中可结合至平滑肌细胞基因启动子区的CArG区从而激活基因的转录[23-24]。SRF在血管中的作用可被共因子myocardin和ELK1调控。多种miRNAs(miR-21[25]、miR-143[26]和miR-145[27]等)参与调控平滑肌细胞的分化及去分化过程。
MicroRNAs(miRNAs)是一类广泛存在于动物、植物及病毒中的长约18~22个核苷酸序列的非编码RNA,通过靶向基因的3’非编码区(3’UTR)调控基因表达[28,29]。miRNAs在胚胎发育[30]、细胞分化[31]、增殖[32]、凋亡[33]、造血[34]及癌症治疗[35-36]等方面有调控作用。另外miRNAs可以调控一些血管相关细胞的细胞行为,如血小板聚集、平滑肌增殖等[37]。miRNA作为一类重要的调控因子,在不同的病理生理状态下表达情况会发生改变,可对相关基因进行精细调控。所以,可以利用这一特性研究miRNA在血管疾病的靶基因及作用机制。
越来越多的文献表明,miR-181家族参与调控血管的生理和病理过程。miR-181家族有四个成员:miR-181a、miR-181b、miR-181c、miR-181d,它们分别有不同的靶基因调控不同的通路[38]。miR-181a和miR-181b是miR-181家族中研究较为清楚且在基因上成簇存在。miR-181家族广泛参与调控急性骨髓白血病致癌过程[39]、免疫系统代谢调控[40]、血管炎症反应[38]、淋巴细胞发育及稳态[41]。其中miR-181a通过靶向NOX4调控内皮细胞增殖,通过调控骨桥蛋白(osteopontin,OPN)表达参与调控动脉粥样硬化的形成[42,43]。miR-181b通过靶向NF-κB信号通路中的importin-α3调节血管炎症[44]。miR-181c是CD4(+)T 细胞的负调控因子[45],miR-181d调节胸腺细胞的急性应激反应[46]。
本研究选取miR-181a/b作为切入点,研究了miR-181a/b在平滑肌细胞表型转化中的功能。结果表明,平滑肌细胞由收缩表型向合成表型转化后,miR-181a/b表达明显上调。miR-181a/b抑制收缩表型基因的表达,促进合成表型基因的表达,同时能促进平滑肌细胞的迁移与增殖能力。而miR-181a/b inhibitor的作用与miR-181a/b的作用相反。
除此之外,在平滑肌细胞中沉默SRF的表达用来验证其功能,SRF沉默后抑制平滑肌收缩基因的表达,促进合成基因的表达,这一结果与文献[16]报道的维持平滑肌收缩功能一致。
通过生物信息学分析,SRF可能是miR-181a/b的靶基因,通过双荧光素酶报告系统验证miR-181a/b可以直接靶向SRF的3’-UTR序列。western blot结果表明miR-181a/b明显降低SRF的蛋白表达,实时定量PCR结果显示miR-181a/b降低SRF的mRNA表达。
本研究首次证明了在HAoSMCs中SRF是miR-181a/b的靶基因,miR-181a/b通过直接靶向SRF调控平滑肌细胞向合成表型转化,是对miRNA调控血管系统复杂网络的补充。本研究结果为血管疾病治疗提供途径和方法。
参考文献
[1]Davis BN,Hilyard AC,Nguyen PH,et al.Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype[J].J Biol Chem,2009,284:3728-3738.
[2]Elia L,Quintavalle M,Zhang J,et al.The knockout of miR-143 and-145 alters smooth muscle cell maintenance and vascular homeostasis in mice:correlates with human disease[J].Cell Death Differ,2009,16:1590-1598.
[3]Sarkar J,Gou D,Turaka P,et al.MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration[J].Am J Physiol Lung Cell Mol Physiol,2010,299:L861- 871.
[4]Albinsson S,Suarez Y,Skoura A,et al.MicroRNAs are necessary for vascular smooth muscle growth,differentiation,and function[J].Arterioscler Thromb Vasc Biol,2010,30:1118-1126.
[5]Rensen SS,Doevendans PA,van Eys GL.Regulation and characteristics of vascular smooth muscle cell phenotypic diversity[J].Neth Heart J,2007,15:100-108.
[6]Kumar MS,Owens GK.Combinatorial control of smooth muscle-specific gene expression[J].Arterioscler Thromb Vasc Biol,2003,23:737-747.
[7]Miano JM,Cserjesi P,Ligon KL,et al.Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis[J].Circ Res,1994,75:803-812.
[8]Mack CP,Owens GK.Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions[J].Circ Res,1999,84:852-861.
[9]Mack CP,Somlyo AV,Hautmann M,et al.Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization[J].J Biol Chem,2001,276:341-347.
[10]van der Loop FT,Schaart G,Timmer ED,et al.Smoothelin,a novel cytoskeletal protein specific for smooth muscle cells[J].J Cell Biol,1996,134:401-411.
[11]Sluiter I,van der Horst I,van der Voorn P,et al.Premature differentiation of vascular smooth muscle cells in human congenital diaphragmatic hernia[J].Exp Mol Pathol,2013,94195-202.
[12]Doevendans PA,van Eys G.Smooth muscle cells on the move:the battle for actin[J].Cardiovasc Res,2002,54:499-502.
[13]Yoshida T,Owens GK.Molecular determinants of vascular smooth muscle cell diversity[J].Circ Res,2005,96:280-291.
[14]Speer MY,McKee MD,Guldberg RE,et al.Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice:evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo[J].J Exp Med,2002,196:1047-1055.
[15]Lai YM,Fukuda N,Su JZ,et al.Novel mechanisms of the antiproliferative effects of amlodipine in vascular smooth muscle cells from spontaneously hypertensive rats[J].Hypertens Res,2012,25:109-115.
[16]Chen J,Kitchen CM,Streb JW,et al.Myocardin:a component of a molecular switch for smooth muscle differentiation[J].J Mol Cell Cardiol,2002,34:1345-1356.
[17]Alexander MR,Owens GK.Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease[J].Annu Rev Physiol,2012,74:13-40.
[18]Orr AW,Hastings NE,Blackman BR,et al.Complex regulation and function of theinflammatory smooth muscle cell phenotype in atherosclerosis[J].J Vasc Res,2010,47:168-180.
[19]Doran AC,Meller N,McNamara CA.Smooth muscle cells in the initiation and early progression of atherosclerosis[J].Arterioscler Thromb Vasc Biol,2008,28:812-819.
[20]Shen JY,Chan-Park MB,He B,et al.Three-dimensional microchannels in biodegradable polymeric films for control orientation and phenotype of vascular smooth muscle cells[J].Tissue Eng,2006,12:2229-2240.
[21]Owens GK,Kumar MS,Wamhoff BR.Molecular regulation of vascular smooth muscle cell differentiation in development and disease[J].Physiol Rev,2004,24:767-801.
[22]Hao H,Gabbiani G,Bochaton-Piallat ML.Arterial smooth muscle cell heterogeneity:implications for atherosclerosis and restenosis development[J].Arterioscler Thromb Vasc Biol,2003,23:1510-1520.
[23]Du KL,Ip HS,Li J,et al.Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation[J].Mol Cell Biol.2003,23:2425-2437.
[24]Miano JM.Serum response factor:toggling between disparate programs of gene expression[J].J Mol Cell Cardiol,2003,35:577-593.
[25]Wang Z,Wang DZ,Hockemeyer D,et al.Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression[J].Nature,2004,428:185-189.
[26]Cordes KR,Sheehy NT,White MP,et al.miR-145 and miR-143 regulate smooth muscle cell fate and plasticity[J].Nature,2009,460:705-710.
[27]Davis BN,Hilyard AC,Lagna G,et al.SMAD proteins control DROSHA-mediated microRNA maturation[J].Nature,2008,454:56-61.
[28]Bartel DP.MicroRNAs:genomics,biogenesis,mechanism,and function[J].Cell,2004,116:281-297.
[29]He L,Hannon GJ.MicroRNAs:small RNAs with a big role in gene regulation[J].Nat Rev Genet,2004,5:522-531.
[30]Darnell DK,Kaur S,Stanislaw S,et al.MicroRNA expression during chick embryo development[J].Dev Dyn,2006,235:3156-3165.
[31]Ivey KN,Srivastava D.MicroRNAs as regulators of differentiation and cell fate decisions[J].Cell Stem Cell,2010,7:36-41.
[32]Brennecke J,Hipfner DR,Stark A,et al.bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila[J].Cell,2003,113:25-36.
[33]Jovanovic M,Hengartner MO.miRNAs and apoptosis:RNAs to die for[J].Oncogene,2006,25:6176-6187.
[34]Montagner S,Deho L,Monticelli S.MicroRNAs in hematopoietic development[J].Bmc Immunol,2014,15:14.
[35]Soifer HS,Rossi JJ,Saetrom P.MicroRNAs in disease and potential therapeutic applications[J].Mol Ther,2007,15:2070-2079.
[36]Dong H,Lei J,Ding L,et al.MicroRNA:function,detection,and bioanalysis[J].Chem Rev,2013,113:6207-6233.
[37]Urbich C,Kuehbacher A,Dimmeler S.Role of microRNAs in vascular diseases,inflammation,and angiogenesis[J].Cardiovasc Res,2008,79:581-588.
[38]Sun X,Sit A,Feinberg MW.Role of miR-181 family in regulating vascular inflammation and immunity[J].Trends Cardiovasc Med,2014,24:105-112.
[39]Su R,Lin HS,Zhang XH,et al.MiR-181 family:regulators of myeloid differentiation and acute myeloid leukemia as well as potential therapeutic targets[J].Oncogene,2014,6:1-14.
[40]Williams A,Henao-Mejia J,Harman CC,et al.miR-181 and metabolic regulation in the immune system[J].Cold Spring Harb Symp Quant Biol,2013,78:223-230.
[41]Henao-Mejia J,Williams A,Goff LA,et al.The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis[J].Immunity,2013,38:984-997.
[42]Scatena M,Liaw L,Giachelli CM.Osteopontin:a multifunctional molecule regulating chronic inflammation and vascular disease[J].Arterioscler Thromb Vasc Biol,2007,27:2302-2309.
[43]Remus EW,Lyle AN,Weiss D,et al.miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells[J].Atherosclerosis,2013,228:168-174.
[44]Sun X,Icli B,Wara AK,et al.MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation[J].J Clin Invest,2012,122:1973-1990.
[45]Xue Q,Guo ZY,Li W,et al.Human activated CD4(+)T lymphocytes increase IL-2 expression by downregulating microRNA-181c[J].Mol Immunol,2011,48:592-599.
[46]Belkaya S,Silge RL,Hoover AR,et al.Dynamic modulation of thymic microRNAs in response to stress.PLoS One,2011,6:e27580.
