实时荧光定量聚合酶链反应检测妊娠晚期孕妇和新生儿B族溶血性链球菌价值的系统评价和Meta分析
2015-04-20郑雪艳
郑雪艳 韦 红
·论著·
实时荧光定量聚合酶链反应检测妊娠晚期孕妇和新生儿B族溶血性链球菌价值的系统评价和Meta分析
郑雪艳 韦 红
目的 评价实时荧光定量PCR(RT-PCR)检测妊娠晚期孕妇和新生儿B族溶血性链球菌(GBS)的价值,为GBS感染早期诊断提供依据。方法检索PubMed、Cochrane图书馆、中国生物医学文献数据库、维普数据库、万方数据库和中国知网,收集RT-PCR用于妊娠晚期孕妇及生后7 d内新生儿GBS并与细菌培养金标准比较的诊断性研究,检索时间均从建库至2015年5月。行文献质量评价,提取诊断参数,采用Meta Disc1.4 软件进行Meta分析,计算汇总敏感度、特异度、阳性似然比和阴性似然比,绘制汇总受试者工作特征(SROC)曲线,计算曲线下面积(AUC),评估RT-PCR检测GBS的诊断价值。结果17篇文献(20项研究)进入Meta分析,其中英文15篇,中文2篇,共纳入研究对象5 660例,5 938个研究标本。①Meta分析结果显示,RT-PCR检测GBS的汇总敏感度为0.92(95%CI:0.90~0.94) , 特异度为0.94(95%CI:0.93~0.95) ,阳性似然比为13.81(95%CI:8.80~21.69) ,阴性似然比为0.11(95%CI:0.06~0.20),SROC AUC为 0.972 1, Q*指数为0.923 3。②分别剔除样本量<100、中文语种、样本采集时间不明确和靶基因不明的文献行敏感性分析,显示剔除文献后各诊断参数95%CI与原数据基本重叠。③各诊断参数的文献间存在显著的统计学异质性,亚组分析提示研究对象和靶基因可解释部分异质性来源,样本类型可能不是异质性的来源。结论现有证据显示,RT-PCR检测GBS价值较高,可作为妊娠晚期孕妇和新生儿诊断GBS感染的重要检测手段之一。
实时荧光定量PCR; B型溶血性链球菌; 诊断; 新生儿; 敏感度; 特异度; 系统评价; Meta分析
B族溶血性链球菌(GBS)是新生儿早发型感染主要的致病菌之一,主要由新生儿经产道时感染。GBS常引起新生儿肺炎、败血症、化脓性脑膜炎等重症感染,危及患儿的生命或残留神经系统不良结局。2002年世界疾病预防控制中心(CDC)和美国儿科学会(AAP)提出开展产前GBS筛查后,新生儿GBS感染率下降了80%;2010年CDC推荐行产前GBS状态的筛查及抗生素预防治疗[1]。有研究表明,GBS感染的孕妇预防性使用抗生素能有效降低新生儿GBS感染发生率[2~4],但晚近的Meta分析表明,对存在高危因素的新生儿产时或产前母亲预防性使用抗生素并不能显著降低新生儿GBS感染[5]。因此早期诊断和治疗仍是最有效降低新生儿GBS感染发生率及病死率的方法。细菌培养是GBS诊断的金标准,但耗时长,早期诊断的价值不大,近年来发展的实时荧光定量PCR(RT-PCR)方法在GBS感染的早期诊断中优势明显,但各文献纳入样本量不大且报道的诊断参数不尽相同,为此本研究检索RT-PCR诊断妊娠晚期孕妇和新生儿GBS感染的相关文献,采用Meta分析方法进行定量综合,以期为新生儿GBS感染的早期诊断提供证据。
1 方法
1.1 文献纳入和排除标准 ①RT-PCR对妊娠晚期孕妇及新生儿GBS感染诊断价值的前瞻性研究;②研究对象为出生7 d内新生儿或妊娠晚期孕妇(孕28周至产时)③文献报道了RT-PCR检测GBS感染的诊断参数,或通过其提供的数据可以做出推算;④诊断金标准为细菌培养发现GBS,且明确说明诊断标本的来源;⑤中文和英文文献。
1.2 文献检索 电子检索PubMed、Cochrane图书馆、中国生物医学文献数据库(CBM)和维普数据库(VIP)、万方数据库、中国知网(CNKI)。英文检索词:Group B streptococcus, GBS, real time PCR;中文检索词:B族链球菌、PCR。检索起止时间为建库至2015年5月10日。以PubMed数据库为例,检索式如下:(“real time PCR” OR RT-PCR) AND (“group B Streptococci” OR GBS)。未手工检索灰色文献。
1.3 文献筛选、资料提取和偏倚风险评价 本文文献筛选、资料提取和质量评价由郑雪艳、韦红完成,意见不统一时协商讨论决定。
1.3.1 文献筛选 首先通过阅读文题和摘要进行初筛,排除明显不符合纳入标准的文献,之后阅读全文决定是否纳入。
1.3.2 资料提取 ①一般情况及临床信息:包括题目、作者、发表年份、国家、研究类型、样本类型、研究对象和靶基因;②诊断参数:诊断四格表参数,包括真阳性值(TP)、假阳性值(FP)、真阴性值(TN)、假阴性值(TN),计算敏感度、特异度、阳性似然比(PLR)和阴性似然比(NLR)等。

1.4 统计学方法 采用Spearman相关分析检验由阈值效应引起的异质性;采用χ2检验评价敏感度和特异度的异质性;Cochrane-Q检验评价PLR、NLR以及诊断比值比(DOR)的异质性,检验水准为α=0.05。采用I2评价异质性程度:I2≤25%为低度异质性,~70%为中等异质性,≥70%为高度异质性。若存在非阈值效应引起的异质性则采用随机效应模型合并分析,反之则采用固定效应模型合并分析。
采用Meta-Disc 1.4软件绘制汇总受试者工作特征(SROC)曲线,计算曲线下面积(AUC)和Q*指数,Q*指数为SROC曲线与直线(敏感度=特异度)相交处的敏感度,Q*指数越大,AUC越接近1,表示诊断试验的准确性和诊断价值越高。
2 结果
2.1 检索结果 初检索到155篇文献,17篇文献[6~22](20项研究)进入Meta分析,其中英文15篇,中文2篇,文献纳入和排除流程见图1。一般情况如表1所示,17篇文献共纳入5 660例研究对象,5 938个研究样本,16篇文献样本来源于孕妇,1篇文献样本来自新生儿。4篇文献RT-PCR采用的靶基因不明确。纳入文献的诊断参数见表2。

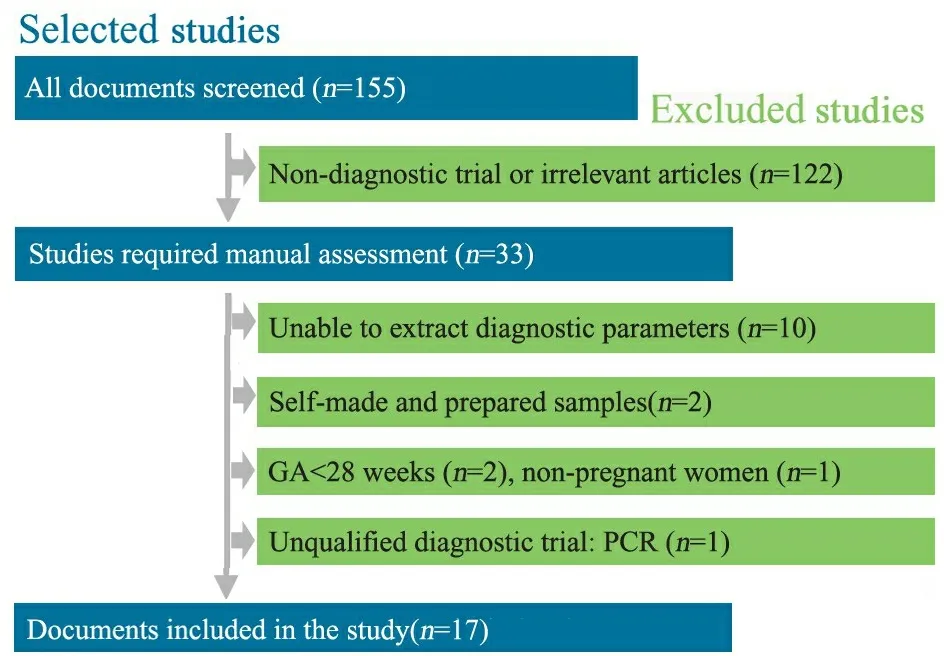
图1 文献筛选流程图
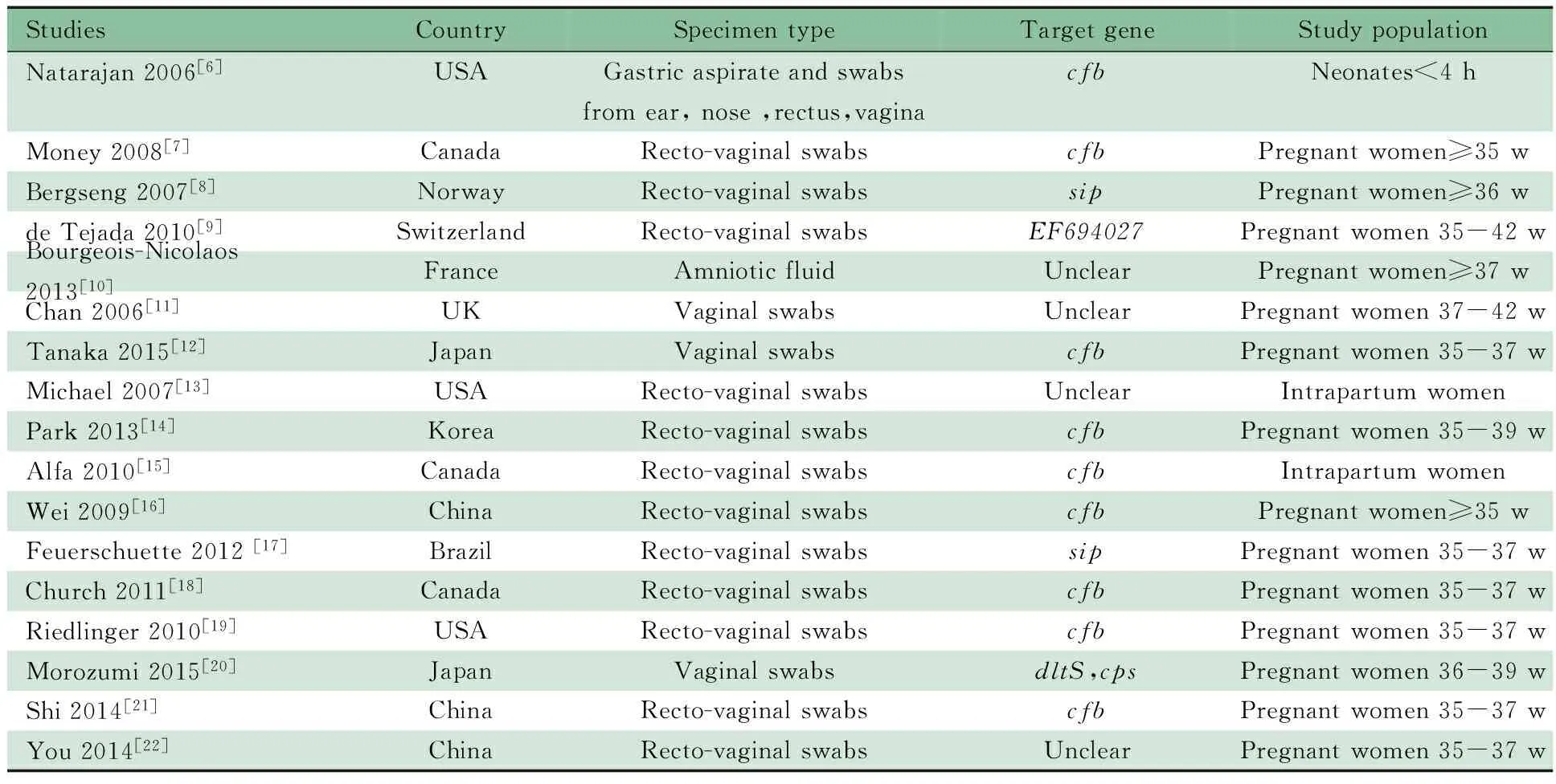
表1 纳入17篇文献的基本情况Tab 1 Characteristics of 17 included studies
Notes w: weeks

表2 纳入17篇文献诊断参数Tab 2 The diagnostic parameters of RT-PCR for GBS detection of 17 included studies
Notes TP:true positive;FP:false positive;TN:true negative;FN:false negative;Sen:sensitivity;Spe:specificity
2.3 发表偏倚 图2B显示,漏斗图比较对称,提示发表偏倚的可能性不大。
2.4 阈值效应 SROC曲线显示(图2C),各文献所对应的点散在分布,不呈“肩臂”状,计算敏感度对数与特异度对数的Spearman 相关系数为0.009,P=0.970,提示不存在阈值效应。
2.5 Meta分析结果 报道敏感度、特异度、PLR和NLR的文献间Q检验P均<0.001,I2均>50%,文献间存在显著的统计学异质性。采用随机效应模型合并结果,Meta分析的结果显示(图3和4),汇总敏感度为0.92 (95%CI: 0.90~0.94) ,特异度为0.94 (95%CI: 0.93~0.95),PLR为13.81(95%CI:8.80~21.69),NLR为0.11(95%CI:0.06~0.20)。SROC AUC= 0.972 1,Q*指数为0.923 3(图2C)。
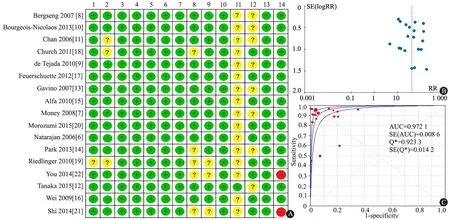
图2 纳入17篇文献的偏倚风险评价结果、发表偏倚和诊断GBS感染的汇总受试者工作特征曲线
Fig 2 Quality, SROC of GBS detection and publication bias analysis of 17 included studies
Notes A: +: yes, -: no; ?: unclear;1:spectrum composition;2:selection criteria;3:reference standard;4:disease progression bias;5: partial verification;6:differential verification;7:incorporation bias;8: index test execution;9:reference standard execution;10:test review bias;11:reference standard review bias;12:clinical review bias;13:uninterruptible test results;14:withdrawals; B: funnel plot for publication bias analysis; C: SROC of RT-PCR in GBS detection

图3 RT-PCR诊断GBS感染的汇总敏感度、特异度
Fig 3 Pooled sensitivity and specificity of RT-PCR in GBS detection
2.6 异质性原因分析 报道各诊断参数的文献间均有统计学异质性,以DOR为效应量行Meta回归(REML法)分析异质性来源。自变量选择:患者类型(孕妇为1,新生儿为0)、样本(阴道直肠拭子/阴道拭子为1,其他为0)、靶基因(cfb为1,其他或不清楚为0),结果提示患者类型是造成非阈值效应的主要原因,RDOR=8.83(95%CI:1.20~64.94),P=0.034 1。
以患者类型、样本来源和靶基因行亚组分析,Meta分析结果显示(表3),新生儿文献的汇总特异度、PLR和NLR无显著统计学异质性,敏感度异质性的P值处于临界值(0.09);靶基因为cfb的文献汇总特异度和PLR有显著的统计学异质性,孕妇、直肠阴道拭子标本的汇总敏感度、特异度、PLR、NLR均有显著统计学异质性。提示患者类型和靶基因可解释部分异质性,样本来源可能不是异质性的来源。
2.7 敏感性分析 分别剔除样本量<100的文献[7,12,13]、中文文献[21,22]、样本采集时间不明的文献[11,13,14,18,19],靶基因不明的文献[11~13,21]行敏感性分析,Meta分析结果显示剔除文献后各诊断参数的汇总95%CI与未剔除文献的结果基本重叠(表4)。
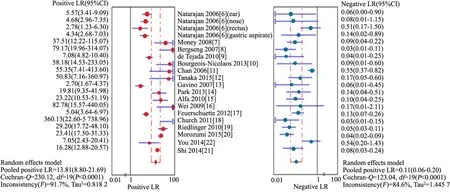
图4 RT-PCR诊断GBS感染的汇总阳性似然比、阴性似然比
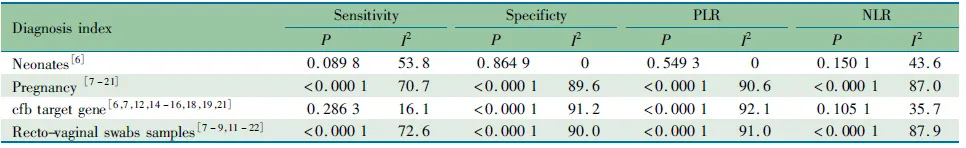
表3 异质性亚组分析结果Tab 3 Subgroup analysis results of heterogeneity
Notes PLR: positive likelihood ratio; NLR: negative likelihood ratio
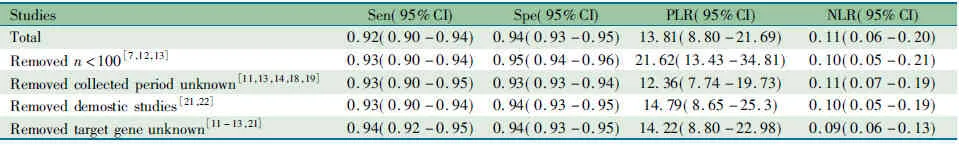
表4 敏感度分析结果Tab 4 The results of sensitivity analysis
Notes Sen:sensitivuty; Spe:specificty;PLR: positive likelihood ratio; NLR: negative likelihood ratio
3 讨论
本文纳入的17篇文献中,1篇可能存在疾病谱偏倚,3篇可能存在选择偏倚,3篇有金标准实施偏倚的可能,5篇有发生待评价试验实施偏倚的可能,16篇可能存在金标准解读偏倚。所有病例均未出现难以解释的中间结果。所有文献发生金标准偏倚、疾病进展偏倚、部分参照偏倚、多重参照偏倚、混合偏倚、试验解读偏倚可能性不大。提示本研究纳入文献的总体质量强度较高。
细菌培养作为GBS感染诊断的金标准,但至少48 h才能得出阳性结果,阴性结果至少也需72 h。GBS的血清学检测,如乳胶凝集试验、酶联免疫吸附试验等,特异度较好(约95%),但报道的敏感度差别较大(9%~82%),且由于抗生素不合理使用,也可能会降低检测的敏感度[23],上述方法已较少应用。核酸分子杂交技术具有较高的敏感度和特异度,但需行预富集培养18~24 h[24]。常规PCR检测至少需要6 h。RT-PCR最短30 min可得到阳性结果[25],150 min可得出阴性结果[14],因此在GBS的早期诊断中优势明显。
本文纳入的文献间存在显著的统计学异质性。考虑可能与靶基因、纳入的研究对象和样本来源有关。目前用于GBS检测的基因有:编码特异性溶血性致病物质CAMP因子(Christie-Atkins-Munch-Petersen factor)的cfb基因[26],编码荚黏膜多糖的cps基因(cps1aH, cps1a/2/3IJ,cps5O 分别编码Ⅰa,Ⅲ 和Ⅴ型GBS)[27],编码表面免疫相关蛋白的sip基因等,其中以cfb为靶基因的RT-PCR研究最多。Meta回归分析显示,研究对象是异质性的来源,为进一步分析异质性来源,以新生儿为研究对象的文献、妊娠晚期孕妇为研究对象的文献、靶基因为cfb基因的文献、直肠阴道拭子为样本的文献行亚组分析,结果提示研究对象和靶基因可解释部分异质性,样本来源不是异质性的原因。考虑异质性可能还与其他原因有关,如孕产妇抗生素使用情况、经产情况、年龄、产检情况、GBS分布的地域性差异、试验操作步骤、实验室条件、仪器和试剂等,但各文献缺乏详尽的描述,故无法进行进一步分析。
本研究结果显示, RT-PCR检测 GBS的汇总敏感度为0.92 (95%CI:0.90~0.94%), 提示其漏诊率为8%;汇总特异度为0.94(95%CI:0.93~0.95) , 提示其误诊率为6%。似然比为同时反映敏感度和特异度的复合指标, 当PLR>10 且NLR<0.1时, 具有非常好的诊断效能;PLR 5~10且NLR<0.2 时,具有较好的诊断效能。本文结果的汇总PLR为13.81(95%CI:8.80~21.69) ,NLR为0.11(95%CI:0.06~0.20),同时SROC AUC为0.972 1,Q*指数为0.923 3。提示RT-PCR对于GBS具有非常好的诊断效能。
本文Meta分析的局限性:①未检索灰色文献,检索语种局限于中文和英文;②各研究间的异质性较大, 无法分析各研究间异质性的原因;③用于新生儿检测的研究很少,仍需更多文献积累。
[1]Baker CJ, Byington CL, Polin RA. Policy statement-Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics, 2011,128(3):611-616
[2]Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol, 2013,121(3):570-577
[3]Todorova-Christova M, Vacheva R, Decheva A, et al. A study on early-onset neonatal group B streptococcal infection, Bulgaria, 2007-2011. Arch Pediatr, 2014,21(9):953-960
[4]Creti R, Berardi A, Baldassarri L, et al. Characteristics of neonatal GBS disease during a multicentre study (2007-2010) and in the year 2012. Ann Ist Super Sanita, 2013,49(4):370-375
[5]Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database Syst Rev, 2014,6:CD007467
[6]Natarajan G, Johnson YR, Zhang F, et al. Real-time polymerase chain reaction for the rapid detection of group B streptococcal colonization in neonates. Pediatrics, 2006,118(1):14-22
[7]Money D, Dobson S, Cole L, et al. An evaluation of a rapid real time polymerase chain reaction assay for detection of group B streptococcus as part of a neonatal group B streptococcus prevention strategy. J Obstet Gynaecol Can, 2008,30(9):770-775
[8]Bergseng H, Bevanger L, Rygg M, et al. Real-time PCR targeting the sip gene for detection of group B Streptococcus colonization in pregnant women at delivery. J Med Microbiol, 2007,56(Pt 2):223-228
[9]de Tejada BM, Stan CM, Boulvain M, et al. Development of a rapid PCR assay for screening of maternal colonization by group B streptococcus and neonatal invasive Escherichia coli during labor. Gynecol Obstet Invest, 2010,70(4):250-255
[10]Bourgeois-Nicolaos N, Cordier AG, Guillet-Caruba C, et al. Evaluation of the Cepheid Xpert GBS assay for rapid detection of group B Streptococci in amniotic fluids from pregnant women with premature rupture of membranes. J Clin Microbiol, 2013,51(4):1305-1306
[11]Chan KL, Levi K, Towner KJ, et al. Evaluation of the sensitivity of a rapid polymerase chain reaction for detection of group B streptococcus. J Obstet Gynaecol, 2006,26(5):402-406
[12]Tanaka K, Iwashita M, Matsushima M, et al. Intrapartum group B Streptococcus screening using real-time polymerase chain reaction in Japanese population. J Matern Fetal Neonatal Med, 2015:1-5
[13]Gavino M, Wang E. A comparison of a new rapid real-time polymerase chain reaction system to traditional culture in determining group B streptococcus colonization. Am J Obstet Gynecol, 2007,197(4):388.e1-4
[14]Park JS, Cho DH, Yang JH, et al. Usefulness of a rapid real-time PCR assay in prenatal screening for group B streptococcus colonization. Ann Lab Med, 2013,33(1):39-44
[15]Alfa MJ, Sepehri S, De Gagne P, et al. Real-time PCR assay provides reliable assessment of intrapartum carriage of group B Streptococcus. J Clin Microbiol, 2010,48(9):3095-3099
[16]Wei CF, She BC, Liang HS, et al. Prenatal Group B Streptococcus test using real-time polymerase chain reaction. Taiwan J Obstet Gynecol, 2009,48(2):116-119
[17]Feuerschuette OM, Serratine AC, Bazzo ML, et al. Performance of RT-PCR in the detection of Streptococcus agalactiae in the anogenital tract of pregnant women. Arch Gynecol Obstet, 2012,286(6):1437-1442
[18]Church DL, Baxter H, Lloyd T, et al. Evaluation of the Xpert(R) group B streptococcus real-time polymerase chain reaction assay compared to StrepB Carrot Broth for the rapid intrapartum detection of group B streptococcus colonization. Diagn Microbiol Infect Dis, 2011,69(4):460-462
[19]Riedlinger J, Beqaj SH, Milish MA, et al. Multicenter evaluation of the BD Max GBS assay for detection of group B streptococci in prenatal vaginal and rectal screening swab specimens from pregnant women. J Clin Microbiol, 2010,48(11):4239-4241
[20]Morozumi M, Chiba N, Igarashi Y, et al. Direct identification of Streptococcus agalactiae and capsular type by real-time PCR in vaginal swabs from pregnant women. J Infect Chemother, 2015,21(1):34-38
[21]Shi CY(时春艳), Zhao YY, Fang L, et al.A multi-center study on realtime polymerase chain reaction assay for group B Streptococcus in pregnant women.Chin J Perinat Med(中华围产医学杂志),2014,17(6):361-364
[22]You YQ(游艳琴), Tong HL, Gao ZY.实时聚合酶链反应技术检测妊娠晚期B族溶血性链球菌的临床价值.Chin J Perinat Med(中华围产医学杂志),2014,17(6):403-405
[23]Deng JH(邓江红), Yang YH. The review of Molecular-based diagnosis and genotype study for group B streptococcus. Chin J Pediatr(中华儿科杂志), 2005,43(11):832-835
[24]Emonet S, Schrenzel J, de Tejada B M. Molecular-based screening for perinatal group B streptococcal infection: implications for prevention and therapy. Mol Diagn Ther, 2013,17(6):355-361
[25]Jost C, Bercot B, Jacquier H, et al. Xpert GBS assay for rapid detection of group B streptococcus in gastric fluid samples from newborns. J Clin Microbiol, 2014,52(2):657-659
[26]Ke D, Menard C, Picard FJ, et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem, 2000,46(3):324-331
[27]Poyart C, Tazi A, Reglier-Poupet H, et al. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol, 2007,45(6):1985-1988
(本文编辑:丁俊杰)
The diagnostic value of real-time PCR in group B Streptococci detection: a systematic review and meta analysis
ZHENGXue-yan,WEIHong
(DepartmentofNeonatology,Children′sHospitalofChongqingMedicalUniversity,MinistryofEducationKeyLaboratoryofChildDevelopmentandDisorders,ChongqingKeyLaboratoryofPediatrics,Chongqing400014,China)
WEI Hong,E-mail:1056670630@qq.com
ObjectiveTo evaluate the diagnostic value of real time polymerase chain reaction(RT-PCR)in group BStreptococci(GBS) detection.MethodsPubMed,Cochrane Library,China Biology Medicine disc(CBM disc),VIP citation databases,Wanfang database, CNKI were searched to recruit studies which evaluated the diagnostic value of RT-PCR compared with the conventional bacteria culturing method in late trimester of pregnant women and neonates aged below 7 days. The data were analyzed by using Meta Disc 1.4 software. The diagnostic value of RT-PCR in GBS detection was evaluated by the pooled sensitivity, pooled specificity, pooled likelihood ratio (LR) and area under curve (AUC) of summary receiver operating characteristic curve (SROC curve) statistical indicators.ResultsSeventeen literatures (twenty researches) were collected and 5 660 cases(5 938 pairs of specimens ) were included in the study . ①The pooled sensitivity and specificity were 0.93(95%CI:0.90-0.94) and 0.94(95%CI:0.93-0.95). The pooled positive likelihood ratio (PLR) and negative likelihood ratio(NLR) were 13.81(95%CI:8.80-21.69) and 0.11(95%CI:0.06-0.20) respectively. SROC area under the curve (AUC) was 0.972 1,Q*was 0.923 3. ②The sensitivity analysis of removing studies with sample capacity<100, domestic studies, studies with unclear period to collect samples and with unclear target gene,showed the confidence interval of the diagnostic parameters almost overlapped with the original data. ③Heterogeneity test showed remarkable heterogeneity. Subgroup analysis showed heterogeneity came from research object and target gene partly and sample type may not be the cause of heterogeneity. ConclusionRT-PCR had great value of diagnostic efficiency,and could be one of the valuable reference tests in early diagnosis of pregnant women in late trimester and neonatal GBS infection.
Real time PCR ; Group Bstreptococci; Diagnose; Neonate; Sensitivity; Specificity; Systematic review; Meta analysis
重庆医科大学附属儿童医院新生儿科,儿童发育疾病研究教育部重点实验室,儿科学重庆市重点实验室 重庆,400014
韦红,E-mail:1056670630@qq.com
10.3969/j.issn.1673-5501.2015.03.006
2015-02-03
2015-05-20)
