基团贡献法估算硅烷及硅氧烷的密度
2015-03-20程大海张引弟
程大海,伍 川,董 红,武 侠,张引弟
(杭州师范大学有机硅化学及材料技术教育部重点实验室,浙江杭州311121)
0 前 言
物质的组成、结构与性能的关系一直都是化学中十分活跃的研究领域[1],国内外学者根据分子组成以及内部原子或官能团之间的相互作用,先后提出了原子贡献法、基团贡献法及化学键贡献法等主要研究方法[2],在物质微观组成、结构与宏观性质之间建立起有机联系,进而可对化合物的热力学性质进行准确预测.通过研究物质性能与内部结构之间的关系,以少量纯物质或混合物的实验数值作为样本,拟合得到构成物质分子的原子或基团的贡献值或参数值,从而可对含有相同种类原子或基团但是物性参数未知的其它纯物质或混合物的密度进行预测计算,极大程度地减少人、财、物和时间的消耗[3].从20 世纪40年代硅烷和聚硅氧烷实现工业化生产以来,因为它们具有优异的光学、热性能及耐辐射性能,广泛应用于各个领域,已成为不可替代的新材料.目前,有机硅化合物微观结构与宏观性质之间的相关研究比较少,王克强[4]利用图论方法探究了硅烷摩尔折射度与分子结构之间的关系,Kupchik[5]报道了利用分子连接性预测烷基硅烷的摩尔折射度,但是这些方法都仅限于研究烷基硅烷化合物的热力学性质,对于取代硅烷和硅氧烷化合物热力学性质与微观结构的关系研究却鲜见报道.因而本文采用基团贡献法对硅烷、取代硅烷和低聚二硅氧烷分子结构与其密度之间的关系进行了探索研究.
1 基本原理和方法
硅原子通常采用sp3杂化方式形成4 个σ 成键轨道,可分别与4 个有机基团连接.若不考虑不同官能团与硅原子的相互作用,可认为硅原子4 个σ 成键轨道对物质的热力学性质具有相同的贡献值[6].对于每个由硅原子与单价原子或官能团构成的σ 键,可视为1 个新的基团,4 个新组合的基团即可构成1 个硅烷分子.分析聚硅氧烷均聚物的结构,构成聚合物主链结构Si-O-Si 单元中的氧原子可被一分为二,氧原子对热力学性质的贡献值平均分配到2 个σ 成键轨道上.将1 个硅原子σ 键与1 个氧原子σ 键组合得到新的基团“-Si-O-”,它可看作是由1/4 个Si 原子与1/2 个O 原子形成的.由此硅氧骨架出发,再连接其它的原子或官能团,则可构建各种硅烷和硅氧烷.按上述方法,新组合的基团列于表1 中.
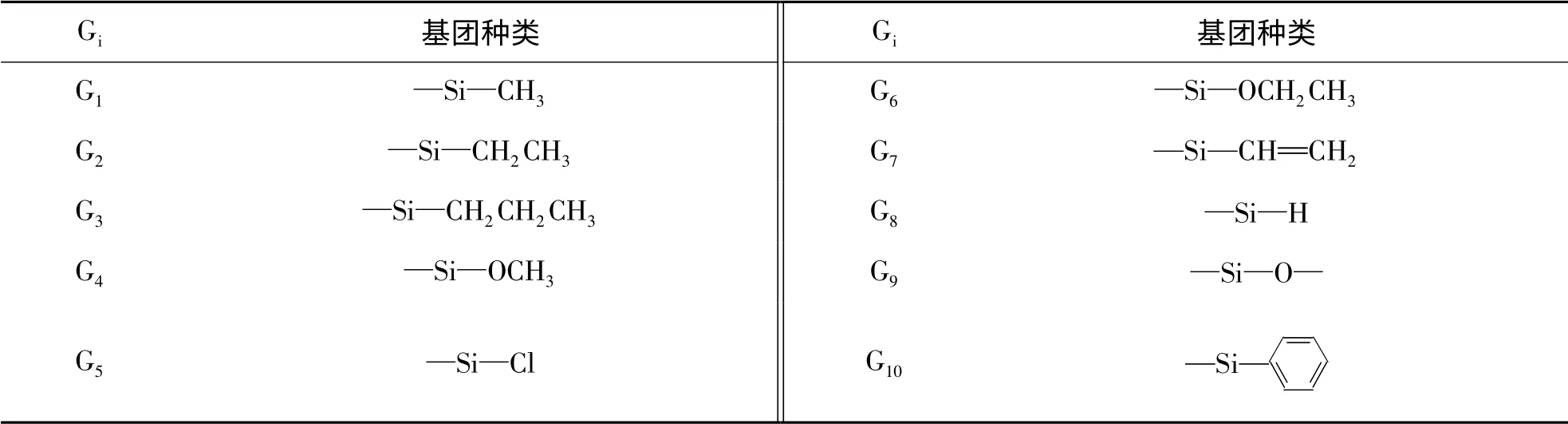
表1 硅烷及硅氧烷分子中新基团的定义Tab. 1 Definition of new groups in silane and polysiloxane molecule
按表1 所定义的基团,这些基团经过组合构成了硅烷、硅氧烷以及直链或支链的聚硅氧烷分子.例如十甲基四硅氧烷可以看作是由10 个“-Si-CH3”与6 个“-Si-O-”组成.同时随着“-Si-O-”不断连接延伸,再连接其它官能团,即可构建成各种聚硅氧烷.因此采用基团贡献法不仅可对硅烷、硅氧烷等小分子有机硅化合物的密度进行计算,也可以预测出线性或空间网状结构的硅油、硅树脂或硅橡胶等有机聚硅氧烷的密度.
密度随分子结构改变而改变,是与分子结构密切相关的物理量,因而密度与上述新组合生成的基团之间必然存在着一定的函数关系,考虑到随着基团的无限增加,而密度趋于一个有限值的事实,确定式(1)为所需的密度函数[7]:

式中的n(Gi)为构成硅烷、硅氧烷分子基团的数目,ci和ωi是与基团特性有关的常数.以110 种硅烷和硅氧烷化合物作为样本,对每一样本化合物采用式(1)建立一个关系式,最终构成一个含有110 个多元一次线性方程的方程组.
采用式(2)计算密度文献值与计算值之间的均方根误差(RMSD):
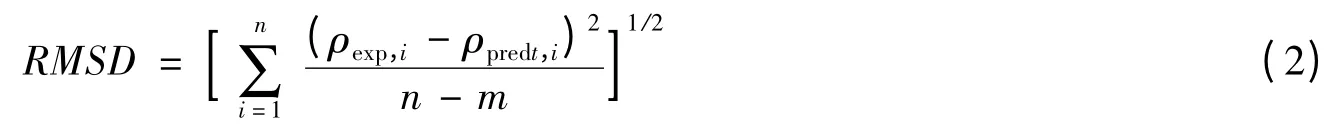
式中n 为密度回归方程组中化合物的数目,m 为回归方程组中回归参数的数目.
2 计算结果及讨论
以表2 中的110 种硅烷和硅氧烷化合物为样本,采用基团贡献法研究硅烷化合物中分子结构与密度之间的关系.采用式(1)描述每个化合物的原子和官能团种类和数量与化合物密度之间的关系,得到110个多元一次线性方程组成的方程组,利用七维高科有限公司生产的1stOpt 优化分析综合工具软件包中的“麦夸特法(Levenberg-Marquardt)+通用全局优化法(Universal Global Optimization-UGO)”组合算法对方程组进行最优化求解,得到各个基团的ci和ωi值,结果列于表3.
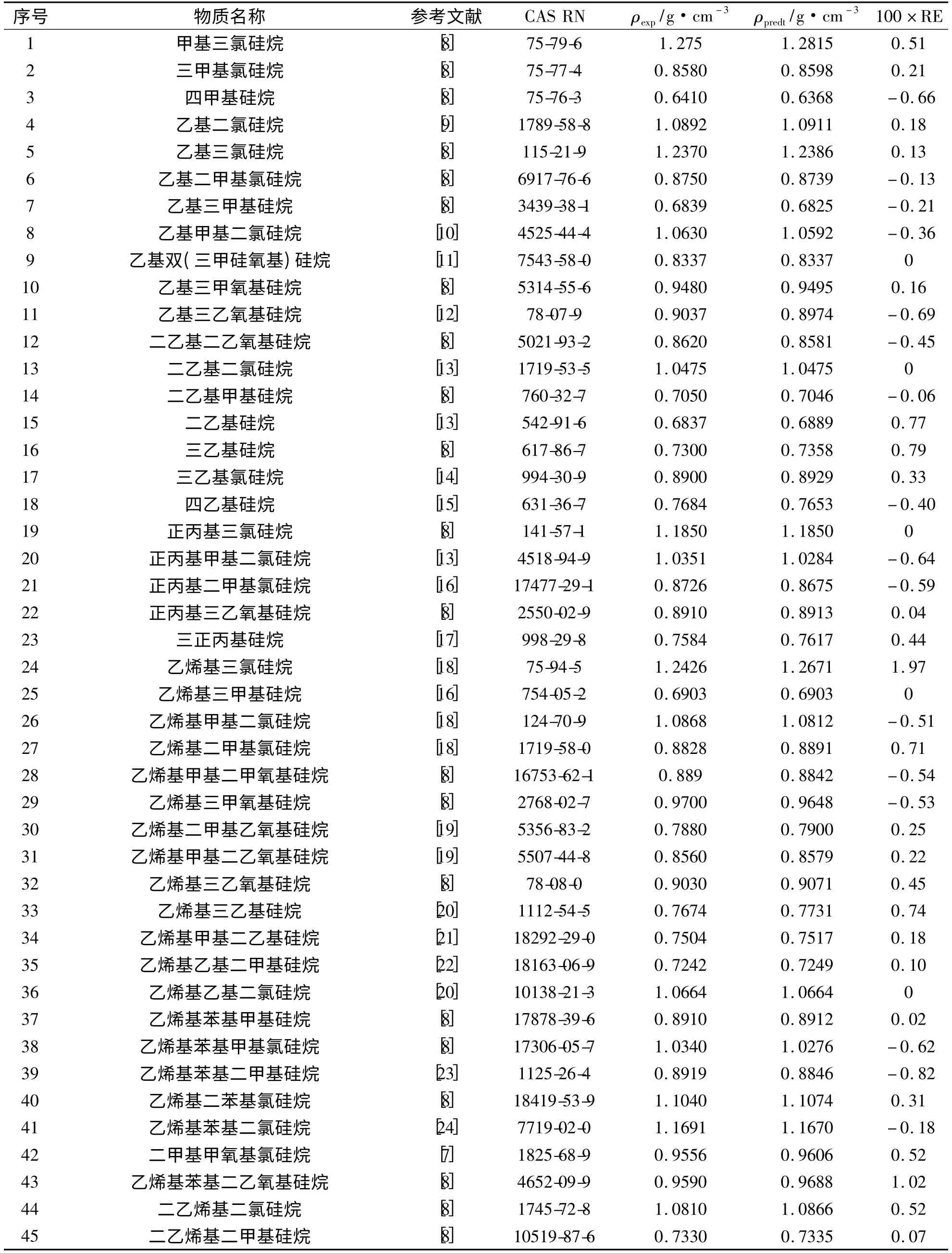
表2 110 种硅烷及硅氧烷化合物的密度Tab. 2 Values of density for 110 kinds of silane and siloxane compounds

续表

续表

表3 有机硅化合物各基团密度计算结果Tab. 3 Calculated values for various groups in silicone compound
利用拟合得到的基团密度常数,从硅烷或硅氧烷的分子结构出发,可以计算得到化合物的密度数据.例如,甲基三甲氧基硅烷(CH3Si(OCH3)3)密度表达式为:

图1 密度预测值与实验值的比较Fig.1 Comparisons between predicted and experimental values for density

将得到的密度常数c1= 0. 6420,c4= 1. 6083,ω1=1.0082及ω4=1.0859 代入上式计算得到甲基三甲氧基硅烷的密度ρpredt=0.9479 g·cm-3,与实验值ρexp=0.9548 g·cm-3非常接近,它们之间的相对误差仅为-0.73%. 计算得到110 个样本的密度数值并分别与其实验值进行对比,结果见表2,计算值与实验数据之间的总体平均相对误差、平均绝对误差及均方根误差分别为0.47%、0.0044 g·cm-3及0.0070 g·cm-3,计算结果显示预测值与实验值之间的偏差小(图1).
为了进一步验证基团贡献法的准确性,随意选取18 个样本,利用回归得到的各基团密度常数,对选取的18 个样本的密度进行计算.结果如表4 和图2 所示.
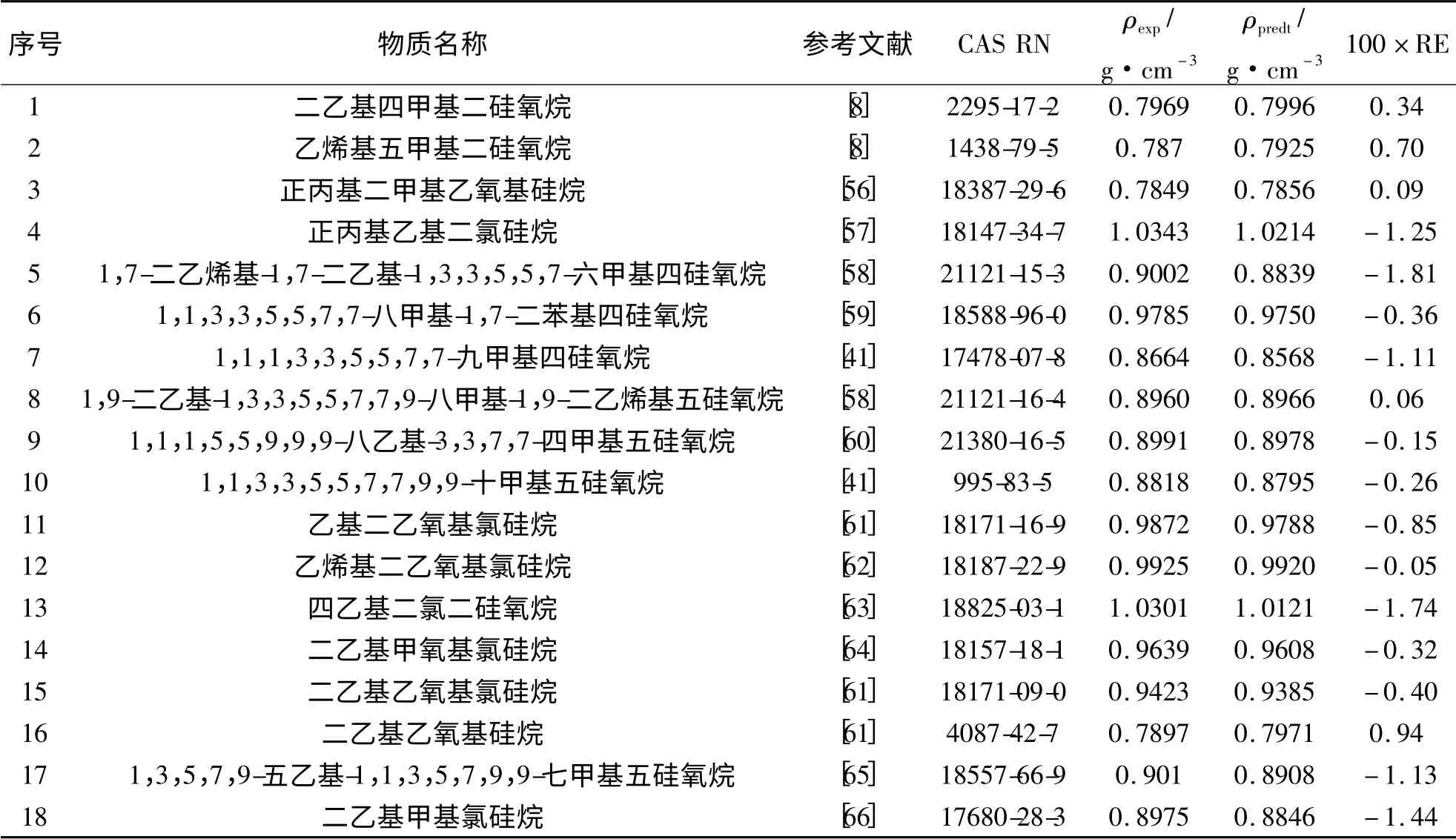
表4 对有机硅化合物密度预测结果Tab. 4 Predicted values obtained from group contribution method for silane
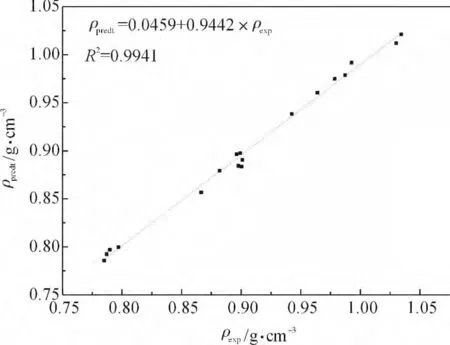
图2 预测样本的密度预测值与实验值的比较Fig.2 Comparisons between predicted and experimental values of density for 18 kinds of silicone compounds
18 个样本化合物预测结果的平均绝对误差是0.0066 g·cm-3、平均相对误差是0.72%、均方根误差是0.0161 g·cm-3.由此可见预测值与实验数据的一致性令人满意.
3 结 论
分析硅烷和硅氧烷分子的结构,对其进行合理拆分,组合成新的基团. 采用基团贡献法,利用最优化组合算法,得到基团对硅烷、硅氧烷的密度常数,通过这些数值,计算硅烷、硅氧烷的密度,并与实验值进行对比,结果表明本文中对基团的拆分及组合是合理的,回归得到的密度常数可以被用来计算硅烷、硅氧烷的密度,实验值与计算值具有良好的一致性,并且由于拆分组合的基团中“-Si-O-”可构成聚硅氧烷的硅氧骨架结构,所以利用本文中的基团贡献法也可为已知结构聚硅氧烷的密度预测提供一个有力工具.
[1]冯瑞英. 炔烃的摩尔体积和摩尔折射度与其分子结构之间关系的理论探讨[J]. 零陵师专学报,1993(3):40-43.
[2]王克强,冯瑞英. 烷烃摩尔体积与分子结构之间定量关系的探讨[J].许昌学院学报,2002,21(2):22-25.
[3]王从岗,陈泽辉,富嘉文,等. 基团贡献法预测石油馏分热力学性质的探索[J].石油炼制与化工,1993,24(5):57-64.
[4]王克强. 硅烷摩尔折射度与其分子结构之间关系的探讨[J].有机化学,1994,14(3):269-274.
[5]Kupchik E. J. Structure-Molar Refraction Relationships of Alkylsilanes Using Molecular Connectivity[J]. Quantitative Structure-Activity Relationships,1985,4(3):123-127.
[6]程大海,伍川,董红. 原子贡献法估算硅烷及硅氧烷的摩尔折射度[J].杭州师范大学学报:自然科学版,2013,12(5):395-403.
[7]王克强. 预测烷烃密度的新方法——基团键贡献法[J].有机化学,1999,19(3):304-308.
[8]Arkles B.Silanes,Silicones and Metal-Organics[J].Gelest Inc:Gelest Catalog,2000:75.
[9]Dolgov B N,Voronkov M G,Borisov S N. Catalytic transformations of a-lkyldichlorosilanes and dialkylchlorosilanes in the presence of a-luminum chloride[J]. Zhurnal Obshchei Khimii,1957,27:709-716.
[10]Kanazashi M. Studies on unsaturated organosilicon compounds. II. Reactions and properties of vinyltrimethylsilane[J]. Bulletin of the Chemical Society of Japan,1955,28(1):44-50.
[11]Borisov S N,Sviridova N G. Cleavage of siloxanes by halosilanes. A new method of synthesis of organosiloxanes[J]. Zhurnal Obshchei Khimii,1959,29:1534-1541.
[12]Freidlina R K,Chukovskaya E T,Tsao I. Addition of silanes to unsaturated compounds in the presence of iron pentacarbonyl[J]. Doklady Akademii Nauk SSSR,1959,127:352-355.
[13]Dolgov B N,Borisov S N,Voronkov M G. Reaction of dialkylchlorosilanes with aluminum chloride[J]. Zhurnal Obshchei Khimii,1957,27:2062-2066.
[14]Valade J,Calas R,Mileo J C. Organosilicon chemistry. Preparation of trialkylchlorosilanes[J]. Compt Rend,1959,249:1769-1770.
[15]Nametkin N C,Sorokin G V,Pozdnyakova M V,et al. Polymerization of ethylene with the titanium tetrachloride-trialkyl aluminum-trialkylsilane systems[J]. Vysokomolekulyarnye Soedineniya,Seriya B:Kratkie Soobshcheniya,1968,10(1):53-55.
[16]Petrov A D. Synthesis of α-trimethylsilylstyrene,1-trimethylsilylcyclohexene,and other unsaturated organosilicon compounds[J]. Zhurnal Obshchei Khimii,1957,27:1535-1539.
[17]Dolgov B N,Vinter G,Komarov V A,et al. Reaction of pentaerythritol with trialkylsilanes in presence of certain metal halides[J]. Bulletin of the Academy of Sciences of the USSR,Division of chemical science,1963,12(12):1978-1983.
[18]Sheludyakov V D. Characteristics of hydrochlorination of vinylsilanes with several vinyl groups at the silicon atom[J]. Zhurnal Obshchei Khimii,1985,55:1345-1350.
[19]Vaisarova V,Chvalovsky V. Organosilicon compounds. LVI. Dipole moments of substituted vinylsilanes and phenylsilanes[J]. Collection of Czechoslovak Chemical Communications,1968,33(3):859-865.
[20]Voronkov M G. Acetylene hydrosilylation reaction[J]. Doklady Akademii Nauk SSSR,1980,254:887-890.
[21]Mironov V F. Relative reactivity of some alkenylsilanes with trichlorosilane[J]. Izvestiya Akademii Nauk SSSR,Seriya Khimicheskaya,1960,4:760-762.
[22]Sommer L H,Bailey D L,Goldberg G M. Vinylsilanes,chlorovinylsilanes,and β-styryltrimethylsilane. Further studies on the α-silicon effect and β-eliminations involving silicon[J]. Journal of the American Chemical Society,1954,76(6):1613-1618.
[23]Kanazashi M. Unsaturated organosilicon compounds. I. Synthesis of organovinylsilanes by Wurtz-Fittig reaction[J]. Bulletin of the Chemical Society of Japan,1953,26(9):493-496.
[24]Steiling L,Wannagat U. Sila drugs,15. Synthesis and properties of silapridinol[J]. Zeitschrift fuer Naturforschung,Teil B:Anorganische Chemie,Organische Chemie,1979,34B:1413-1417.
[25]Mironov V F,Petrov A D,Maksimova N G. Organomagnesium synthesis of 2-and 3-(Trimethylsilyl)acrylic acids and vinyl derivatives of silicon,germanium,and tin[J]. Russian Chemical Bulletin,1959,8(11):1864-1870.
[26]Sheludyakov V D,Zhun V I,Polyakova M V,et al. Some data on the synthesis of vinylsilanes[J]. Zhurnal Obshchei Khimii Some data on the synthesis of vinylsilanes,1980,50(4):868-871.
[27]Zakharkin L I. Reduction of alkylc hlorosilanes with sodium hydride in the presence of triethylaluminum[J]. Russian Chemical Bulletin,1960,9(12):2079-2080.
[28]Molchanova T V,Kopylov A I,Burde N L,et al. Synthesis of some aromatic silanes[J]. Trudy Khimiko-Metallurgicheskogo Instituta,Akademiya Nauk Kazakhskoi SSR,1966,13:17-24.
[29]Lehnert R,Porzel A,Ruehlmann K. Preparation of siloxanes. 9. Position of the equilibrium chlorodimethylphenylsilane + water . dblharw.hydroxydimethylphenylsilane + hydrochloric acid in dioxane-benzene-d6[J]. Zeitschrift fuer Chemie,1988,28(5):190-192.
[30]Ruehlmann K,Grosse-Ruyken H,Scheller D,et al. Synthesis of siloxanes. XIV. Reaction of chlorosilanes with silanols in acid medium[J].Journal of Organometallic Chemistry,1988,356(1):39-47.
[31]Malnova G N,Mikheev E P,Klebansky A L,et al,Filimonova N P. Catalytic phenylation of hydrogen-containing alkylchlorosilanes with benzene[J]. Doklady Akademii Nauk SSSR,1957,117:623-625.
[32]Westermark H. Preparation of some organic silicon hydrides[J]. Acta Chemica Scandinavica,1954,8(10):1830-1834.
[33]Nagel R,Post H W. Silico-organic compounds. XXI. Alkyl and phenyI derivatives of yinyltrichlorosilane[J]. Journal of Organic Chemistry,1952,17:1379-1381.
[34]Lukevics E,Pudova O A,Dzintara M. Organometallic derivatives of sulfur-containing heterocycles. XIII. Dehydrocondensation of thienylsilanes with hydroxyl-containing compounds in the presence of organic bases[J]. Zhurnal Obshchei Khimii,1984,54(2):339-342.
[35]Koristek S,Langner J,Cermak J. Preparing organoalkoxysilanes and organoaryloxysilanes:Czech,CS 122624[P]. 1967-04-15.
[36]Voronkov M G. Alkoxysilanes. XIII. Reaction of siloxanes with alkoxysilanes. New method of synthesis of alkoxysilanes and siloxanes[J].Zhurnal Obshchei Khimii,1959,29:907-915.
[37]Andrianov K A,Delazari N V. Reactions of chloro (trimethylsiloxy)silanes[J]. Russian Chemical Bulletin,1961,10(12):2028-2031.
[38]Müller R,Köhne R,Sliwinski S.Über Silikone L Definierte Methylsiloxanes mit Si-H Bindungen[J]. Journal für Praktische Chemie,1960,11(5-6):336-340.
[39]Tanaka T,Tasaka A,Okawara R,et al. Lower members of methylmethoxypolysiloxanes with one to five silicon atoms[J]. Technology Reports of the Osaka University,1957,7:193-197.
[40]Krolevets A A,Antipova V V,Popov A G,et al. New syntheses of alkoxysilanes and their properties[J]. Zhurnal Obshchei Khimii,1988,58(10):2274-2281.
[41]Greber G,Metzinger L. Polysiloxane hydrides:West Germany,DE 1085875[P]. 1960-07-28.
[42]Lebedev E P,Fedorov A D. Cocondensation of organodisilazanes and organochlorosilanes with organosilanols and siloxanols[J]. Zhurnal Obshchei Khimii,1979,49(1):147-150.
[43]Kovalev I F,Ozolins L,Voronkov M G,et al. Integral intensities and depolarization ratios of Raman lines of (CH3)3SiOSi(CH3)3,(CH3)3SiOC(CH3)3,and(CH3)3COC(CH3)3[J]. Akademiya Nauk SSSR,Otdelenie Fiziko-Matematicheskikh Nauk,1967,3:301-307.
[44]Andreev D N,Kukharskaya E V. Condensation of hexamethyldisiloxane in a high-voltage silent discharge of sonic frequency[J]. Doklady Akademii Nauk SSSR,1960,134:817-820.
[45]Mironov I V,Siraeva I N,Kiladze T K,et al,Rakhmankulov D L. Hydrosilylation of 4,4-dimethyl-1,3-dioxane[J]. Zhurnal Obshchei Khimii,1981,51(12):2700-2704.
[46]Andrianov K A,Kochetkova A S,Khananashvili L M. Copolymerization of methylvinylsiloxane oligomers with methylhydridosiloxanes[J]. Zhurnal Obshchei Khimii,1968,38(1):175-178.
[47]Petrov A D,Vdovin V M. Reaction of symmetric tetraalkyldisiloxanes with dialkenyldialkylsilanes and symmetric tetraalkyldialkenyld isiloxanes[J]. Izvestiya Akademii Nauk SSSR,Seriya Khimicheskaya,1959,4:939-941.
[48]McCusker P A,Ostdick T. Reactions of haloboranes with organocyclosiloxanes. I. Boron chloride with methyl and ethyl trimer and tetramer[J].Journal of the American Chemical Society,1958,80(5):1103-1106.
[49]Borisov S N,Sviridova N G,Orlova V S. Reaction of silanols with hydrosilanes[J]. Zhurnal Obshchei Khimii,1966,36(4):687-692.
[50]Sliwinski S. Silicones. XCVI. Preparation of 1,3,3,5-tetramethyl-1,1,5,5-tetraphenyl-and 1,3,5-trimethyl-1,1,3,5,5-pentaphenyltrisiloxane[J]. Journal fuer Praktische Chemie (Leipzig),1966,32(1-2):31-36.
[51]Andreev D N,Lavrinovich L I. Resistance of Si-C bonds in dimethyldialkylsilanes to the action of sulfuric acid[J]. Zhurnal Obshchei Khimii,1968,38(12):2743-2751.
[52]Topchiev A V,Nametkin N S,Kartasheva L I. Reaction of ethyl bromide with silicon[J]. Russian Chemical Bulletin,1958,7(8):922-925.
[53]Sadykh-Zade S I,Petrov A D. Synthesis and reactions of butadienylsilanes[J]. Zhurnal Obshchei Khimii,1958,28:1542-1547.
[54]Patnode W I,Wilcock D F. Methylpolysiloxanes[J]. Journal of the American Chemical Society,1946,68:358-363.
[55]Borisov S N,Voronkov M G,Dolgov B N. The disproportionation of trialkylsilanes[J]. Bulletin of the Academy of Sciences of the USSR,Division of chemical science,1957,6(11):1416-1418.
[56]Westermark H. The exchange of ethoxy groups in methylethoxysilanes for alkyl groups[J]. Svensk Kemisk Tidskrift,1952,64:283-284.
[57]Ponomarenko V A,Sokolov B A,Petrov A D. Addition of methyldichlorosilane and ethyldichlorosilane to allyl halides[J]. Doklady Akademii Nauk SSSR,1956,106:76-79.
[58]Andrianov K A,Gavrikova L A,Rodionova E F. Synthesis and investigation of polymerization reactions of divinylsiloxane oligomers and their copolymerization with isoprene[J]. Izvestiya Akademii Nauk SSSR,Seriya Khimicheskaya,1968 (8):1786-1791.
[59]Brevnova T N,Semenov V V. Oxidation of 1-phenyl-2-chlorotetramethyldisilane by peroxide compounds[J]. Zhurnal Obshchei Khimii,1979,49(1):142-147.
[60]Andrianov K A,Nogaideli A I,Khananashvili L M,et al. Reaction of 1,5-dihydroorganotrisiloxanes with organosilanols[J]. Russian Chemical Bulletin,1968,17(9):2040-2041.
[61]Kumada M. Preparation of ethoxychlorosilanes and their alkyl derivatives by ethoxy-chlorine interconversions[J]. Journal of the Institute of Polytechnics,Osaka City University,1952,2(Ser. C):139-146.
[62]Alksne V,Grinevich K P. Alkenylalkoxychlorosilanes[J]. Latvijas PSR Zinatnu Akademijas Vestis,Kimijas Serija,1964 (2):249-252.
[63]McCusker P A,Ostdick T. Reactions of haloboranes with organocyclosiloxanes. I. Boron chloride with methyl and ethyl trimer and tetramer[J].Journal of the American Chemical Society,1958,80(5):1103-1106.
[64]Andrianov K A,Zhdanov A A,Kashutina E A. Reaction of diethylmethoxychlorosilane with sodium derivatives of acetylacetone and acetoacetic ester[J]. Zhurnal Obshchei Khimii,1962,32:297-301.
[65]Dow Corning Ltd. Ethylmethylpolysiloxanes:Great Britain,GB 659012[P].1951-10-17.
[66]Shostakovskii M F,Kochkin D A,Shikhiev I A. Synthesis and transformation of oxygen-containing organosilicon compounds. VII. Synthesis and some transformations of silanols[J]. Zhurnal Obshchei Khimii,1955,V25:622-626.
