手性Betti碱/二乙基锌催化不对称硅氢加成反应
2015-03-20彭家建厉嘉云肖文军徐艺凇
王 磊,彭家建,白 赢,厉嘉云,肖文军,徐艺凇,陈 峰
(杭州师范大学有机硅化学及材料技术教育部重点实验室,浙江杭州311121)
0 引言
19 世纪末20 世纪初,Betti 首先报道了1-(α-氨基苄基)-2-萘酚化合物的合成方法,开启了人们研究Betti 碱的大门[1].通过改进曼尼希反应制备Betti 碱衍生物是一种很重要的方法,该反应条件温和[2]. 在过去的几十年里,人们对Betti 碱类化合物的研究越来越重视,其中一个重要原因在于手性Betti 碱类衍生物可作为有效的手性催化剂或手性辅助剂[3-12]. 此外,由于氨基和酚羟基可以被转化成各种各样的化合物,Betti 碱类衍生物在有机合成的许多领域都扮演着重要角色[14-15].
Betti 最先报道了醛类和碘甲烷的烷基化反应,在合适的手性Betti 碱存在下,有机锂和有机镁试剂都能和羰基类化合物发生具有立体选择性的烷基化反应[16-18].最近关于羰基类化合物的立体选择性烷基化的研究有了重大发现,发现在一定的手性配体存在下,有机锌试剂也能很好的和羰基类化合物发生具有立体选择性的烷基化反应.
正是由于上述手性Betti 碱在羰基类化合物烷基化中表现出的高立体选择性,我们尝试利用手性Betti碱作为配体,考察其和金属配位后催化羰基化合物的不对称硅氢加成反应的催化性能.
1 实验部分
1.1 主要实验方法
四氢呋喃用钠丝处理后蒸馏,三乙氧基硅烷蒸馏收集134 -135 ℃馏份.
产物用GC9800 型气相色谱仪分析,填充柱的规格是SE-30.2 m×2.5 mm×0.25 μm,氢气为载气,流速:1 mL/min;柱升温程序:初温70 ℃,维持1 min,以10 ℃/min 升至100 ℃,然后以25 ℃/min 升至250℃,进样口温度为250 ℃.分析结果由2010 型色谱数据处理软件按面积归一化法计算.

图1 手性Betti 碱结构Fig. 1 Structure of chiral Betti base
1.2 手性Betti 碱的合成[19-21]
以配体1 为例:将2-萘酚(0.29 g,2.0 mmol),苯甲醛(0.26 g,2.4 mmol),(S)-1-苯乙胺(0.26 g,2.1 mmol)三者混合均匀,氮气保护下80 ℃搅拌10 h.用TLC 检测当反应结束后,停止加热.等混合物冷却至室温加入5 mL 乙醇,这时会有产物(白色晶体)析出.收集白色晶体用无水乙醇洗涤3 次(3 mL×3),再用正己烷/乙酸乙酯重结晶,得到无色晶体1,产率90%.
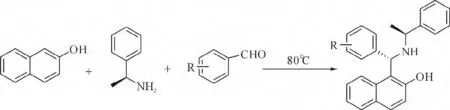
图2 手性Betti 碱合成Fig. 2 Synthesis of chiral Betti base
配体1:colorless crystals:90% yield.1H NMR (400 MHz,CDCl3)δ 13.83 (s,1H),7.87-7.73 (m,2H),7.54-7.35 (m,4H),7.35-7.16 (m,10H),5.53 (s,1H),3.95 (q,J =6.3 Hz,1H),2.37 (s,1H),1.55 (d,J=6.3 Hz,3H).13C NMR (101 MHz,CDCl3)δ 157.53,143.23,141.64,132.81,129.96,129.26,129.13,128.99,128.87,128.17,128.07,127.92,126.86,126.62,122.62,121.29,120.29,113.30,60.43,56.73,23.15.
配体2:white crystals:85% yield.1H NMR(400 MHz,CDCl3)δ 14.03 (s,1H),7.89-7.09 (m,15H),5.62 (s,1H),4.03 (d,J =6.3 Hz,1H),2.37 (s,4H),1.60 (d,J =6.3 Hz,3H).13C NMR (101 MHz,CDCl3)δ 157.59,143.30,141.74,138.96,132.91,129.97,129.21,129.15,129.05,128.93,128.53,128.10,126.93,126.67,125.08,122.66,121.42,120.38,113.48,60.57,56.77,23.19,21.69.
配体3:white crystals:85% yield.1H NMR (400 MHz,CDCl3)δ 13.97 (s,1H),7.84 (dd,J =8.0,13.5 Hz,2H),7.52-7.01 (m,13H),5.80 (s,1H),3.98 (d,J =6.3 Hz,1H),2.04 (s,4H),1.61(d,J=6.3 Hz,3H).13C NMR (101 MHz,CDCl3)δ 158.04,142.62,138.94,135.03,132.79,131.03,129.89,129.12,129.07,129.00,128.92,128.29,128.19,127.39,127.03,126.78,122.67,121.02,120.27,114.01,56.77,21.73,18.32.
配体4:white crystals:85% yield.1H NMR (400 MHz,CDCl3)δ 13.88 (s,1H),7.86-7.75 (m,2H),7.42-7.35 (m,4H),7.31-7.18 (m,6H),6.97 (dd,J=7.7,1.5 Hz,1H),6.86 (d,J=8.4 Hz,1H),6.74 (t,J=7.5 Hz,1H),5.89 (s,1H),3.93 (d,J=6.5 Hz,1H),3.75 (s,3H),2.58 (s,1H),1.52 (d,J=6.9 Hz,3H).13C NMR (101 MHz,CDCl3)δ 158.27,156.62,143.00,132.93,129.79,129.54,129.32,128.79,128.78,128.46,128.28,127.75,127.37,126.52,122.53,121.48,121.15,120.04,113.26,110.44,56.84,55.10,54.39,22.61.
配体5:white crystals:80% yield.1H NMR (400 MHz,CDCl3)δ 13.82 (s,1H),7.81-7.71 (m,2H),7.49-7.37 (m,5H),7.31-7.14 (m,10H),5.47 (s,1H),3.92 (d,J =6.3 Hz,1H),2.34 (s,1H),1.52 (d,J=6.3 Hz,3H),1.26 (s,9H).13C NMR (101 MHz,CDCl3)δ 157.53,150.94,143.36,138.73,132.88,129.86,129.12,129.00,128.89,128.04,127.57,126.89,126.58,126.16,122.60,121.42,120.35,113.66,60.03,56.70,34.63,31.47,23.21.
1.3 催化酮的硅氢加成反应[22]
氮气保护下,25 mL 烧瓶中加入0.08 mol 的手性Betti 碱配体(1,2,3,4 或5),5 mL 四氢呋喃,搅拌溶解后,再加入0.28 mL 二乙基锌(3.5equiv),搅拌4 h 后,在室温条件下,加入1 mmol 潜手性酮和5 mmol的三乙氧基硅烷,反应20 h 后,向混合溶液中加入5 mL 质量分数为15%的KOH 溶液水解4 h.加入少量二氯甲烷,收集有机层,无水硫酸钠干燥,过滤,用旋转蒸发仪旋干得到粗产物,过柱子得到纯产物.
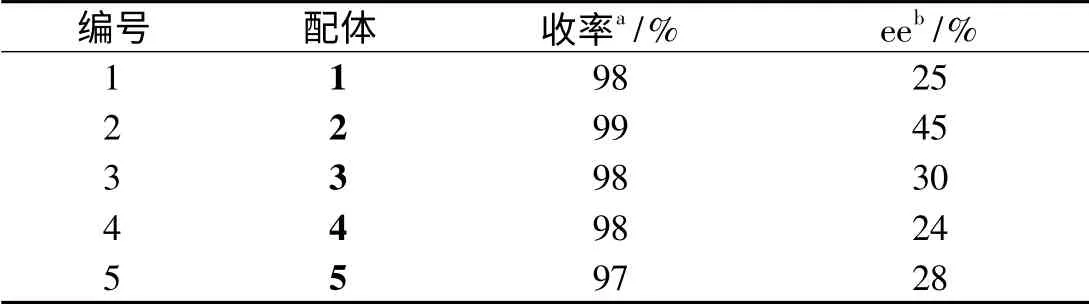
表1 苯乙酮与三乙氧基硅烷不对称硅氢加成Tab. 1 Asymmetric hydrosilylation of acetophenone with HSi(OEt)3
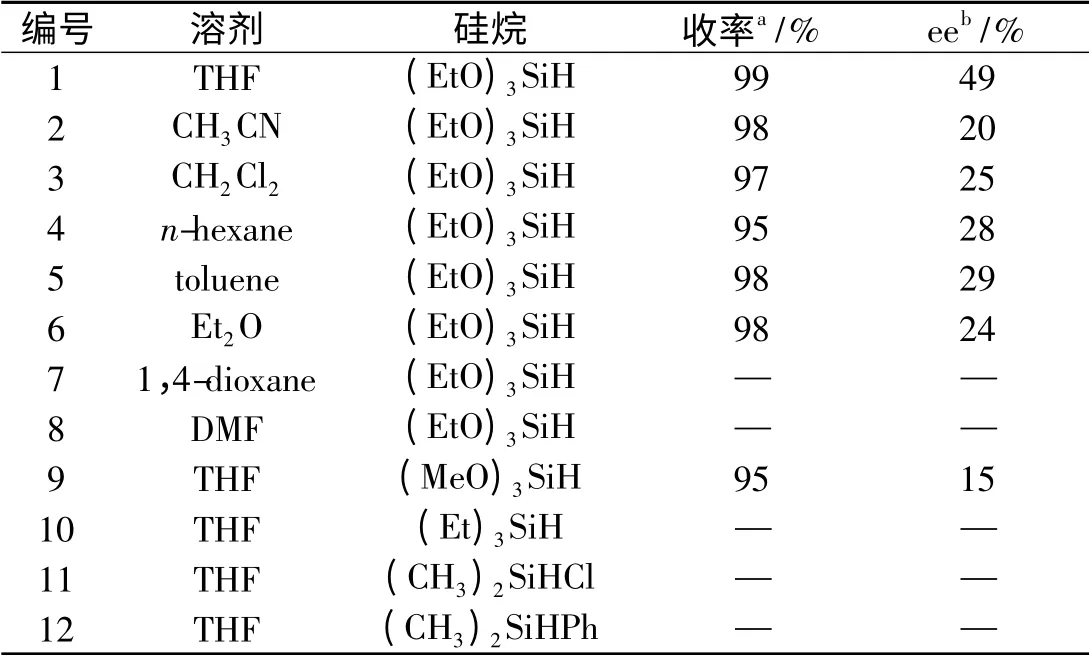
表2 溶剂及硅烷对硅氢加成反应影响Tab. 2 Solvent and silane screening for the asymmetric hydrosilylation
2 结果与讨论
2.1 不同Betti 碱对硅氢加成反应的影响
室温条件下,我们分别用Betti 碱1-5作配体,测试ZnEt2/不同配体催化剂对苯乙酮和三乙氧基硅烷不对称加成反应的影响,结果列于表1. 实验表明,制备配体的原料芳香族醛的苯环上带有不同取代基时,对于催化剂的立体选择性有较大影响.即使是相同的取代基但是在苯环上的位置不同时,其催化效果也不尽相同.
2.2 不同溶剂和硅烷对硅氢加成的影响
为了研究不同溶剂对Betti 碱/ZnEt2体系催化剂催化性能的影响,在室温下,三乙氧基硅烷作氢源,在不同溶剂中用Betti碱2/ZnEt2做催化剂,催化苯乙酮的硅氢加成反应,结果列于表2.实验表明不同溶剂对于Betti 碱2/ZnEt2催化剂体系催化活性没有太大影响,在几种常见的溶剂中,该催化体系均能很好的催化硅氢加成反应的进行,但是不同溶剂对于该催化体系的立体选择性还是有不同影响的,这可能和溶剂的极性有关.当选用四氢呋喃做溶剂时,该催化体系立体选择性最好.实验还证实只有当三乙氧基硅烷当氢源时效果最好,这可能是由于三乙氧基容易使Si 上的H 解离出来以及空间位阻较大造成的.
2.3 共溶剂和温度对硅氢加成反应的影响
在四氢呋喃作溶剂的条件下,我们在不同温度下以Betti 碱2/ZnEt2作催化剂催化苯乙酮和三乙氧基硅烷的硅氢加成反应,结果列于表3,实验表明温度对催化剂的活性和立体选择性均没什么影响.接下来我们又研究了共溶剂法对该催化剂的影响,数据表明催化剂的活性和立体选择性和t-BuOH 的量有着密切的关系.随着t-BuOH 用量的不断增加,催化剂的活性和立体选择性明显降低.
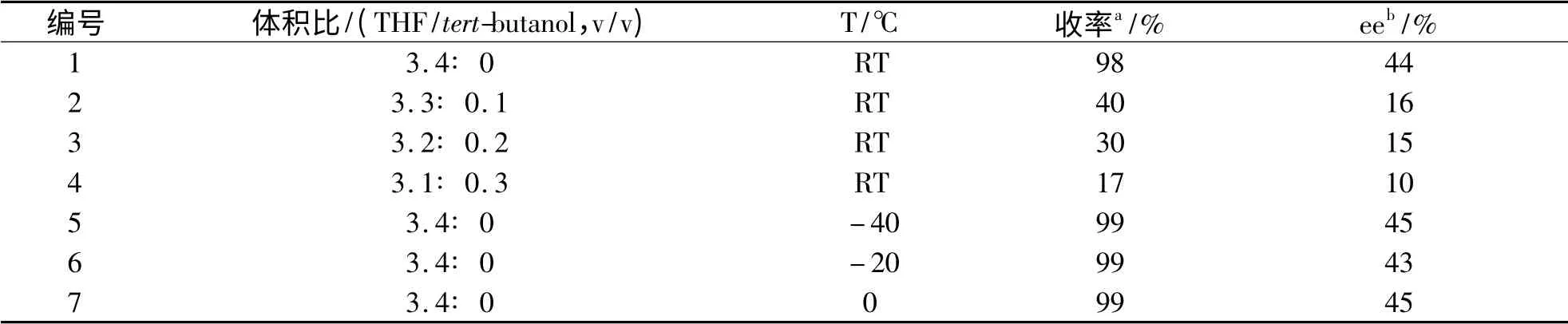
表3 四氢呋喃/叔丁醇体积比及反应温度对不对称硅氢加成反应的影响Tab. 3 The volume rate of THF/tert-butanol and the reaction temperature screening for the asymmetric hydrosilylation
2.4 不同底物对硅氢加成反应的影响
室温条件下,四氢呋喃作溶剂,选用Betti 碱2/ZnEt2作催化剂催化多种潜手性芳香酮和三乙氧基硅烷的硅氢加成反应,结果列于表4.该催化体系对于不同芳香族酮的催化活性都很好,但立体选择性相对来说还不能令人满意.

表4 不同芳基酮不对称硅氢加成Tab. 4 The asymmetric hydrosilylation of different aryl ketones
3 结 论
合成了5 个手性Betti 碱化合物,将其作为手性配体用于二乙基锌催化潜手性酮的不对称硅氢加成反应.Betti 碱/ZnEt2催化剂体系均可以得到较高的酮转化率,但其不对称选择性ee 值最高仅达到49%.反应温度和溶剂对转化率影响较小,但不同溶剂对其立体选择性有影响.所考察的不同硅烷中,使用三乙氧基硅烷作氢源,所得到的收率及立体选择性优于三甲氧基硅烷及三乙基硅烷作氢源时的结果.
[1]Betti M. ɑ-Naphthol phenylaminomethane[J]. Org Synth Colloid,1941,1:381-383.
[2]Szatmári I,Fueloep F. Synthesis and transformations of 1-(a-aminobenzyl)-2-naphthol derivatives[J].Curr Org Synth,2004,1(1):155-165.
[3]Cardellichio C,Ciccarella G,Naso F,et al.The Betti base:absolute configuration and routes to a family of related chiral nonracemic bases[J].Tetrahedron:Asymmetry,1998,9(20):3667-3675.
[4]Cardellicchio C,Ciccarella G,Naso F,et al. Use of readily available chiral compounds related to the Betti base in the enantioselective addition of diethylzinc to aryl aldehydes[J]. Tetrahedron,1999,55(51):14685-14692.
[5]Cimarelli C,Mazzanti A,Palmieri G,et al. Solvent-free asymmetric aminoalkylation of electron-rich aromatic compounds:stereoselective synthesis of aminoalkylnaphthols by crystallization-induced asymmetric transformation[J]. J Org Chem,2001,66(14):4759-4765.
[6]Liu D X,Zhang L C,Wang Q C S,et al.The application of chiral aminonaphthols in the enantioselective addition of diethylzinc to aryl aldehydes[J]. Org Lett,2001,3(17):2733-2735.
[7]Cimarelli C,Palmieri G,Volpini E. A practical stereoselective synthesis of secondary and tertiary aminonaphthols:chiral ligands for enantioselective catalysts in the addition of diethylzinc to benzaldehyde[J]. Tetrahedron:Asymmetry,2002,13(22):2417-2426.
[8]Lu J,Xu X,Wang S,et al. Novel preparation of nonracemic 1-[a-(1-azacycloalkyl)benzyl]-2-naphthols from Betti base and their application as chiral ligands in the asymmetric addition of diethylzinc to aryl aldehydes[J]. J Chem Soc Perkin Trans 1,2002(24),2900-2903.
[9]Lu J,Xu X,Wang S,et al.Synthesis of chiral ligands derived from the Betti base and their use in the enantioselective addition of diethylzinc to aromatic aldehydes[J]. Tetrahedron Lett,2002,43(46):8367-8369.
[10]Ji J X,Qui L Q,Yip C W,et al.A convenient,one-step synthesis of optically active tertiary aminonaphthol and its applications in the highly enantioselective alkenylations of aldehydes[J]. J Org Chem,2003,68(4):1589-1590.
[11]Ji J X,Wu J,Au-Yeung T T L,et al.Highly enantioselective phenyl transfer to aryl aldehydes catalyzed by easily accessible chiral tertiary aminonaphthol[J]. J Org Chem,2005,70(3):1093-1095.
[12]Dong Y,Li R,Lu J,et al. An efficient kinetic resolution of racemic Betti base based on an enantioselective N,O-deketalization[J]. J Org Chem,2005,70(21):8617-8620.
[13]Wang X,Dong Y,Sun J,et al.Nonracemic Betti base as a new chiral auxiliary:application to total syntheses of enantiopure (2S,6R)-dihydropinidine and (2S,6R)-isosolenopsins[J]. J Org Chem,2005,70(5):1897-1900.
[14]Szatmári I,Hetényi A,Lázár L,et al.Transformation reactions of the Betti base analog aminonaphthols[J]. J Heterocyclic Chem,2004,41(3):367-373.
[15]Heydenreich M,Koch A,Klod S,et al. Synthesis and conformational analysis of naphth[1’,2’:5,6][1,3]oxazino[3,2-c][1,3]benzoxazine and naphth[1’,2’:5,6][1,3]oxazino[3,4-c][1,3]benzoxazine derivatives[J]. Tetrahedron,2006,62(48):11081-11089.
[16]Noyori R,Kitamura M. Enantioselective addition of organometallic reagents to carbonyl compounds:chirality transfer,multiplication,and amplification[J]. Angew Chem,1991,30(1):49-69.
[17]Noyori R,Tokunaga M,Kitamura M. Stereoselective organic synthesis via dynamic kineti resolution[J]. Bull Chem Soc Jpn,1995,68(1):36-36.
[18]Tarbell D S,Paulson M C. Attempted asymmetric syntheses involving the Grignard reagent in optically active solvents[J]. J Am Chem Soc,1942,64(12):2842-2844.
