CHD8基因新发突变的孤独症谱系障碍1例报道并文献复习
2015-04-20郑清文詹国栋赵薇薇邹小兵
郑清文 詹国栋 唐 春 毕 欣 赵薇薇 邹小兵
·论著·
CHD8基因新发突变的孤独症谱系障碍1例报道并文献复习
郑清文1詹国栋1唐 春1毕 欣2赵薇薇2邹小兵1
目的 提高对孤独症谱系障碍(ASD)CHD8基因突变患儿临床表型和基因型的认识。方法回顾性分析1例存在CHD8基因突变的ASD患儿的临床资料,并文献复习。结果①男,3岁3月龄,头围55.0 cm(>4s),身高107.0 cm(>2s),体重23.5 kg(>2s)。有典型的ASD表现,运动明显落后,有慢性便秘史。②提取患儿及其父母静脉血DNA,以二代高通量测序技术对4 813个临床相关基因的外显子区进行测序,共检测到变异8 982个,经筛选流程筛选ASD相关的EHMT1、PCDH9、NLGN4X的错义突变,CHD8的无义突变(c.307C>T, p.Gln103*)有致病可能。Sanger测序显示患儿EHMT1、PCDH9的突变来源于父亲,NLGN4X的突变来源于母亲,患儿父母未发现CHD8的突变。③目前国外共报道了CHD8基因与神经发育障碍有关的15个重要突变;CHD8基因突变患者的临床表型包括ASD表现(87%)、身材高大(86%)、大头畸形(80%)、慢性便秘或间歇性腹泻(80%)、运动落后(67%)和睡眠异常(67%)等,本文病例临床表型与文献报道较为一致。结论CHD8基因是ASD重要的易感基因;ASD患儿合并大头畸形、生长过快,且有慢性便秘或间歇性腹泻时应考虑CHD8基因突变可能,可行基因检测协助诊断。
孤独症谱系障碍;CHD8基因; 大头畸形; 生长过快; 二代测序技术
1 病例资料

母亲孕早期出现阴道流血,服中药及卧床休息后流血停止;定期产检,未见异常,无妊娠期发热,否认猫、狗、放射线等不良接触史。否认家族中有神经精神发育异常者。
查体:身高107.0 cm(>2s),体重23.5 kg(>2s),头围55.0 cm(>4s)。前额宽且突出。心音有力,律齐,未闻及病理性杂音;腹软,未扪及包块,未见脐疝、腹股沟疝;脊柱、指/趾端未见畸形;手掌皮纹未见异常。
实验室检查:肝功能、血脂、甲状腺功能、血浆皮质醇(上午8时)、胰岛素样生长因子-1未见异常;左手X线正位片示:过度生长,骨龄发育正常(图1)。睡眠脑电图、头颅MRI、听力检查、腹部和心脏超声未见异常。FMR1基因CGG重复次数正常;染色体基因芯片分析(CytoScan HD芯片,Affymetrix,USA)未发现致病性拷贝数变异(CNVs)。
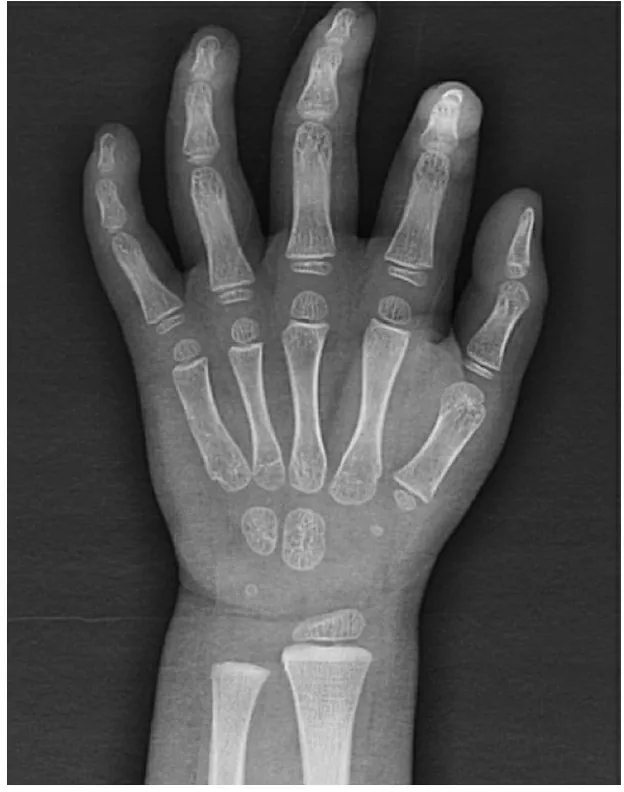
图1 患儿左手X线正位片
Fig 1 Anterioposterior radiograph of the patient′s left hand
Notes Overgrowth but normal bone age
孤独症诊断会谈问卷(ADI-R):社会互动异常15分(截止分10分),沟通异常17分(截止分8分),局限、重复、刻板的行为模式3分(截止分3分),36月龄前发展异常的迹象3分(截止分1分)。孤独症诊断观察量表(ADOS):沟通7分[孤独症截止分4分,孤独症谱系障碍(ASD)截止分2分],相互性社会互动7分(孤独症截止分7分,ASD截止分4分),游戏1分,刻板行为和局限兴趣2分。Gesell发育评估:粗动作发育龄30月龄,发育商76;细动作发育龄22.5月龄,发育商57;应物能发育龄25月龄,发育商64;言语能23月龄,发育商59;应人能30月龄,发育商76。诊断为ASD。
经患儿家长同意和我院伦理委员会审核,采用EDTA抗凝管抽取患儿及其父母静脉血各2 mL,并抽提基因组DNA(QIAamp DNA提取试剂盒,QIAGEN公司)。提取的DNA用DNA酶片段化后用磁珠法纯化,随后进行PCR扩增并连接上接头序列,经TruSight One Sequencing Panel(llumina Inc,美国)2次捕获及纯化,再经PCR扩增和纯化,获得的最终文库在MiSeq测序仪(illumina Inc,USA)上进行测序。其中TruSight One Sequencing Panel根据人类基因突变数据库(HGMD Professional)、在线人类孟德尔遗传数据库(OMIM)、GeneTests网站 (www.genetests.org)、Illumina等公司的其他商业化试剂盒的信息,纳入了4 813个与已知临床表型相关的基因,其中包含102个ASD相关基因(http://www.illumina.com/products/trusight-one-sequencing-panel.ilmn)。
实验产生1.0 Gb数据,平均测序深度为114.8×,10×以上的捕获区覆盖率达98.10%,平均插入片段大小237 bp,共检测到变异8 982个。经筛选流程筛选(图2),并结合患儿临床资料和生物信息学软件预测结果,对各个基因的功能、变异情况以及遗传模式进行分析发现,ASD相关的EHMT1、PCDH9、NLGN4X的错义突变、CHD8的无义突变(c.307C>T, p.Gln103*)有致病可能(表1)。图3为二代高通量测序发现患儿CHD8基因存在突变。
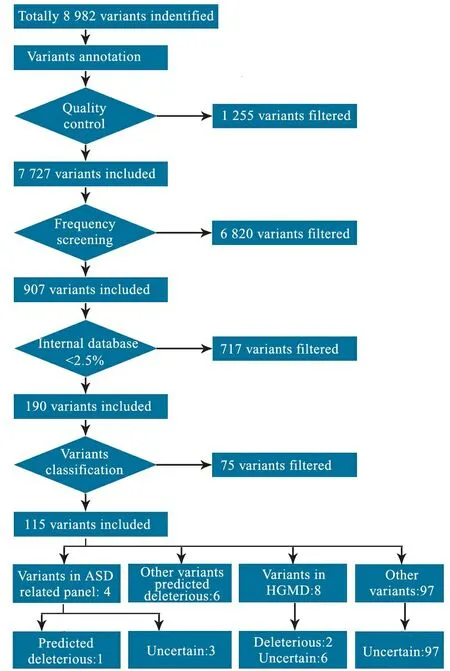
图2 高通量数据筛选流程图
Fig 2 The filtering process of high-throughput sequencing data in this study
Notes Internal database of KingMed Center for Clinical Laboratory Co., Ltd.
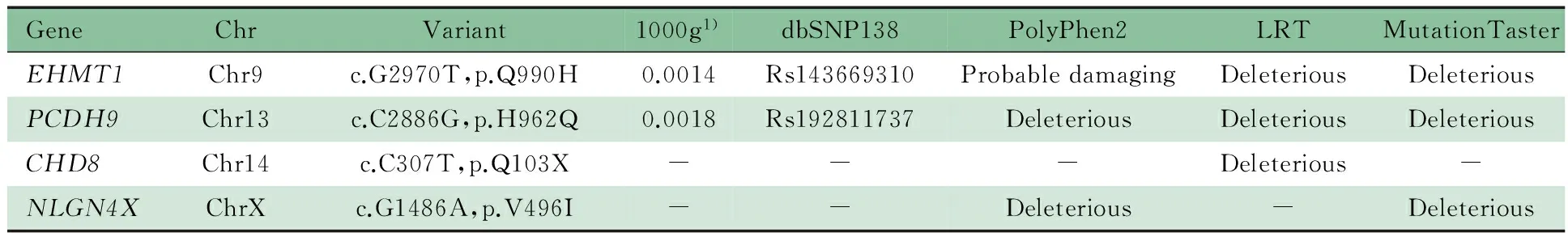
表1 本文病例ASD相关基因候选变异总结Tab 1 Summary of candidate causative variants in ASD related genes in this patient
Notes 1)1000 Genome Project

图3 本文病例二代高通量测序发现CHD8基因存在突变
Fig 3CHD8 mutation was identified via next-generation sequencing
Sanger测序的PCR引物序列及退火温度如表2所示。该家系Sanger测序结果(图4)显示,患儿EHMT1、PCDH9的突变来源于父亲,NLGN4X的突变来源于母亲。双亲未发现CHD8的突变,患儿携带的可能为新发突变,不能除外其父母为性腺嵌合体的可能。
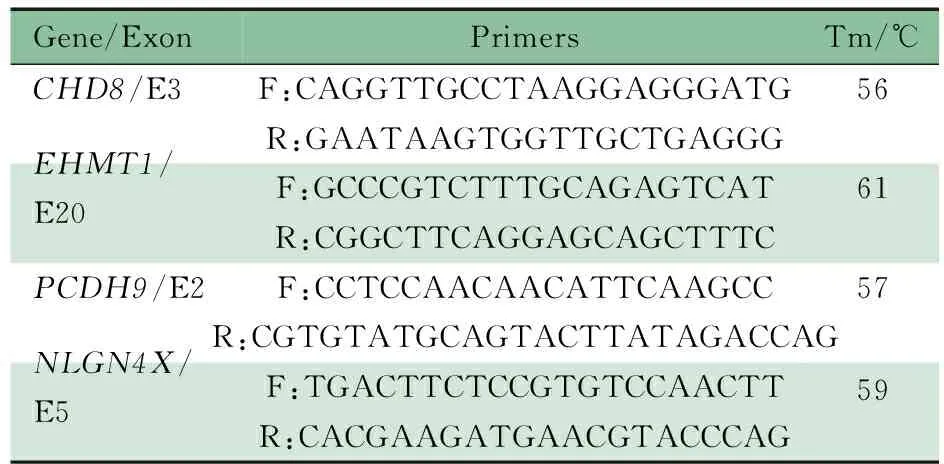
表2 本文病例可疑致病基因外显子编码区引物序列及退火温度Tab 2 PCR primers and Tm used in this study
2 文献复习
以“((“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields]) OR (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autistic”[All Fields])) AND CHD8[All
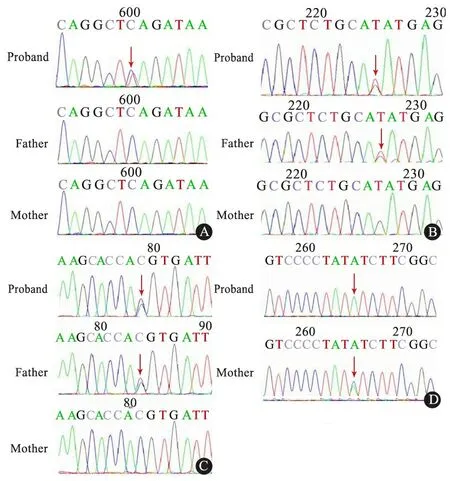
图4 本文病例及其父母可疑致病基因突变位点Sanger测序图
Fig 4 Sanger sequencing validation of candidate causative variants in this family
Notes A:CHD8,c.307C>T, p.Gln103* was found in proband; B:EHM1, c.2970G>7,9.Gln990His were found in proband and his father; C:PCDH9, c.2886C>G,p.His962Gln were found in proband and his father; D:NLGN4X, c.1486G>A, p.Val496Ile were found in proband and his mother
Fields]”为检索式检索PubMed数据库,以检索词“CHD8”与“孤独症”检索万方数据库和中国知网,检索时间均为建库至2015年1月31日,共检索到英文文献13篇、中文文献9篇;排除其中不相关文献10篇,12篇文献中关于“CHD8基因突变与ASD”的临床相关研究3篇。
Bernier等[1]通过对3 730例临床诊断为发育迟缓(DD)或ASD的患者进行CHD8基因测序,并结合O′Roak的研究小组前期外显子测序(209例ASD患者)[2]和靶基因检测(2 446例ASD患者)[3]的结果,汇总了CHD8已知的与神经发育障碍有关的15个重要突变(表3),而在累计近9 000名正常对照中未发现CHD8基因的截断突变(包括无义突变、错义突变、移码突变、剪接突变、整码突变等)。本文患儿的CHD8基因无义突变c.307C>T, p.Gln103*是新发现的突变,为首次报道。对既往Bernier等[1]和O′Roak等[2,3]已报道的15例CHD8基因突变患者的临床表型进行总结,结果如表4所示。本文患儿大头畸形、前额突出,生长较同龄儿童快,有典型的ASD表现,语言、运动和智力落后于同龄儿童,且有慢性便秘病史,其临床表型与表4描述的临床表型较为一致。
表3 已知与神经发育障碍有关的CHD8基因重要突变
Tab 3Summary of CHD8 mutations associated with neurodevelopmental disorders (from 5′ to 3′)
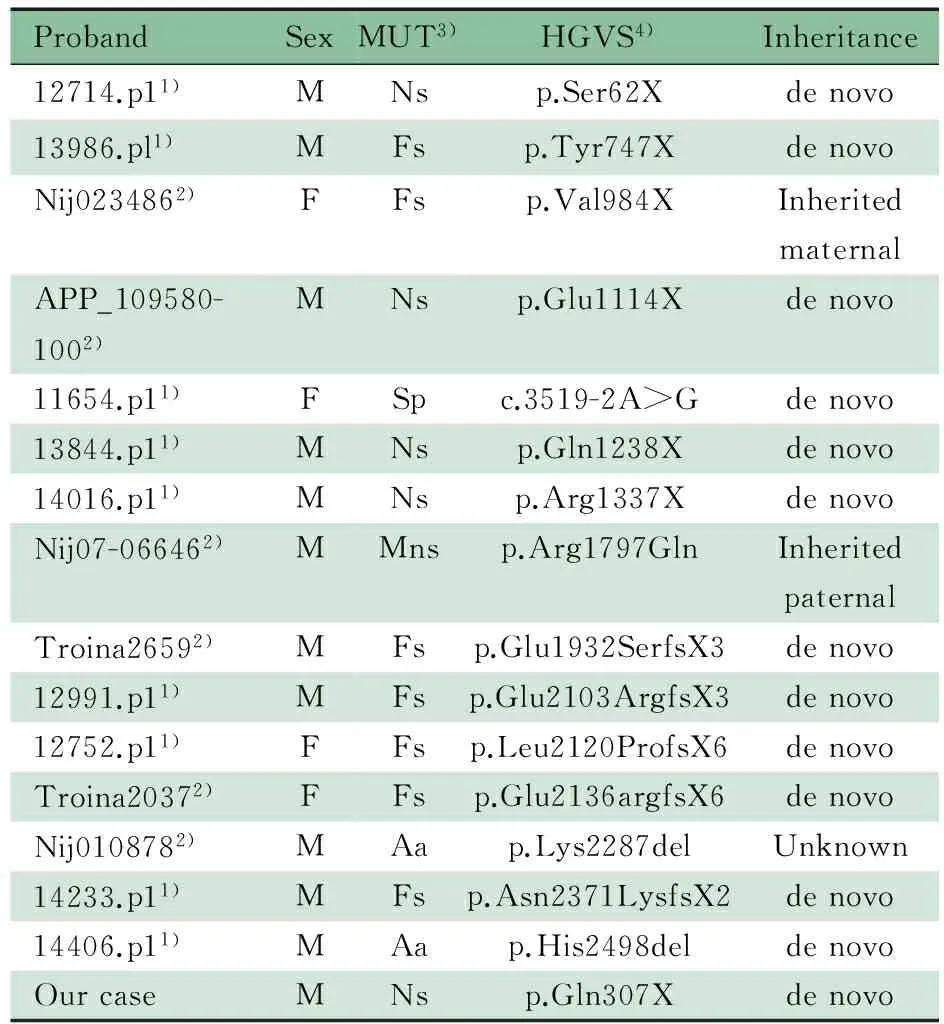
ProbandSexMUT3)HGVS4)Inheritance12714.p11)MNsp.Ser62Xdenovo13986.pl1)MFsp.Tyr747XdenovoNij0234862)FFsp.Val984XInheritedmaternalAPP_109580-1002)MNsp.Glu1114Xdenovo11654.p11)FSpc.3519-2A>Gdenovo13844.p11)MNsp.Gln1238Xdenovo14016.p11)MNsp.Arg1337XdenovoNij07-066462)MMnsp.Arg1797GlnInheritedpaternalTroina26592)MFsp.Glu1932SerfsX3denovo12991.p11)MFsp.Glu2103ArgfsX3denovo12752.p11)FFsp.Leu2120ProfsX6denovoTroina20372)FFsp.Glu2136argfsX6denovoNij0108782)MAap.Lys2287delUnknown14233.p11)MFsp.Asn2371LysfsX2denovo14406.p11)MAap.His2498deldenovoOurcaseMNsp.Gln307Xdenovo
Notes MUT:mutation. 1) Patient′s mutation was previously reported by O′Roak et al[2,3];2) Patient′s mutation was previously reported by Bernier et al[1];3) Ns: nonsense; Fs: Frameshift; Sp: splice; Mns: missense-near-splice site; Aa: single amino-acid deletion;4) HGVS: human genome variant sequence
3 讨论
ASD的临床表型和基因型十分复杂,迄今确切发病机制尚不清楚,但已明确遗传因素在其中发挥重要的作用。
表4 CHD8突变患者的临床表型[%(n/N)]
Tab 4 Brief description of phenotypic features of patients with CHD8 mutation[%(n/N)]
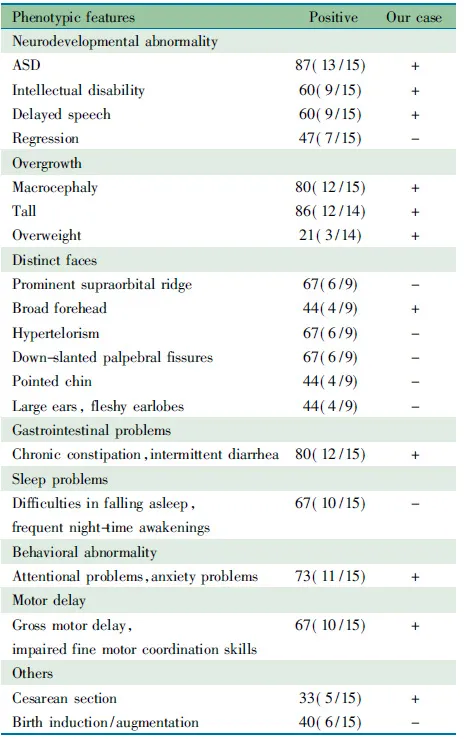
PhenotypicfeaturesPositiveOurcaseNeurodevelopmentalabnormalityASD87(13/15)+Intellectualdisability60(9/15)+Delayedspeech60(9/15)+Regression47(7/15)-OvergrowthMacrocephaly80(12/15)+Tall86(12/14)+Overweight21(3/14)+DistinctfacesProminentsupraorbitalridge67(6/9)-Broadforehead44(4/9)+Hypertelorism67(6/9)-Down-slantedpalpebralfissures67(6/9)-Pointedchin44(4/9)-Largeears,fleshyearlobes44(4/9)-GastrointestinalproblemsChronicconstipation,intermittentdiar-rhea80(12/15)+SleepproblemsDifficultiesinfallingasleep,frequentnight-timeawakenings67(10/15)-BehavioralabnormalityAttentionalproblems,anxietyproblems73(11/15)+MotordelayGrossmotordelay,impairedfinemotorcoordinationskills67(10/15)+OthersCesareansection33(5/15)+Birthinduction/augmentation40(6/15)-
Notes +: present; -: absent
将异质性明显的疾病根据某些特征分为不同的亚型,可提高研究对象的同质性,有利于病因的发现和致病机制的阐述。既往研究发现,ASD群体中大头围(>同年龄同性别P97)的比例为11%~30%[4~6],高于正常人群,且头围与ASD的某些临床表型如社交障碍的严重程度、智力低下等似乎存在联系[7, 8],提示可将大头围作为ASD的一种分型标准。
近年来从亚型的角度分析,已发现某些基因(如PTEN[9, 10]、CHD8[1]、RAB39B[11]等)在伴大头畸形的ASD患者发病中起重要作用。本文患儿大头畸形、生长过快,有明显的ASD特征,伴随运动发育落后和慢性便秘,FMR1基因CGG重复次数正常,初步排除了脆性X染色体综合征,染色体基因芯片分析未发现致病性CNVs的存在。为明确诊断,运用二代高通量测序方法对4 813个临床相关基因的外显子区进行测序,重点关注与ASD有关的基因,发现患儿EHMT1、PCDH9、NLGN4X、CHD8基因存在突变,其中EHMT1、PCDH9来源于未患病父亲,且dbSNP及千人基因组数据库有收录(EHMT1:rs143669310,0.0014;PCDH9:rs192811737,0.0018),不考虑为患儿的致病原因。NLGN4X的突变来源于母亲,该基因突变引起的ASD可认为是X连锁隐性遗传,但未见报道过度生长、大头畸形等表型。结合本文患儿的临床表型认为NLGN4X的变异可能并不是其致病原因,而根据既往关于CHD8基因的文献报道[1~3],CHD8杂合无义突变c.307C>T,p.Gln103*(NM_020920.3)使编码产物变短而破坏蛋白质原有功能,更有可能是患儿ASD相关的重要遗传学改变。
CHD8基因位于14q11.2区,总长约46 kb,由37个外显子组成;其编码产物通过结合β-catenin募集组蛋白H1,改变染色质空间结构而发挥转录抑制作用,是Wnt/β-catenin信号通路重要的调节物[12, 13],而该通路对神经元增殖和分化、突起生长和突触形成作用较大[14~18],与ASD发病显著相关[19~21]。O′Roak等于2012年首次对209例ASD患者及其未患病同胞进行外显子组测序,发现2例患者存在CHD8基因突变[2];随后相关研究已证实,CHD8新发的截断突变是ASD发病的重要危险因素[1, 3, 22]。此外,Talkowski等[23]在携带染色体平衡易位的ASD患者中发现了CHD8的破坏,也提示了该基因对于ASD发病的重要作用。
Sugathan等[24]在神经前体细胞模拟CHD8基因功能性的杂合缺失发现,CHD8基因调节许多功能各异但与ASD相关的基因(SCN2A、DLG2、SHANK3、MBD3等),以及与突触形成、神经元分化、细胞黏附等有关的基因(LAMA4、NCAM1、MEGF10等)。突变的CHD8可通过影响上述基因而影响神经系统的正常发育。chd8基因敲除的斑马鱼模型再现了大头畸形[1, 24]和胃肠道动力不足[1]的临床表型。通过神经元特殊染色发现,前脑和中脑神经元前体细胞的过度增生导致这2个部位体积增大,造成头部过度生长;而发育过程中肠道神经丛增殖或是迁移障碍导致肠道神经元减少,影响正常的胃肠道功能。细胞和动物研究结果均说明CHD8破坏会导致神经元发育的缺陷。
针对CHD8基因异常的ASD患者,除通过特殊教育和训练提高认知、社交和适应能力外,还应关注患者的胃肠道问题和睡眠状况。此外,既往研究表明,CHD8与胃肠道、皮肤等部位多种肿瘤密切相关[25~28];Bernier的研究中1例41岁的CHD8突变患者合并肿瘤,另1例将突变传给患儿的父亲罹患直肠癌[1],这提示注意肿瘤的早期征象可能是CHD8突变患者日后定期随访的又一重点。
总之,CHD8基因是ASD重要的易感基因;ASD患儿合并大头畸形、生长过快,伴有慢性便秘或间歇性腹泻时应考虑存在CHD8基因突变可能,可行基因检测协助诊断。
[1]Bernier R, Golzio C, Xiong B, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 2014, 158(2): 263-276
[2]O′Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature, 2012, 485(7397): 246-250
[3]O′Roak BJ, Vives L, Fu W, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science, 2012, 338(6114): 1619-1622
[4]Woodhouse W, Bailey A, Rutter M, et al. Head circumference in autism and other pervasive developmental disorders. J Child Psychol Psychiatry, 1996, 37(6): 665-671
[5]van Daalen E, Swinkels SH, Dietz C, et al. Body length and head growth in the first year of life in autism. Pediatr Neurol,2007,37(5):324-330
[6]Lainhart JE, Bigler ED, Bocian M, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A, 2006, 140(21): 2257-2274
[7]Davis JM, Keeney JG, Sikela JM, et al. Mode of genetic inheritance modifies the association of head circumference and autism-related symptoms: a cross-sectional study. PLoS One, 2013, 8(9): e74940
[8]Sacco R, Militerni R, Frolli A, et al. Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry, 2007, 62(9): 1038-1047
[9]Marchese M, Conti V, Valvo G, et al. Autism-epilepsy phenotype with macrocephaly suggests PTEN, but not GLIALCAM, genetic screening. BMC Med Genet,2014,15:26
[10]Klein S, Sharifi-Hannauer P, Martinez-Agosto JA. Macrocephaly as a clinical indicator of genetic subtypes in autism. Autism Res, 2013, 6(1): 51-56
[11]Giannandrea M, Bianchi V, Mignogna ML, et al. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet, 2010, 86(2): 185-195
[12]Thompson BA, Tremblay V, Lin G, et al. CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Mol Cell Biol, 2008, 28(12): 3894-3904
[13]Nishiyama M, Skoultchi AI, Nakayama KI. Histone H1 recruitment by CHD8 is essential for suppression of the Wnt-beta-catenin signaling pathway. Mol Cell Biol, 2012, 32(2): 501-512
[14]Machon O, Backman M, Machonova O, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol, 2007, 311(1): 223-237
[15]Zhou CJ, Borello U, Rubenstein JL, et al. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience, 2006, 142(4): 1119-1131
[16]Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol, 2012, 4(2)[17]Farias GG, Godoy JA, Cerpa W, et al. Wnt signaling modulates pre- and postsynaptic maturation: therapeutic considerations. Dev Dyn, 2010, 239(1): 94-101
[18]Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci, 2007, 30(6): 268-275
[19]Krumm N, O′Roak BJ, Shendure J, et al. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci, 2014, 37(2): 95-105
[20]Okerlund ND, Cheyette BN. Synaptic Wnt signaling-a contributor to major psychiatric disorders?. J Neurodev Disord, 2011, 3(2): 162-174
[21]Hormozdiari F, Penn O, Borenstein E, et al. The discovery of integrated gene networks for autism and related disorders. Genome Res, 2015, 25(1): 142-154
[22]Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature, 2012, 485(7397): 242-245
[23]Talkowski ME, Rosenfeld JA, Blumenthal I, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell, 2012, 149(3): 525-537
[24]Sugathan A, Biagioli M, Golzio C, et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc Natl Acad Sci U S A, 2014, 111(42): E4468-E4477
[25]Sawada G, Ueo H, Matsumura T, et al. CHD8 is an independent prognostic indicator that regulates Wnt/beta-catenin signaling and the cell cycle in gastric cancer. Oncol Rep, 2013, 30(3): 1137-1142
[26]Tahara T, Yamamoto E, Madireddi P, et al. Colorectal carcinomas with CpG island methylator phenotype 1 frequently contain mutations in chromatin regulators. Gastroenterology, 2014, 146(2): 530-538
[27]Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature, 2014, 505(7484): 495-501
[28]Kim MS, Chung NG, Kang MR, et al. Genetic and expressional alterations of CHD genes in gastric and colorectal cancers. Histopathology, 2011, 58(5): 660-668
(本文编辑:丁俊杰)
《中国循证儿科杂志》主要栏目及其特点
述评:在高度精练又不失全面地评价某一临床或基础研究热点问题的基础上,前瞻性地指出研究可能的切入点和方向。
专家对谈录:是国内外或境内外专家共同参与的谈话式栏目。选题为最新的基础或临床研究问题。
论著:本刊论著文稿的内容应遵循以下类型医学研究报告规范撰写。
队列研究、病例对照研究和横断面研究以观察性流行病学研究报告的质量(Strengthening the reporting of observational studies in epidemiology,STROBE) 为标准,STROBE原文见www.strobe-statement.org。STROBE中文解读见《中国循证儿科杂志》,2010,5(3):223-227
观察性研究的Meta 分析(Meta-analysis of observational studies in epidemiology,MOOSE)报告规范原文见JAMA,2000, 283(15): 2008-2012。随机对照试验Meta分析报告质量(the quality of reporting of Meta-analysis of randomized controlled trial, QUORPM)原文见Lancet,1999,354(9193):1896-1900。MOOSE和QUOROM中文解读见《中国循证儿科杂志》,2010,5(1):60-63
非劣效性和等效性随机对照试验的报告规范(Consolidated standards of reporting trials,CONSORT)原文见http://www.consort-statement.org。CONSORT中文解读见《中华流行病学杂志》,2006,12(27):1086-1088
非随机对照设计报告规范(Transparent reporting of evaluations with nonrandomized designs,TREND)原文见Am J Public Health,2004,94(3):361-366。TREND中文解读见《中华流行病学杂志》,2006,4(27):408-410
诊断试验准确性研究的报告规范(Standards for reporting of diagnostic accuracy,STARD)原文见www.stard-statement.org。STARD中文解读见《中华流行病学杂志》,2006,10(27):910-912
随机对照试验报告规范(CONSORT)原文见http://www.consort-statement.org。CONSORT中文解读见《中国循证儿科杂志》,2010,5(2):146-150
遗传关联性研究报告规范(STREGA)原文见http://www.strega-statement.org。STREGA中文解读见《中国循证儿科杂志》,2010,5(4):304-307
病例报告:应遵循病例报告的报告规范(CARE)撰写,原文见http://www.care-statement.org/index.html。CARE中文解读见《中国循证儿科杂志》,2014,9(3):216-219
A novel CHD8 gene mutation in a patient with autism spectrum disorder and literature review
ZHENGQing-wen1,ZHANGuo-dong1,TANGChun1,BIXin2,ZHAOWei-wei2,ZOUXiao-bing1
(1TheThirdAffiliatedHospitalofSunYat-senUniversity,Guangzhou510630; 2KingMedCenterforClinicalLaboratoryCo.,Ltd.,Guangzhou510330,China)
ZOU Xiao-bing,E-mail:zouxb@vip.tom.com; ZHAO Wei-wei,E-mail:lab-zhaoweiwei@kingmed.com.cn
ObjectiveTo draw attention to the phenotype and genotype of patients with autism spectrum disorders (ASD) carryingCHD8 mutation.MethodsThe clinical data of one patient with ASD carryingCHD8 mutation, including clinical manifestations, laboratory findings, genetic testing results and family investigation were summarized and analyzed, and relevant literatures aboutCHD8 mutation were reviewed in this article.Results①The 3-year-and-3-month boy was presented with macrocephaly (head circumference was 55.0 cm,Z>4s), overgrowth (height was 107.0 cm,Z>2s; weight was 23.5 kg,Z>2s) and typical autistic behavior as well as significant motor delay and gastrointestinal(GI) problems (chronic constipation). No additional patient was found in his family. ②Targeting 4 813 genes associated with known clinical phenotypes, a total of 8 982 variances were found in the patient via next-generation high-throughput DNA sequencing. Missense mutations of autism-related genesEHMT1,PCDH9,NLGN4Xand nonsense mutation ofCHD8 [c.307C>T, p.Gln103*(NM_020920.3)] were probably pathogenic after filtering unrelated variances.EHMT1,PCDH9 mutations were identified in his father andNLGN4Xmutation in his mother, butCHD8 mutation was not identified in his parents via Sanger sequencing. ③To date a total of 15 independentCHD8 mutations associated with neurodevelopmental disorders has been reported worldwide. Common phenotypic features of patients withCHD8 disruptions include autism(87%), overgrowth(86%), macrocephaly(80%), GI complaints(80%), motor delay(67%, especially fine motor coordination) and sleep problems(67%).ConclusionThe mutation ofCHD8 is an important risk factor of ASD. ASD patients with macrocephaly, overgrowth and GI problems should be considered as carryingCHD8 mutation and genetic testing is recommended to confirm the diagnosis.
Autism spectrum disorder ;CHD8; Macrocephaly; Overgrowth; Next-generation sequencing
国家重点基础研究发展计划(973计划):2012CB517901
1 中山大学附属第三医院 广州,510630; 2 广州金域医学检验中心 广州,510330
邹小兵,E-mail:zouxb@vip.tom.com;赵薇薇,E-mail:lab-zhaoweiwei@kingmed.com.cn
10.3969/j.issn.1673-5501.2015.03.012
2015-03-03
2015-05-27)
