羟基自由基和鸟嘌呤-胞嘧啶碱基对反应的密度泛函理论研究
2015-12-29李敏杰李重杲陆文聪
李敏杰 刁 玲 寇 莉 李重杲 陆文聪
(上海大学化学系,创新药物研究中心,上海200444)
羟基自由基和鸟嘌呤-胞嘧啶碱基对反应的密度泛函理论研究
李敏杰*刁 玲 寇 莉 李重杲 陆文聪
(上海大学化学系,创新药物研究中心,上海200444)
为了解决年龄衰老、基因突变和癌症等问题,理解DNA的氧化损伤机理非常重要.本文利用密度泛函方法和极化连续介质模型在液相条件下研究了羟基自由基夺取鸟嘌呤-胞嘧啶(GC)碱基对上5个氢原子的反应机理.研究结果表明,所有的脱氢反应路径都是放热过程,热力学上五个脱氢反应路径形成自由基的稳定性顺序是(H2b-GC)•>(GC-H4b)•>(GC-H6)•>(GC-H5)•~(H8-GC)•,其中H2b反应路径的能量变化最大,说明该反应平衡时的转化率最高.动力学上,相对于反应复合物的局部反应能垒大小顺序是H2b<H4b<H5<H6<H8,可以看出在H2b夺取路径中能垒最小,表明了该反应能在室温下迅速完成,和实验结果一致.综合考虑热力学和动力学方面的分析,发现H2b夺取反应最容易进行,次之是H4b夺取反应,然后是H6和H5.而H8的夺取反应很难发生,和实验观察到的8位加成产物现象一致.
DNA氧化损伤;羟基自由基;鸟嘌呤-胞嘧啶碱基对;反应机理;密度泛函理论
www.whxb.pku.edu.cn
1 In troduc tion
It is known that free radicals and low-energy electrons are constantly formed in living organisms due to ionization ofwater byγ-ray,X-ray,and UV radiations.1-3In particular,hydroxyl radical(•OH),w ith a very short lifetime,playsan important role in radiation-induced nucleic acid(DNA/RNA)damageby reacting w ith nucleic acid components.The damage brings about deleterious biological effects such as cancer,mutations,aging,and apoptosis by altering the DNA/RNA sequence in living organisms.4-8For this reason,the reactions of hydroxyl radicalw ith nucleic acid bases,nucleosides,and nucleotides have been extensively studied experimentally and theoretically.9-37
The reaction of nucleic acid w ith hydroxyl radicalmainly includes additionmodes on the base unitand hydrogen atom abstraction from the basesand the sugar units.Approximately half of the damage induced by OH radical(•OH)occurs on the bases among the nucleic components,11-13asgiven in chemicalequations (1)and(2).

The necleobases are the structural unitswhich carry genetic information in DNA and RNA.Muchwork has been devoted to unveil theaddition and hydrogen abstraction reactionsof hydroxyl radicalw ith the bases.11-36It iswell established that hydroxyl radicaladds to the C8,C5,or C4 positions in purine bases(adenine and guanine),and to the C5=C6 double bond in pyrimidine bases(thymine,cytosine and uracil),which give rise to the formation of radical adductsw ith oxidizing or reduction capabilities.18-30The radical adducts can undergo subsequent inter-base hydrogen rearrangement,ring-opening or intra-or inter-molecular hydrogen abstraction,or cross-link reactionsw ith otherbiomacromolecules.12,31-33
For hydrogen abstraction,Chatgilialoglu etal.13,28reported that themain abstraction of OH radicalw ith guanine is from N2 amino group using pulse radiolysisw ith optical detection and the same resultwas obtained by Mundy29and Abolfath30et al.using theoretical methods.Sevilla et al.19reported that the hydrogen abstraction pathways from N1 and N2 amino groupsof guanineare competitive route.The hydrogen abstractions from adenine by hydroxyl radical reported by Schaefer etal.11are exotherm ic.The sequenceof dehydrogenation kinetically isC6 amino group>N9>C2>C8.11To cytosine,the C4 amino group isenergeticallymore favorable than C5 and C6 for the dehydrogenation process.23,26The reaction of hydroxyl radicalw ith thym inewas studied24,26,27and the hydrogen of C5methylgroup would be themosteasily abstracted one.26,27Theabstraction from N1 ismore preferred than the N3 for uracil.25Furthermore,the propertiesof nucleobase radicalswere studied by Schaefer11and othergroups.
The canonicalWatson-Crick base pairs are guanine-cytosine (GC,Fig.1)and adenine-thymine(AT)in DNA.Thymine is replaced by uracil in RNA.Base pairsare thebuilding blocksof the DNA double helix and contribute to the folded structure of both DNA and RNA.Some efforts have beenmade to understand the structures and energetics of hydroxyl radical addition reaction w ith Watson-Crick base pairs.The structures and stabilities of dehydrogenation GC radicalsare respectively studied theoretically by Schaefer etal.37The effectsof the hydroxyl radicaladdition on double proton transfer reactions in the guanine-cytosine base pair were also studied theoretically by Zhang and Eriksson.22The proton transfer reaction isproposed to beamutationmechanism.
All of the previous theoretical predictions have contributed positively to the understanding of hydroxyl radical reactionsw ith nucleic acid bases in the DNA and RNA damage.Even though hydrogen abstractionsby hydroxyl radicalw ith GC and AT base pairs(Fig.1)may notbe known the prevailing reaction tillnow, it is important to study all theways of oxidative DNA damage caused by hydroxyl radical.
However,to the bestof our know ledge,there isno theoretical study reported so far regarding the hydrogen abstraction process. The aim of thiswork is to systematically study themechanisms of hydrogen abstraction of GC base pairby hydroxyl radical.The structures and energetics of different reaction pathways[GC+•OH→reactant complexes→transition states(TSs)→product complexes→GC dehydrogenated radicals+H2O]are investigated using the B3LYP/DZPmethod.The solvation effectsare treated using the polarization continuum model(PCM).The corresponding reaction pathways,structures,and energetics are provided,whichwould shed lighton related biochemicalexperiments.
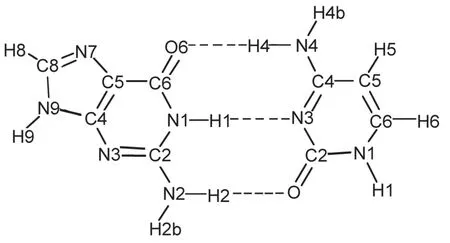
Fig.1 Schem atic of theWaston-Crick guanine-cytosine(GC) base pairw ith atom-numbering scheme
2 Com pu tationalm ethods
All calculationswere performed using the Gaussian 09 program.38Geometry optimizations and frequency calculationswere carried out using the B3LYP hybrid density functional39,40w ith double-ζquality basis setsw ith polarization and diffuse functions (DZP++).41Localminimaand transition stateswere identified by imaginary frequencies.Thermodynamic correctionswere obtained from the frequency calculations.Intrinsic reaction coordinate (IRC)calculations were performed to confirm transition states properly connecting reactantsand products.Solventeffectswere taken into consideration by employing the self-consistent reaction field(SCRF)method w ith the polarized continuum modelat the levelof M 06/DZP++theory.42The dielectric constantε=78.4was used for the aqueous solution.Correction for basis set superposition errors(BSSE)were estimated at the same level.In all the cases,the reference state is 1mol·L-1,298 K.In our previousstudies,we have theoretically exam ined the redox potentials of nucleobases and themetabolites to understand the charge/electron transfer processes involved in nucleic acids.43
The binding energies for reactant complexes[(GC)…OH]•(BE1)and for product complexes[(GC)-H…H2O]•(BE2)were predicted according to the follow ing definitionsgiven in equations (3)and(4):
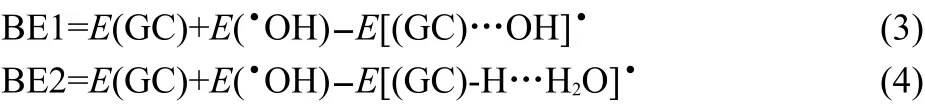
The dissociation energies for reactantcomplexes[(GC)…OH]•(DE1)and for product complexes[(GC)-H…H2O]•(DE2)were evaluated using follow ing equations:

The reaction energies(ΔE)were calculated as the differences of the energies between the reactants and products respectively given in equation(7):

The local barrier energies(ΔE≠)can be obtained as the differences of energies between the transition states and reactant complexesas depicted in equation(8).


Fig.2 Different pathways for hydroxyl radical reaction w ith GC base pair
3 Resu lts and d iscussion
Seven hydrogen atoms forGC base pair can be abstracted by hydroxyl radical.Three hydrogen atomsare attached to guanine, H2b,H8,and H9.And four hydrogen atoms are attached to cytosine,H1,H4b,H5,and H6.In the real configuration of DNA, N1 atom of the cytosine and N9 atom of guanine are always bonded to a carbon atom of the deoxyribose,instead of a hydrogen atom,so the two hydrogen abstraction reactions attacked by hydroxyl radicalare not considered here.In this study,we have examined other five hydrogen abstraction reactions resulting from hydroxyl radical attacking the GC base pair(Fig.2),and five corresponding radicals are formed,(H2b-GC)•,(H8-GC)•,(GCH4b)•,(GC-H5)•,and(GC-H6)•radicals.
3.1GC
The optimized geometries of GC base pair are presented in Fig.3.The interatom ic distances of the intermolecular hydrogen bond in GC base pairobtained in ourwork are in good agreement w ith the theoretical structures reported by Schaefer etal.44,45The interatomic distances of N1(G)…N3(C),N2(G)…O2(C),and O6(G)…N4(C)in GC base pair obtained in our work are consistentw ith the results reported by Zhang and Eriksson.22Thus the structure ofG.C base pair is reasonable in thisarticle.
Themolecular charge distribution in terms of natural bond orbital(NBO)analysis for GC is shown in Fig.4.Five H-atoms are bonded to nitrogen(N)or carbon(C)atoms.The charges are 0.418e,0.406e,0.229e,0.219e,and 0.194e for H2b,H4b,H5,H6, and H8,respectively.Obviously,the H-atomsbonded to N-atoms aremore positive charge than those bonded to C-atoms.The relativeorder isH2b>H4b>H5>H6>H8.
3.2Dehyd rogenation rad icals
The five dehydrogenation GC radicals by hydroxyl radicalare presented in Fig.5.As to the conformationsof the radicals,the coplaner configuration is not changed.The bond lengths of intermolecularhydrogen bondswere found to beelongated in nitrogen centered radicals(H2b-GC)•and(GC-H4b)•.No obvious deformations are found for bond lengths of hydrogen bonds in carbon centered radicals(H8-GC)•,(GC-H5)•,and(GC-H6)•. The changes forbond lengthsof thehydrogen bonds in nitrogen centered radicalsmay result in DNA lesions,since the intermolecularhydrogen bondsare responsible for the GC base pair.
The relative energiesof GC dehydrogenation radicals respected to GC base pairare summarized in Table1.The(H2b-GC)•and (GC-H4b)•radicals are produced by the hydrogen abstractiondifferently from the exocyclic amino group of guanine and cytosine.The(H2b-GC)•radical is themost stable radical.The followed is the(GC-H4b)•radical.The(GC-H5)•radical,the mostunstable one,ishigher than the(H2b-GC)•radical in energy by 66.52 kJ·mol-1.The energies of the carbon centered radicals ((GC-H5)•,(GC-H6)•,and(H8-GC)•)arehigher than thoseofnitrogen centered radicals((H2b-GC)•)and(GC-H4b)•).The stability orderof these radicalsis(H2b-GC)•>(GC-H4b)•>(GC-H6)•>(H8-GC)•>(GC-H5)•.These resultsare in good agreementw ith those obtained by Bera and Schaefer.46The sequence isnotaltered when considering the zero pointenergy(ZPE)corrections.According to our calculations,H2b may be easier abstracted by hydroxyl radical than H4b,H5,H6,and H8.

Fig.3 Optim ized geom etriesof GC base pair
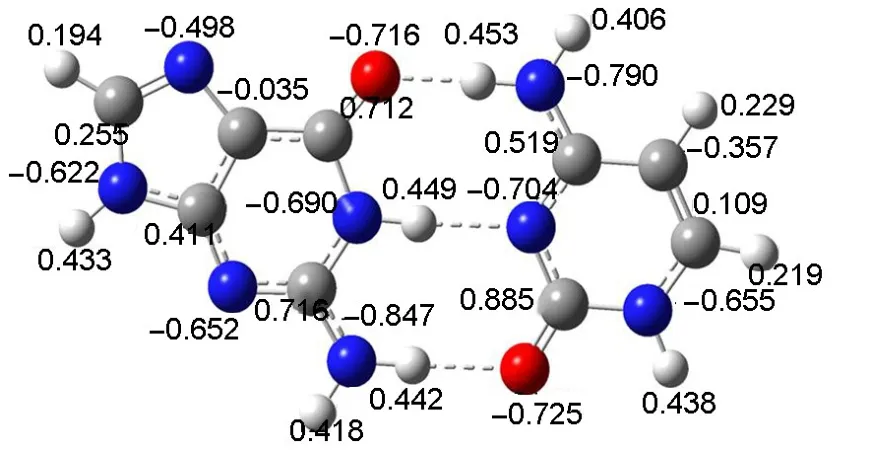
Fig.4 M olecu lar charge distribution in term sof NPA charges(e)
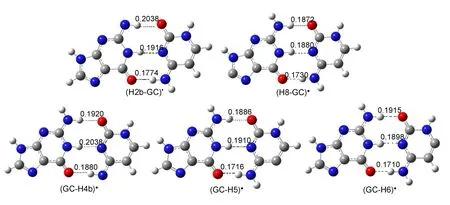
Fig.5 Op tim ized geometriesof GC dehyd rogenation radicals

Tab le1 Relativeenergies respected to GC base pair of theGC radicals
3.3 H2b-GC abstrac tion
H2b atom may be the easiest attacked by hydroxyl radical, since the atom is themostpositive charged hydrogen atom.The optimized geometries of the reactant complex,TS,and product complex for the reaction pathway of the OH radicalattacking H2b are shown in Fig.6.The OH radicalattaches to guanine at the N3 and H2b through two hydrogen bonds in reactantcomplex 1,with the distancesof 0.1780 and 0.2431 nm for N3…H and O…H2b, respectively.As the dehydrogenation reaction proceeds to TS1,the O…H2b distance decreases to 0.1404 nm,while the N2…H2b bond lengthens from 0.1014 to 0.1106 nm.When productcomplex 1 is formed,theO…H2b distance further decreases to 0.0983 nm, while the N2…H2b bond lengthens to 0.01955 nm.
For this pathway,the relative energies in aqueous solution are also shown in Fig.6.Reactantcomplex 1 hasa binding energy of 22.34 kJ·mol-1relative to separated reactantsGC plus•OH.The localenergy barrierof reaction from reactantcomplex 1 to TS1 is predicted to be 1.09 kJ·mol-1,itseems that the reaction for H2b abstraction in kinetics view would be rapid.A fter the H2b is abstracted,the productcomplex 1 is formed by thehydrogen bond (H2b…N2)of the resultingwaterand the new ly formed N2-GC radical.Theenergy of productcomplex is100.37 kJ·mol-1lower than thatof separated reactants.Compared to the reactant complex,the productcomplex is78.03 kJ·mol-1lower in energy.The corresponding dissociation energy for productcomplex 1 to N2-GC radicaland H2O is9.79 kJ·mol-1.Meanwhile,the separated N2-GC radical plus H2O lies 90.58 kJ·mol-1below separated reactants.
3.4 H8-GC abstrac tion
The hydrogen abstraction process from C8 atom isalso studied.The optim ized geometries and relative energies are displayed in Fig.7.Reactant complex 2 is a hydroxyl radical adduct characterized by experiments,12instead of hydrogen bond interaction complex in other reaction pathways.The conjugation of the entire system is disrupted and N7=C8 double bond(0.1310 nm)is broken to N7―C8 bond(0.1452 nm).The distance of C8―O bond is 0.1414 nm.As the reaction progresses,the C8―O bond breaksand H8m igrates away from C8,the C8…H bond(0.1304 nm)and H8…O hydrogen bond(0.1197 nm)form in TS2.In product complex 2,the water and the C8-GC radical are held together through O6…H and N7…H hydrogen bonds w ith the distancesof 0.1984 and 0.2347 nm,respectively.

Fig.6 Op tim ized geom etriesand energy surfaces for the reaction of hydroxyl radicalattacking on H 2b of GC

Fig.7 Op tim ized geometriesand relativeenergies for the hydroxyl radicalattacking on H8 of GC
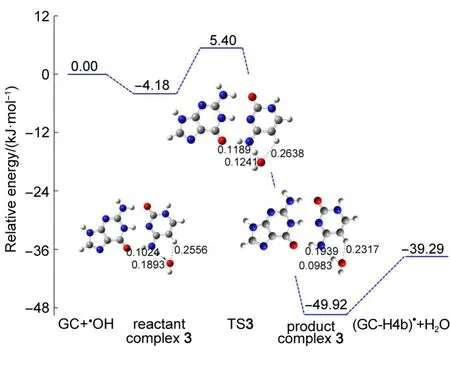
Fig.8 Optim ized geom etriesand relativeenergies for the hydroxyl radicalattacking on H4 ofGC
The energy of reactantcomplex 2 is136.57 kJ·mol-1lower than thatof separated GC plus•OH.Itis themoststable one among all the reactant complexes.The local barrier ismuch higher than thoseofother reaction pathwaysw ith the valueof153.14 kJ·mol-1. TS2 lies16.57 kJ·mol-1above separated reactants in energy.The product complex lies101.63 kJ·mol-1above the corresponding reactantcomplex.The dissociation energy for product complex 2 is 18.20 kJ·mol-1relative to C8-GC radical pluswater.The H8 abstraction process(GC+•OH→C8-GC•+H2O)isexothermic by ca 16.74 kJ·mol-1.
3.5 GC-H4b abstrac tion
H4b atom is the secondedmost positively charged hydrogen atom,itmay be also easily abstracted by OH radical.In order to attack H4b atom,the OH radical binds to cytosine by two hydrogen bonds.As shown in Fig.8,the one is H4b hydrogenbonded to OH radical,the other is H5 linked to oxygen atom of OH radical.As the reaction progresses,thehydrogen bond lengths of O…H4b decrease from 0.1893 nm in reactant complex 3,to 0.1241 nm in TS3,then to 0.0983 nm in product complex 3. Meanwhile,the N4…H4b gradually increases along the pathway, from 0.1024 nm in reactantcomplex 3,to 0.1189 nm in TS3,to 0.1939 nm in product complex 3.The H5…O hydrogen bond is formed in productcomplex 3 and the length is0.2317 nm.
The binding energy between the GC base pairand the attacking OH radical is predicted to be4.18 kJ·mol-1,see Fig.8.There isa localenergy barrier from reactantcomplex 3 to TS3with the value of9.58 kJ·mol-1.Theenergy of TS3 is5.40 kJ·mol-1higher than that of the separated reactants.The H5…O and N4…H4b hydrogen bonds are formed in the product complex and the dissociation energy ispredicted to be10.63 kJ·mol-1.Theenergy of the productcomplex is45.74 kJ·mol-1lower than thatof the reactant complex.The H4b abstraction process is exothermic by 39.29 kJ· mol-1.
3.6 GC-H5 abstrac tion
Thehydrogen abstraction reaction by OH radical from C5 site to form GC-C5 radicalwas also studied.Fig.9 shows the optimized geometries of reactant complex 4,TS4,and productcomplex 4 of the reaction pathway.The OH radicalbinds to GC at the H4b and H5 positions through two hydrogen bonds to form the reactantcomplex 4.TheO…H5 distance is0.2556 nm,a long hydrogen bond,while the O…H4b distance is0.1893 nm.A long the reaction pathway,the O…H5 bond decreases to 0.1195 nm in the TS4,and further shortens to 0.0969 nm in the product complex,and in themeantime the C5―H5 bond length increases from 0.1085 nm in the reactant complex,to 0.1195 nm in TS4,and finally the bond cleaves in the product complex.The O…H4b bond cleaves in TS4 and formsw ith the distance of 0.1987 nm in productcomplex 4.
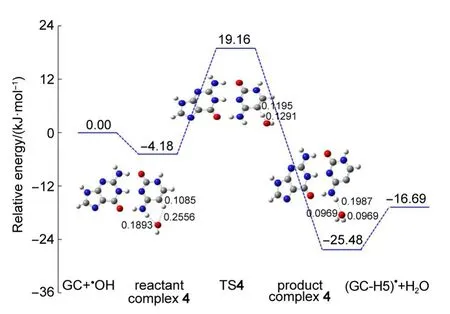
Fig.9 Optim ized geometriesand relativeenergies for the hyd roxyl radicalattacking on H 5 of GC
The stability energy of the reactant complex by two hydrogen bonds is computed to be4.18 kJ·mol-1compared to separated GC plushydroxyl radical(Fig.9).The localactivation energy is23.34 kJ·mol-1and theenergy of TS4 is19.16 kJ·mol-1above separated reactants.The corresponding dissociation energy is calculated to be8.79 kJ·mol-1relative to GC-C5 radicalpluswater.Theproduct complex and the separated products lie above the corresponding reactantcomplex and separated reactantsby 21.30 and 16.69 kJ· mol-1in energy,respectively.
3.7 GC-H6 abstrac tion
The abstraction process of H6 involves reactant complex 5, TS5,and productcomplex 5(Fig.10).OH radicalbinds to cytosine through two bonds.The one is the H1-bonded to the attacking OH radical(O…H1 distance is0.2053 nm),the other isO2 linked to hydrogen atom of OH radical(O2…H length is0.1745 nm).As the reaction progresses,the two hydrogen bonds cleave and the new hydrogen bond of O…H6(0.1217 nm)forms in TS5,the O2…H(0.1872 nm)and O…H1(0.1860 nm)bonds form in product complex 5,and in themeantime the C6…H6 bond increases from 0.1088 nm in reactantcomplex 5 to 0.1278 nm in the TS5,and further cleaves in productcomplex 5.
The binding energy for reactant complex 5 is 19.87 kJ·mol-1compared to the separated GC plus hydroxyl radical.The local activation energy(reactant complex 5→TS5)is 39.49 kJ·mol-1. This localbarrier ishigher than those for theabstraction processes of H2b,H4b,and H5,though it ismuch lower than the H8 abstraction barrier.The TS5 lies19.62 kJ·mol-1above the separated reactants in energy.The formation of O2…H and O…H1 bonds stabilizes productcomplex 5,and the corresponding dissociation energy ispredicted to be18.37 kJ·mol-1.Theenergy lies28.12 kJ· mol-1below thatof reactantcomplex 5.The abstraction process isexothermic by ca29.62 kJ·mol-1.

Fig.10 Optim ized geometriesand relativeenergies for the hyd roxyl radicalattacking on H 6 of GC
3.8 Energitics
The energy profiles along the five reaction pathway[GC+•OH→reactantcomplexes→TS→productcomplexes→(GC-H)•+ H2O]are shown in Fig.11.The reaction energiesand localbarrier energies in aqueous solution are listed in Table 2.Thermodynamically,all thehydrogen abstraction processesare exothermic. The H2b abstraction processhas the highest reaction energiesw ith the data of 90.58 kJ·mol-1.The reaction energies forH4b,H5,H6, and H8 abstraction processes are 39.29,16.69,29.62,16.74 kJ· mol-1,respectively.According to our calculations,the abstraction order is H2b>H4b>H6>H5~H8 from a thermodynamic view.The H2b and H4b would be themore thermodynam ically favorableabstraction sites than H6,H5,and H8.In addition,the localbarrier of H2b abstraction process is the lowest in all the studied pathwayswith the valueof 1.09 kJ·mol-1.Theother localbarriersare 9.58,23.34,39.49,153.14 kJ·mol-1for H4b,H5,H6,H8 abstraction processes,respectively.In kinetics,the abstraction order is H2b>H4b>H5>H6>H8,which is consistentwith the theoretical results reported by Yadav and M ishra.26Both in thermodynamics and in kinetics,H2b abstraction process is themost favorable reaction pathway among all the hydrogen abstraction reactions and it is followed by H4b abstraction pathway.Themain path of hydrogen abstraction is from the exocyclic NH2of guanine initiated by OH radical also reported experimentally by Chatgilialoglu etal.13,28Xia etal.23also reported thatH4b site is more abstracted than H5 site of cytosine by OH radical thermodynamically and kinetically.In comparison w ith other sites,H8 abstraction process has the least reaction probably,which is consistentwith experimentally observed hydroxylation adduct,12nothydrogen abstraction radical.
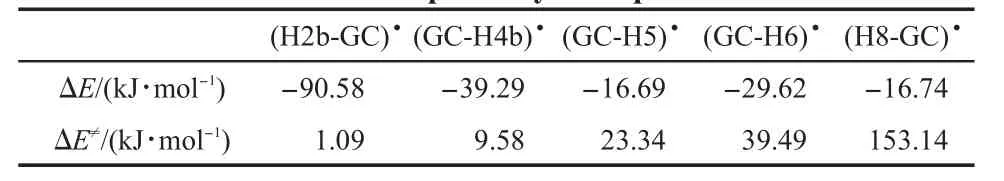
Tab le2 Reaction energies(ΔE)and localbarrier energies(ΔE≠) for fiveabstraction pathways in aqueoussolution
4 Conc lusions
In thiswork,we have studied five possible hydrogen abstraction reactions by hydroxyl radical in GC base pair in aqueous solution at theM 06/DZP++//B3LYP/DZP++levelof theory.All the reactions in GC base pair are thermodynamically exothermic. The stability of dehydrogenation radical is in the sequence:(H2b-GC)•>(GC-H4b)•>(GC-H6)•>(GC-H5)•~(H8-GC)•,the reaction energy in H2b reaction process is the lowest compared w ith the other pathways,indicated that reaction conversion of(H2b-GC)•would be the highest.In kinetics,the localbarrier energy in the abstraction pathways is in the sequence of H2b<H4b<H5<H6<H8, observing that thebarrierenergy in H2b abstraction process is the lowest among all the discussed processes,suggested that this pathway in kinetics view would be themost rapid.H2b abstraction processwould be themost favorable reaction pathway,and the compatible pathway is H4b abstraction and it is followed by H5 and H6 abstraction pathways.In comparisonw ith other sites, H8 abstraction process is probably themost impossible reaction pathway,which is consistentw ith experimentally observed hydroxylation adduct.Moreover,the NBO charge analysis further verifies thatH2b and H4b atomsw ithmore positive charges can bemore easily attacked by hydroxyl radical,while H8w ith the mostnegative charges is themostdifficultattacked by hydroxyl radical.This study assists further research for a better interpretation of the dehydrogenationmechanism in DNA double helix.
(1)Bao,S.D.;Wu,Q.L.;M cLendon,R.E.;Hao,Y.L.;Shi,Q.; Hjelmeland,A.B.;Dewhirst,M.W.;Bigner,D.D.;Rich,J.N. Nature 2006,444,756.doi:10.1038/nature05236
(2)O'Donovan,P.;Perrett,C.M.;Zhang,X.H.;Montaner,B.;Xu, Y.Z.;Harwood,C.A.;M cGregor,J.M.;Walker,S.L.; Hanaoka,F.;Karran,P.Science 2005,309,1871.doi:10.1126/ science.1114233
(3)Shukla,L.I.;Adhikary,A.;Pazdro,R.;Becker,D.;Sevilla,M. D.Nucleic AcidsRes.2004,32,6565.doi:10.1093/nar/gkh989
(4)Steenken,S.Chem.Rev.1989,89,503.doi:10.1021/ cr00093a003
(5)von Sonntag,C.Free-Radical-Induced DNADamage and Its Repair;Springer Verlag:Berlin,2006.
(6)Dizdaroglu,M.;Jaruga,P.Free RadicalRes.2012,46,382.doi: 10.3109/10715762.2011.653969
(7)Antonchenko,V.Y.;Kryachko,E.J.J.Phys.Chem.A 2005, 109,3052.
(8)D'Souza,J.S.;Dharmadhikari,J.A.;Dharmadhikari,A.K.; Rao,B.J.;Mathur,D.Phys.Rev.Lett.2011,106,118101.doi: 10.1103/PhysRevLett.106.118101
(9)Zhang,R.B.;Eriksson,L.A.Chem.-Eur.J.2009,15,2394. doi:10.1002/chem.v15:10
(10)Kanvah,S.;Joseph,J.;Schuster,G.B.;Barnett,R.N.;Clevland, C.L.;Landman,U.AccountsChem.Res.2010,43,280.doi: 10.1021/ar900175a
(11)Cheng,Q.;Gu,J.;Compaan,K.R.;Schaefer,H.F.Chem.-Eur. J.2010,16,11848.doi:10.1002/chem.201001236
(12)Candeias,L.P.;Steenken,S.Chem.-Eur.J.2000,6,475.
(13)Chatgilialoglu,C.;D'Angelantonio,M.;Kciuk,G.;Bobrowski, K.Chem.Res.Toxicol.2011,24,2200.doi:10.1021/tx2003245
(14)Frances-Monerris,A.;Manuela,M.;Roca-Sanjuan,D.J.Chem. Phys.2013,139,071101.doi:10.1063/1.4818727
(15)Tan,R.;Wang,D.;Hu,L.;Zhang.F.S.Int.J.Quantum Chem. 2014,114,367.doi:10.1002/qua.24567
(16)Wagner,J.R.;Cadet,J.AccountsChem.Res.2010,43,564.doi: 10.1021/ar9002637
(17)Agnibotri,N.;M ishra,P.C.Chem.Phys.Lett.2011,503, 305.doi:10.1016/j.cplett.2011.01.042
(18)Llano,J.;Eriksson,L.A.Phys.Chem.Chem.Phys.2004,6, 4707.doi:10.1039/b410922h
(19)Kumar,A.;Pottiboyina,V.;Sevilla,M.D.J.Phys.Chem.B 2011,115,15129.doi:10.1021/jp208841q
(20)Jena,N.R.;M ishra,P.C.J.Phys.Chem.B.2005,109,14205. doi:10.1021/jp050646j
(21)Catterall,H.;Davies,M.J.;Gilbert,B.C.;Polack,N.P. J.Chem.Soc.Perk.Trans.2 1993,2039.
(22)Zhang,R.B.;Eriksson,L.A.J.Phys.Chem.B 2007,111, 6571.doi:10.1021/jp071772l
(23)Ji,Y.J.;Xia,Y.Y.;Zhao,M.W.;Huang,B.;Li,F.J.Mol. Struct.-Theochem 2005,723,123.doi:10.1016/j. theochem.2005.02.039
(24)Ji,Y.J.;Xia,Y.Y.;Zhao,M.W.;Li,F.;Huang,B.Int.J.Quantum Chem.2005,101,211.
(25)Prasanthkumar,K.P.;Suresh,C.H.;Aravindakumar,C.T. Radiat.Phys.Chem.2012,81,267.doi:10.1016/j. radphyschem.2011.11.001
(26)Yadav,A.;M ishra,P.C.Int.J.Quantum Chem.2013,113, 56.doi:10.1002/qua.24050
(27)Wu,Y.;Mundy,C.J.;Colvin,M.E.;Car,R.J.Phys.Chem.A 2004,108,2922.doi:10.1021/jp0363592
(28)Chatgilialoglu,C.;D'Angelantonio,M.;Guerra,M.;Kaloudis, P.;Mulazzani,Q.G.Angew.Chem.Int.Edit.2009,48,2214. doi:10.1002/anie.v48:12
(29)Mundy,C.J.;Colvin,M.E.;Quong,A.A.J.Phys.Chem.A 2002,106,10063.doi:10.1021/jp0212904
(30)Abolfath,R.M.;Biswas,P.K.;Rajnarayanam,R.;Brabec,T.; Kodym,R.;Papiez,L.J.Phys.Chem.A 2012,116,3940.doi: 10.1021/jp300258n
(31)Vieira,A.J.S.C.;Steenken,S.J.Phys.Chem.1991,95, 9340.doi:10.1021/j100176a056
(32)Ceron-Carrasco,J.P.;Jacquem in.D.RSCAdvances2012,2, 11867.doi:10.1039/c2ra22389a
(33)Pottiboyina,V.;Kumar,A.;Sevilla M.D.J.Phys.Chem.B 2011,115,15090.doi:10.1021/jp207873a
(34)Chaban,G.M.;Wang,D.;Huo,W.M.J.Phys.Chem.A 2015, 119,377.doi:10.1021/jp508771g.
(35)Gao,Y.;Chen,X.;Zhong,L.;Yao,W.;Li.S.Org.Biomol. Chem.2014,12,5891.doi:10.1039/C4OB00168K
(36)Frances-Monerris,A.;Manuela,M.;Roca-Sanjuan,D.J.Phys. Chem.B 2014,118,2932.doi:10.1021/jp412347k
(37)Lind,M.C.;Richardson,N.A.;Wheeler,S.E.;Schaefer,H.F. J.Phys.Chem.B 2007,111,5525.doi:10.1021/jp0714926
(38)Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;etal.Gaussian 09, Revision A.01;Gaussian Inc.:Wallingford,CT,2009.
(39)Becke,A.D.J.Chem.Phys.1993,98,5648.doi:10.1063/ 1.464913
(40)Becke,A.D.Phys.Rev.A 1988,38,3098.doi:10.1103/ PhysRevA.38.3098
(41)Rienstra-Kiracofe,J.C.;Tschumper,G.S.;Schaefer,H.F.; Nandi,S.;Ellison,G.B.Chem.Rev.2002,102,231.doi: 10.1021/cr990044u
(42)Cadet,J.;Douki,T.;Ravanat,J.L.AccountsChem.Res.2008, 41,1075.doi:10.1021/ar700245e
(43)Li,M.J.;Liu,W.X.;Peng,C.R.;Lu,W.C.Acta Phys.-Chim. Sin.2011,27(3),595.[李敏杰,刘卫霞,彭淳荣,陆文聪.物理化学学报,2011,27(3),595.]doi:10.3866/PKU. WHXB20110333
(44)Gupta,A.;Jaeger,H.M.;Compaan,K.R.;Schaefer,H.F. J.Phys.Chem.B 2012,116,5579.doi:10.1021/jp211608b
(45)Zhang,J.D.;Schaefer,H.F.J.Chem.Theor.Comput.2006,3, 115.
(46)Bera,P.P.;Schaefer,H.F.Proc.Natl.Acad.Sci.U.S.A.2005, 102,6698.doi:10.1073/pnas.0408644102
Hyd roxy lRad icalReac tion w ith the Guanine-Cytosine Base Pair: A Density Func tional Theo ry Study
LIMin-Jie*DIAO Ling KOU Li LIZhong-Gao LUWen-Cong
(Innovative Drug Research Center,DepartmentofChemistry,ShanghaiUniversity,Shanghai200444,P.R.China)
To address problems such as aging,mutation,and cancer,it is ofgreat im portance to understand the damagemechanism ofDNA induced by hydroxyl radical.In this study,the abstraction reactionmechanism ofhydroxyl radicalwith guanine-cytosine(GC)base pair in aqueous phase under the polarized continuum model (PCM)has been explored by using density functional theory(DFT).The results indicated thatall the abstraction reactions in GC base pairwere thermodynam ically exotherm ic,and the stability of dehydrogenation radicals decreased in the orderof(H2b-GC)•>(GC-H4b)•>(GC-H6)•>(GC-H5)•~(H8-GC)•.The reaction energy ofH2b abstraction pathway was the lowest among all investigated pathways,thus indicating that the reaction conversion of(H2b-GC)•was the highest.In the five hydrogen abstraction pathways,the localenergy barriers w ith respect to the corresponding reactantcomp lexes increased in the follow ing order:H2b<H4b<H5<H6<H8, thereby suggesting that the H2b abstraction pathway was themost rapid.Thus,H2b abstraction process was themost likely favorable reaction pathway.Another compatible pathwaywould be the H4b abstraction,followed by H6 and H5 abstraction pathways in thermodynam ics and in kinetics.H8 abstraction processwas the least favorable pathway,as consistentw ith the formation ofhydroxylation adductobserved experimentally rather than the hydrogen abstraction radical.
DNAoxidative damage;Hydroxyl radical;Guanine-cytosine base pair; Reactionmechanism;Density functionaltheory
O641 [Communication]
10.3866/PKU.WHXB201504171
Received:February 9,2015;Revised:April17,2015;Published onWeb:April17,2015.
∗Corresponding author.Email:minjieli@shu.edu.cn;Tel:+86-10-66133513.
The projectwassupported by the NationalNatural Science Foundation of China(21273145).
国家自然科学基金(21273145)资助项目
©Editorialofficeof Acta Physico-Chim ica Sinica