在气液界面上压缩诱导聚(L-乳酸)膜的结构变化
2015-12-29陈启斌刘洪来
王 丽 季 姗 陈启斌 刘洪来
(华东理工大学化学系,化学工程国家重点实验室,上海200237)
在气液界面上压缩诱导聚(L-乳酸)膜的结构变化
王 丽 季 姗 陈启斌*刘洪来
(华东理工大学化学系,化学工程国家重点实验室,上海200237)
聚乳酸(PLA)是一种环境友好及生物可降解的聚合物,其界面性质受到了广泛关注.本文以Langm uir-Blodgett(LB)膜天平、原子力显微镜(AFM)研究了聚(L-乳酸)(PLLA)在气液界面上的性质.表面压-面积(π-A)等温曲线的结果表明,在膜压缩的初始阶段,表面压逐渐增大;当膜压为9.0mN·m-1时,曲线出现了一个平台,其重复单元的面积大约在0.11-0.17 nm2之间.原子力显微镜的结果发现,在压缩过程中,膜结构发生了明显的变化:平台刚出现时,膜内出现了大量的纤维结构;在平台区内,界面上出现了多层膜结构.特别地,当表面压为20.0mN·m-1时,PLLA在界面上可形成约6.0 nm厚的薄膜.由此可见,PLLA等温线中的平台与其膜结构的变化紧密相关.这有别于普通双亲分子的性质,即这类双亲分子π-A等温线中的平台通常表示它们在二维空间上发生了单分子膜的相转变.
聚(L-乳酸);界面;单分子膜;相转变;结构特征
1 In troduc tion
Biomaterialshave played an important role in the treatmentof disease and the improvementof health care throughouthistory,in particular,since theadventof the synthetic polymersat theend of the nineteenth century.1Although thesematerials,which are almost adopted from other areas of science and technology,are desirable to use inmedical treatments,therapy,diagnosis,and so forth,how to achieve their biocompatibility,mechanical properties,degradation and numerous other functions still remains a challenge.Moreover,in order tominimize wastemanagement caused by synthetic nondegradable polymers and reduce their impacton the environments,there are increasing demands for the utilization of degradablemacromolecules.2Recently,themost w idely researched biodegradable polymers were aliphatic polyesters,e.g.,poly(glycolide),poly(lactic acid)(PLA),poly(3-hydroxybutyrate),and poly(ε-caprolactone),which have nontoxic and biocompatible properties both as polymers and as their degradation products.3-10Therefore,biodegradable polymershave attracted a great deal of attention over the past few decades as potential replacements for synthetic nondegradable or slow ly degrading polymers.On theotherhand,up to now therehasbeen an increased emphasison theenhancementofmaterial properties w ith the structuresengineered atnanometer scalesand the technology for fabricating the material surface.Moreover,the microscopic structureson thematerial surfaceshavea considerable influence on bulk behaviors,such as adhesions,m igrations,and proliferations.11In general,the critically interfacial layer is typically only a few nanometers'thickness,and sometimes even molecular scales,and thus a detailed physical and chem ical characterization of the interfaces isnecessary for understanding the interfacialbehavior.
The Langmuir-Blodgett(LB)technique offers the possibility to fabricate highly ordered filmsw ith the desired controlover their thickness,morphologies,and surface roughnesson the orderat the molecular level,12which are desirable foranumberof applications such asmodelsurfaces for protein adsorption,tissueengineering, drug delivery,modified substrates for supportedmembranes,and microelectronic and opticaldevices.1,13-17The successof the classic LB technique draws on the property of amphiphilicmolecules when spread and compressed at the air/water interface to form a compact monolayer w ith hydrophobic and hydrophillic parts directed to air and water,respectively.Polymer monolayers, spread at the air/water interface,are under active investigation in order to help ourunderstanding of the factorscontrolling polymerpolymerand polymer-interface interactions,because their details concerningmolecular orientations,monomer conformations,and interaction energiesof polymersare in principledeterminablewith the aid of the surface film balance techniqueand ancillary spectroscopic andm icroscopicmethods.
Among degradable polymers,PLA can be produced from the renewable resources such as corn,wheatstarch,and sugarbeets or the ring-opening polymerization of lactides,meanwhile it can break down to commonmetabolites.Furthermore,PLA issuperior to other polyesters in many aspects such as thermal and mechanical properties and transparency of the processedmaterials.7Based on both characteristics of PLA,wewere interested in its interfacialproperties.Due to the chirality of the lactidemonomer, PLA can form polyenantiomers,i.e.,poly(L-lactic acid)(PLLA) and poly(D-lactic acid)(PDLA).So far,there have been quite a few of papersdealingw ith the behaviorsof PLA(including PLLA and PDLA)at the air/water interface.18-22However,the structural transition of PLLA during compressing the polymeric filmsat the air/water interface is still unclear.In this work,therefore,our objectivewas to study the change in the film structure of PLLA during the compressing process.
2 Materials and m ethods
2.1 Materials
PLLA(Mn=14000 g·mol-1,polydispersity index(PDI)=1.12) was purchased from Polymer Source,Inc.,and used w ithout further purification.Waterw ith the resistivity of 18.2MΩ·cm was purified by a M illi-Q pluswater purification system(ultra pure UVF,ShanghaiHitech Co.,Ltd.)containing one carbon stage and two ion-exchange stages.Chloroform,analyticalgrade chemicals(>99%),wasused as the solventof the spreading solution.
2.2 π-A iso therm m easu rem en t
Surface pressure-area(π-A)isothermswere obtained using a model612D computer-controlled Langmuir film balance(Nima Technology,Coventry,UK),equipped w ith dual barriers and a Wilhelmy plate sensing device.The subphase temperaturewas maintained at20°C using a circulating temperature controller.
PLLA stock solutionswere prepared using chloroform to give final concentrationsof 0.5mmol·L-1relative to the repeatunit.In a typicalexperiment,polymer filmswere fabricated by spreading ~30μL of the stock solution dropw ise over the M illi-Q water surface.Compression was initiated after a delay of 15 m in to allow the complete evaporation of the spreading solventand the compression ratewaskeptat20 cm2·min-1,with a constantbarrier movement.π-A isothermswere recorded during the compressing process.
2.3 LB film p reparation
Themonolayerwas transferred atdifferentsurface pressuresof 5.0,9.0,9.2,9.6,and 20.0mN·m-1and the deposition ratewas 2 mm·m in-1.One-layer LB films for atomic forcemicroscopy (AFM)observation were transferred onto freshly cleaved atomically flatm ica surfaces by a vertical dippingmethod while the monolayerwas held atconstantsurface pressure,and they were dried in air at room temperature and then put into a desiccator. The transfer ratio of theupstrokewasabout0.90-1.05.
2.4 A tom ic fo rcem ic roscopym easu rem en t
TheAFM topographic imageswere carried outon amodelAJIII(Aijian Nanotechnology Inc.,China)m icroscope in tapping modew ith a triangularm icrofabricated cantilever(M ikro Masch Co.,Russia)with a length of(130±5)μm,a Sipyramidal tipw ith Au reflective side and a spring constantof 2.5-10.0 N·m-1.A resonance frequency in the range of 55-500 kHzwas used and resonance peaks in the frequency response of the cantilevertypically at 123.33 kHz were chosen for a tapping mode oscillation.The curvature radius of the tip was less than 10 nm.The experiments were operated under ambient conditions.Prior to AFM measurements,LB filmswere dried in a desiccator at least for24 h.
2.5 Film thicknessm easu rem en ts
The AFM iswellsuitable for the directmeasurementof heights andmorphologiesof surface features,especiallywhen the surface height is less than 50 nm.The thickness of LB films can commonly be obtained by profilometry w ith AFM.That isa partof LB films removed via scratching the surfacew ith an AFM tip or a razorblade so as to expose the substrate,so that the step heights above the substrate could be determined using AFM directly. Herein,an AFM probe tip itselfwasused to remove a portion of the LB film by first creating an approximate square holew ith a size of 1μm×1μm in the film via a contactingmode.In brief,the operating procedure of the thicknessmeasurementof LB films is as following.Firstly,these films could be imaged nondestructively in anormal tappingmode by choosing generalAFM parameters carefully(set point of~0 V,scan speed of~1 Hz).Next,the scanning mode was shifted to a contacting mode and these parameters can bealtered so as to cause the removalof theadhered material,accomplished by scanning repeatedly after increasing the setpoint to nearly themaximum value and the scan speed to 10 Hz(herein,the scanned areawas setat1μm×1μm).Then,the removed film could be verified by increasing the scan sizewhile returning to thenormal imaging parameters in the tappingmode, as shown in Fig.S1(Supporting Information).The resulting image,was used to analyze in the cross section to gain the step heightof the film.

Fig.1 Surface p ressure--area(π-A)isotherm of poly (L-lactic acid)(PLLA)at theair/water interface
3 Resu lts and d iscussion
3.1 π-A iso therm
Theπ-A isotherm of PLLA is shown in Fig.1.This curvewas measured at least five times to confirm reproducibility.The isotherm exhibits a high compressibility region,i.e.,a pre-plateau feature,a sharp break point followed by a relatively horizontal plateau,and then a post-plateau inflection pointand a low compressibility region followed on as the compression proceeds. Herein,theareaof thebreak pointisaround 0.17 nm2·repeatunit-1and the plateau appearsata surface pressure of about9.0mN·m-1. After the plateau,the constant compression leads to a sharp increase in the surface pressure and extrapolation of the steeply linear portion of this curve to zero surface pressure gives a limiting areaof~0.09 nm2·repeatunit-1.More interestingly,this value of~0.09 nm2·repeat unit-1is remarkably less than the crosssectional area of an alkyl chain,i.e.,~0.21 nm2.12-14This result may be attributed to two possibilities:one is the solubility of the polymer,leading to the partly solving PLLA in the subphase during the compression;the other is the formation of themultilayer structure of PLLA.Since PLLA is insoluble in water,the smallareaof the repeatunit isa likely consequenceof the latter case.On theotherhand,it isusual thata polymer film is formed by stacks of non-monomolecular structures.Actually,since this area of~0.09 nm2·repeat unit-1is based on the repeat unit of lactide,if PLLA forms amonomolecular film,the value of the repeatunitshould be larger than thatof the cross-sectionalareaof the alkyl chain,that is to say,it isunlikely thatPLLA can form a truemonomolecular film at the air/water interface.Up to now, only few works have reported the formation ofmonomolecular Langmuir film of polyheterocyclics.23Accordingly,PLLAmight form multilayer or complicated structuresat the air/water interface,leading to the reducingmean area of the repeatunit.This w ill be confirmed by AFM results as follow ing.In addition,it should benoted thatwe discovered that the compressing ratehad a negligible influence on suchπ-A isotherms(see Fig.S2(Supporting Information)),thus the value of the compressing ratewas chosen at20 cm2·m in-1.
In general,plateau regions in isotherms are usually associated w ith the formation of a new phase for conventionalamphiphiles, such as lipidsand surfactants.Many researchershad investigated the properties of such amphiphiles at the air/water interface and suggested that Langmuir filmsof them exhibita liquid expanded (LE)to a liquid condensed(LC)phase transition in themonolayer region.24-26In Fig.1,the presenceof a break point followed by a plateau regionmight thusmean that the plateau corresponds to the formation of a new phase.Therefore,five pointswere selected in this work to monitor the change in film structures during the compression,i.e.,theposition before the plateau(A,5.0mN·m-1), the beginning(B,9.0mN·m-1),middle(C,9.2mN·m-1),and term inal(D,9.6mN·m-1)sites in the plateau region and the point after the plateau(E,20.0mN·m-1).
3.2 Mo rpho logies o f PLLA LB film s
AFM is a useful tool for obtaining information on surface morphology,particularly for LB films.PLLA LB films were deposited on themica atdifferentsurface pressuresandmeasured by AFM,asshown in Fig.2.
The PLLA LB films were firstly transferred onto themica substratesw ith a high film transfer ratio by the vertical dipping method easily and conveniently.In all cases,AFM imagesalmost show the fullarea coverage of the one-layer LB film.This indi-cates that themorphology of the film formed at theair/water interface is preserved upon the vertical upstroke.27As shown in Fig.2A,when PLLA film wascompressed up to 5.0mN·m-1at the air/water interface,a relatively even and featureless film w ith some irregularly dispersed holes was observed.The height of these filmsbased on the defectswasabout2.2 nm.This implies that before the plateau,PLLA primarily adopts a random coil conformation and formsa featurelessmonolayer at the air/water interface.Herein,the gradualsurface pressure increase in the preplateau region is suggestive of a compressible,fluid-like film in this region.
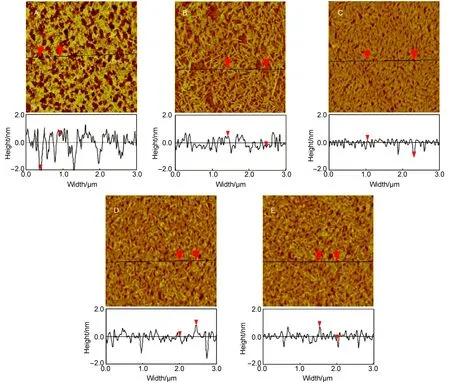
Fig.2 AFM imagesof PLLA Langmuir-Blodgett(LB)films transferred atdifferentsurface pressures
At theonsetof the plateau(the surface pressure is increased up to~9.0mN·m-1),a certain amount of the fibrillar structure is apparently observed,while the featureless polymer thin film of the underlying layer could still be observed at the same time,as shown in Fig.2B.Theheightandwidth of the fibrilsareabout1.0 nm and from~20 to 60 nm,respectively.Here,the practicalwidth of the fibrilshould be re-calculated ow ing to the presence of the AFM tip enlargementeffect.If oneassumes that the tip isa half sphere of diameter(D)and that the fibril isa cylinderw ith a diameter of d,the apparentw idth W,obtained from AFM images, can be expressed by the relation W=2(Dd)1/2.28Given W=20 nm and the value of D is20 nm,the actual diameter d of the PLLA fibril is close to 5.0 nm(in the case of W=60 nm,d=45 nm).In this plateau region,compression the PLLA film over the water surface doesnotcontribute to the surface pressure remarkably,but it results in the change in surfacemorphology from the featureless film to nanofibrillarstructures.In themiddleof theplateau(π=9.2 mN·m-1),aworm likeor vermiculate pattern emerges in thewhole view and this film is relatively uniform,while the featureless polymer thin film of the underlying layer isnotdetected.Herein, the apparentw idth of theworm like structure isalso in the range from 20 to 60 nm.In contrast,the apparentlymean heightof this structure decreases from~0.6 to 1.0 nm,whichmay be due to the closer packing fibersas the film is compressed.At the end of the plateau,compressing the film resulted in the fact thataminor partof fibrils was forced to hump up from the relatively uniform worm like structure,as shown in Fig.2D.A fter this transition,the surface pressure rapidly increases due to the formation of a compacting or solid-like film.When the surface pressure is increased up to 20.0mN·m-1,a relatively flat film is derived from the humped fibrils.Taken together,themorphological change in the film structures indicates that PLLA formsamultilayer film at the air/water interface,rather than amonomolecular film,which w illbe confirmed by the thicknessof the PLLA LB film from the profilometry w ith AFM.The formation of such amultilayer film isa likely resultof this fact that PLLA is lack of a strong polar group in the repeatunit.In this case,thehydrophilic segmentof the lactidemonomer could not be steadily confined at the air/ water interface.PLLA isdifferent from theamphiphilic copolymersor polymeric surfactants,which are of the structure nature of smallmolecule surfactants(<1 kDa).As for such amphiphilic copolymersor polymeric surfactants,thehydrophilic block or the polarhead groups reside readily in the subphase due to theirgood affinity w ithwater.29,30In contrast,PLLA tends to form intra-or inter-molecular aggregates due to the intra-and inter-molecular interactionsof itself and the relatively strong hydrophobicity of the repeatmonomer.Themultilayer structure is closely consistent w ith the resultof theπ-A isotherm and explain why the area of the repeat unit ismuch smaller than expected.Therefore,this finding suggests that it is the amphiphilic nature of the polymer which isa key point for itself to form monomolecular film at the air/water interface.In addition,it is noted that the rootmean square roughness(Rrms)measured on all these images is less than 1.0 nm,indicating that these LB filmsare fairly flat.
3.3 Film thickness
Fig.3A displaysa largerscanning imagew ith asizeof 10μm× 10μm at the surface pressure of 20.0mN·m-1,the roughnessof which hasa slight increase comparedwith thatin 3.0μm×3.0μm, namely that Rrmsis0.318 nm.Fig.3B showshow the film thickness wasmeasured using the AFM.The darker region in the image corresponds to the substrate,where the film was removed by a probe tip.Here,PLLA LB filmswere subjected to perturbation w ith the AFM probe tip,such that repeated scanning under the highest operating forces feasible w ith the cantilevers we used could scrape the adhered PLLA off.This can be verified by the fact that very little,if any,materialwas left(more details seen Fig.S3(Supporting Information)),as shown in the lower partof Fig.3B,the cross-section profile along the corresponding line.For an accuratemeasurementof film thickness,itwas important that the film was completely removed in this region,butat the same time such scratching did notscrape off the substrate.Itwas noticed that therewere high ridgesat the step edges,whichwere due to the buildup ofmaterial thatwas removed from the substrate. Thus,they should be ignored in themeasurementof thickness. Moreover,since neither the film surface nor the scraped region was perfectly flat,as would be expected,the thickness measurementwas thus conducted by using average cross-section analysis from differentareasof several independentpositions(Fig. S3).The darker region is where the average cross section was taken,from which one can obtain the step height thatcorresponds to the thicknessof PLLA LB film.The resulting valueof the film thickness is ca6.0 nm,which strongly supports that PLLA does form multilayer filmswhen compressed after the plateau at theair/ water interface.
Itmustbe pointed out that image bow or tilt is typically presented during AFM scanning,due either to the sample alignment or to the piezoelectric scannersemployed.These bowsand tilts in Fig.2 and Fig.3A were typically removed bym inimal postprocessing,i.e.,simply first-order flattening.However,secondorder flatteningwas carried out in Fig.3B,but the tiltof the cross section profile in the darker region could notbe removed because of the existence of high ridges at the edges.Anyhow,it has a negligible effecton determining the resulting film thickness,as shown in Fig.S3.
Taken together,π-A isotherm,film thickness,and AFM images indicated thatPLLA formedmultilayer structuresafter the plateau at the air/water interface.When the surface pressure started to increase,the featureless film structure displayed and the film thicknesswas about 2.2 nm.These facts suggested that PLLA primarily adopted a random coil conformation,leading to the formation of the featurelessmonolayer at the air/water interface in the pre-plateau region.The area at the inflection pointof the plateau isaround 0.17 nm2·monomer-1,which is consistentw ith the value reported by Klass et al.20This value also indicates a special property of PLLA at the air/water interface.Unlike conventional amphiphilicmolecules at the air/water interface w ith their hydrophilic parts anchored onto the subphase and hydrophobic parts pointing toward air,PLLAmolecules tend to reside freely on the subphase due to the relatively weak hydrophilic character of an including estergroup in the repeatunit.Thus,at the initial compressing stage,PLLA has a tendency to adopt a random coil conformation at the air/water interface.At the beginning of the plateau,a numberof fibrillar structureswere presented and theamountof the fibrilswasapparently increased as the film was compressed.Such plateausareoften associatedwith phase transitionsand are indicatorsof phase coexistence for those conventional amphiphiles in a two-dimensional plane.12-14,24,25Bulk PLLA crystallization studies show thatPLLA or PDLA can crystallize to form the left-handed and right-handed 103helices, respectively,known as theα-form,while blends of PLLA and PDLA form 31helices,termed as theβ-form.31,32However,it turned out that the further compression would lead to the formation of a bilayerand even a trilayer in thiswork,where the top layer is composed of compacting fibrils.In otherwords,PLLA molecules assemble into a well-defined arrangement,in which PLLA molecules are forced out of the interface to form such multilayer.These findings indicate thatPLLAmoleculesareordered as the film is compressed in the plateau region so that a nanoscale surface pattern w ith well-ordered fibrillar structures is formed.Moreover,such fibrils almost lay flat in the plane of the film,leading to the fairly even and uniform surface.The negli-gible increase in the surface pressure in the plateau region indicates that there exists a low level of van derWaals interaction between the fibrils in the film.In addition,according to the ratio of the practicaldiameter to the heightof fibrils,PLLAmolecules have a tendency to laterally associate during compressing.As compression continues,the surface pressure increases rapidly when the area of the repeatunit isabout0.11 nm2·repeatunit-1, corresponding to the closely packing of such fibrils,where multilayers are formed completely.Suchmultilayer structure can be strongly verified by the resultof the film thickness.Furthermore,the compacting fibrilsmake the film stiffer,giving rise to an increase in surface pressure,which explainswhy the surface pressurekeepssteeply increasing in the region of0.06-0.11 nm2· repeatunit-1.The schematic self-assembling processof PLLA at the air/water interface is given in Fig.4,which can be used to explain the resultsof theπ-A isotherm and AFM images,namely that the fact that the area per the repeatunit is smaller than 0.2 nm2is a likely resultof the formation ofmultilayers.Suchmultilayer is composed of the top layers(i.e.,the pink part in the schematic diagram)stemmed from fibrillar structures and the underlying layer w ith an even film(i.e.,the green part in the schematic diagram).In the underlying layer,PLLA molecules readily adopta random coil conformation,while in top layers,the compression can induce the formation of fibrillar structures.

Fig.3 Thicknessm easurementobtained by scratching to rem ove allPLLA LB film from an area asπ=20.0m N·m-1

Fig.4 Schematic processof the PLLA structural transition at the air/water interface during the comp ression
4 Conc lusions
In summary,PLLA can form a stablehyperthin film withwelldefined structureat theair/water interface.A numberof fibrilsare observed over thewater surface at the onsetof the plateau.Intriguingly,compression can induce a transition in the PLLA film structure from a featureless layer to amultilayer.More importantly,the surface pattern is composed of the closely packing fibrils,which is relatively uniform and even.These studiesprovide a new approach for controlling surfacemorphology w ith a biodegradable polymer commonly used for drug delivery and tissue engineering.In addition,it is also necessary to distinguish between the structural transition of the plateau in PLLA LB films and conventional amphiphiles,because the latter commonly corresponds to a phase separation in the two-dimensionalplane.
Suppo rting In fo rm ation:available free of charge via the internetathttp://www.whxb.pku.edu.cn.
(1)Langer,R.;Tirrell,D.A.Nature 2004,428,487.doi:10.1038/ nature02388
(2)Ha,C.;Gardella,J.A.,Jr.Chem.Rev.2005,105,4205.doi: 10.1021/cr040419y
(3)Lee,W.;Iwata,T.;Gardella,J.A.,Jr.Langmuir2005,21, 11180.doi:10.1021/la051137b
(4)Fischer,A.M.;Frey,H.Macromolecules2010,43,8539.doi: 10.1021/ma101710t
(5)Kawalec,M.;Adamus,G.;Kurcok,P.;Kowalczuk,M.;Foltran, I.;Focarete,M.L.;Scandola,M.Biomacromolecules2007,8, 1054.doi:10.1021/bm061155n
(6)Kulinski,Z.;Piorkowska,E.Polymer2005,46,10290.doi: 10.1016/j.polymer.2005.07.101
(7)Urayama,H.;Kanamori,T.;Fukushima,K.;Kimura,Y. Polymer2003,44,5635.doi:10.1016/S0032-3861(03)00583-4
(8)Hu,J.;Sun,X.;Ma,H.;Xie,C.;Chen,Y.E.;Ma,P.X. Biomaterials2010,31,7971.doi:10.1016/j. biomaterials.2010.07.028
(9)Shao,J.;Wang,Y.;Chen,X.;Hu,X.;Du,C.Colloids Surf.B 2014,120,97.doi:10.1016/j.colsurfb.2014.05.021
(10)Ni,S.;Lee,W.;Li,B.;Esker,A.R.Langmuir2006,22, 3672.doi:10.1021/la060084a
(11)Fukuhira,Y.;Kitazono,E.;Hayashi,T.;Kaneko,H.;Tanaka, M.;Shimomura,M.;Sumi,Y.Biomaterials2006,27,1797. doi:10.1016/j.biomaterials.2005.10.019
(12)Petty,M.C.Langmuir-Blodgett Films:An Introduction; Cambridge University Press:Cambridge,1996;pp 1-37.
(13)Gaines,G.L.;Roberts,G.Insoluble Monolayersat Liquid-Gas Interfaces;JohnWiley&Sons,Inc.:New York,1966;pp 136-202.
(14)Ulman,A.An Introduction to Ultrathin Organic Films:From Langmuir-Blodgett to Self-Assembly;Academ ic Press:Boston, 1991;pp 101-105.
(15)Penner,T.L.;Motschmann,H.R.;Armstrong,N.J.; Ezenyilimba,M.C.;Williams,D.J.Nature 1994,367,49.doi: 10.1038/367049a0
(16)Allen,D.;Westerblad,H.Science 2004,305,1112.doi:10.1126/ science.1103078
(17)Wijekoon,W.M.K.P.;Wijaya,S.K.;Bhawalkar,J.D.;Prasad, P.N.;Penner,T.L.;Armstrong,N.J.;Ezenyilimba,M.C.; Williams,D.J.J.Am.Chem.Soc.1996,118,4480.doi:10.1021/ ja953974d
(18)Pelletier,I.;Pézolet,M.Macromolecules2004,37,4967.doi: 10.1021/ma035949v
(19)Bourque,H.;Laurin,I.;Pézolet,M.;K lass,J.M.;Lennox,R. B.;Brown,G.R.Langmuir2001,17,5842.doi:10.1021/ la0009792
(20)Klass,J.M.;Lennox,R.B.;Brown,G.R.;Bourque,H.; Pézolet,M.Langmuir2003,19,333.doi:10.1021/la020606w
(21)Sato,G.;Nishitsuji,S.;Kumaki,J.J.Phys.Chem.B 2013,117, 9067.doi:10.1021/jp403195g
(22)Gurarslan,A.;Tonelli,A.E.Macromolecules2011,44,3856. doi:10.1021/ma200530w
(23)Bjørnholm,T.;Greve,D.R.;Reitzel,N.;Hassenkam,T.;Kjaer, K.;Howes,P.B.;Larsen,N.B.;Bøgelund,J.;Jayaraman,M.; Ewbank,P.C.;M cCullough,R.D.J.Am.Chem.Soc.1998, 120,7643.doi:10.1021/ja981077e
(24)Casillas-Ituarte,N.N.;Chen,X.;Castada,H.;A llen,H.C. J.Phys.Chem.B 2010,114,9485.doi:10.1021/jp1022357
(25)Picas,L.;Suárez-Germà,C.;TeresaMontero,M.;Domènech, Ò.;Hernández-Borrell,J.Langmuir2012,28,701.doi:10.1021/ la203795t
(26)Romão,R.I.S.;Ferreira,Q.;Morgado,J.;Martinho,J.M.G.; Gonçalvesda Silva,A.M.P.S.Langmuir2010,26,17165.doi: 10.1021/la103029d
(27)Genson,K.L.;Vaknin,D.;Villacencio,O.;M cGrath,D.V.; Tsukruk,V.V.J.Phys.Chem.B 2002,106,11277.doi:10.1021/ jp026244i
(28)Fang,J.;Knobler,C.M.;Gingery,M.;Eiserling,F.A.J.Phys. Chem.B 1997,101,8692.doi:10.1021/jp971057j
(29)Wang,M.H.;Janout,V.;Regen,S.L.Langmuir2012,28, 4614.doi:10.1021/la204985d
(30)Perepichka,I.I.;Borozenko,K.;Badia,A.;Bazuin,C.G.J.Am. Chem.Soc.2011,133,19702.doi:10.1021/ja209502d
(31)De Santis,P.;Kovacs,A.J.Biopolymers1968,6,299. doi:10.1002/bip.1968.360060305
(32)Okihara,T.;Tsuji,M.;Kawaguchi,A.;Katayama,K.I.;Tsuju, H.;Hyon,S.H.;Ikada,Y.J.Macromol.Sci.Phys.B 1991,30, 119.doi:10.1080/00222349108245788
Struc tu ral Transition o f Po ly(L-lac tic acid)Film Induced by Com p ression at Air/Water In terface
WANG Li JIShan CHEN Qi-Bin*LIU Hong-Lai
(State Key Laboratory ofChemical Engineering,DepartmentofChemistry,EastChina University of Science and Technology,Shanghai200237,P.R.China)
Poly(lactic acid)(PLA)has attracted considerable interest as an environmentally friend ly and biodegradable polymer.The properties ofpoly(L-lactic acid)(PLLA)atan air/water interfacewere studied based on the Langmuir-Blodgett(LB)film balance and atom ic forcem icroscopy(AFM).The surface pressure-area (π-A)isotherm indicated that the surface pressure of PLLA initially increased as the interfacial film was com pressed;atπ=9.0mN·m-1,a p lateau was observed in theπ-A isotherm,in which the area of the repeat unitwas in the approximate range 0.11-0.17 nm2.The AFM results showed that there is a clear structural transition in the PLLA film during the com pression:(i)at the beginning of the p lateau,a numberof fibrils are presentat the air/water interface and(ii)multilayer structures(at leastbilayer,i.e.,the underlying layerand top layer consisting of fibrils)is formed in the plateau region.In particular,whenπ=20.0mN·m-1,a thin film ofPLLA of thickness about6.0 nm was fabricated.Our findings suggest that the plateau in the PLLAπ-A isotherm is closely related to a change in the film structure from monolayer tomultilayerat the air/water interface.This is significantly different from the behaviorof conventionalamphiphiles,because the p lateau in amphiphilesπ-A isotherm is equivalent to a phase transition ofmonolayers derived from amphiphiles in a two-dimensionalplane.
Poly(L-lactic acid);Interface;Monomolecular film;Phase transition; Structure characteristic
O647;O641
icle]
10.3866/PKU.WHXB201504013 www.whxb.pku.edu.cn
Received:January 29,2015;Revised:April1,2015;Published onWeb:April1,2015.
∗Corresponding author.Email:qibinchen@ecust.edu.cn;Te/Fax:+86-21-64252921.
The projectwassupported by the NationalNatural Science Foundation of China(21273074)and FundamentalResearch Funds for the Central Universitiesof China.
国家自然科学基金(21273074)及中央高校基本科研业务费专项资金资助项目
©EditorialofficeofActa Physico-Chimica Sinica