Li6SrLa2Nb2O12:Pr3+的发光性质
2014-08-02于潘龙田莲花
于潘龙,田莲花
(延边大学理学院 物理系,吉林 延吉 133002)
0 引言
汞在气体放电光源中起着非常重要的作用,然而汞对环境具有很大的潜在危害,因此无汞灯等照明灯的研究和生产具有重要意义[1-3].目前使用在无汞灯中的发光材料主要由三基色(如红色为Y2O3:Eu3+,绿色为CeMgAl11O19:Tb3+,蓝色为BaMgAl10O17:Eu2+)混合成白光[4],或在基质中掺入Eu2+离子(在400~600 nm间有宽带发射,可发白光),但该方法存在封装困难、发射峰的半宽与背景滤波片的波长范围不吻合[4-6]等问题;因此,研究出一种既能发射白光又使发射峰的半宽较窄的发光材料具有重要意义.

1 实验
采用高温固相法制备Li6SrLa2Nb2O12:Pr3+,Li2CO3、SrCO3、Nb2O5、La2O3、Pr2O3均为分析纯.依据化学式Li6SrLa2Nb2O12:Pr3+,按照化学计量准确称量并充分研磨后装入坩埚,在高温烧炉中以1 000 ℃加热2 h.
采用TD-2500型转靶X射线衍射仪分析物相,所用的阳极金属为Cu靶,X射线的波长为0.154 nm.激发光谱和发射光谱采用日立F-7000荧光光谱仪测定.结构图采用Klaus Brandenburg设计的Diamond软件制作.实验中的全部测量均在室温下进行.
2 结果与讨论
2.1 X射线衍射分析

图1为通过Diamond制作的Li6SrLa2Nb2O12的结构图.从图中可以看出,NbO6八面体共边共顶相连形成二维NbO6层,两层之间通过短的Nb—O键相连,每个Nb5+周围被6个O2-包围;Li+单独存在于基团周围,而Sr2+和La3+的格位被4个NbO6八面体所包围,配位数为8[18].当Pr3+掺入Li6SrLa2Nb2O12时,由于Pr3+半径(1.126 Å)和La3+半径(1.160 Å)相似,且两者都是三价离子,因此,Pr3+可以替换La3+的格位.

图1 通过Diamond制作的Li6SrLa2Nb2O12的结构图
图2为基质与掺入不同浓度Pr3+的Li6SrLa2Nb2O12:Pr3+的X射线衍射图谱,图中x代表Li6SrLa2Nb2O12基质中掺杂Pr3+的摩尔浓度.从图中可以看到,掺入低浓度Pr3+的Li6SrLa2-xNb2O12:xPr3+的X射线衍射图谱测试结果与Li6SrLa2Nb2O12的标准无机晶体结构数据库(ICSD)的卡片(卡号161387)相吻合,这说明Pr3+已替换了La3+离子的格位,且对基质晶格没有影响.

图2 (a) Li6SrLa2Nb2O12和(b)—(f) Li6SrLa2-xNb2O12:xPr3+(x=0.3、0.5、1、3、5 mol%)的X射线衍射图谱
2.2 Li6SrLa2Nb2O12:Pr3+荧光粉的发光性质
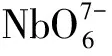
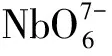

图3 Li6SrLa1.995Nb2O12:Pr3+(0.5 mol%)在491 nm下的激发光谱(a)和在232、280 nm激发下的发射光谱(b)
图4给出了Li6SrLa2-xNb2O12:xPr3+的2个发射峰(491 nm和610 nm处)的强度随Pr3+摩尔浓度(x)的变化曲线.由图可知,随着Pr3+浓度的增加,两个发射峰的强度先升高,当x=0.5 mol%时,样品的发光强度达到最大值,而后由于浓度猝灭,浓度大于0.5 mol%时,发光强度下降.
通过Li6SrLa1.995Nb2O12:Pr3+(0.5 mol%)的发射光谱,得到如图5所示的色度图,其色坐标为(0.228,0.282),颜色接近白光,因此可以考虑用于照明.

图4 Li6SrLa2-xNb2O12:xPr3+(x=0.3、0.5、1、3、5 mol%)的491 nm和610 nm发射峰峰值随Pr3+浓度的变化图(激发波长为280 nm)
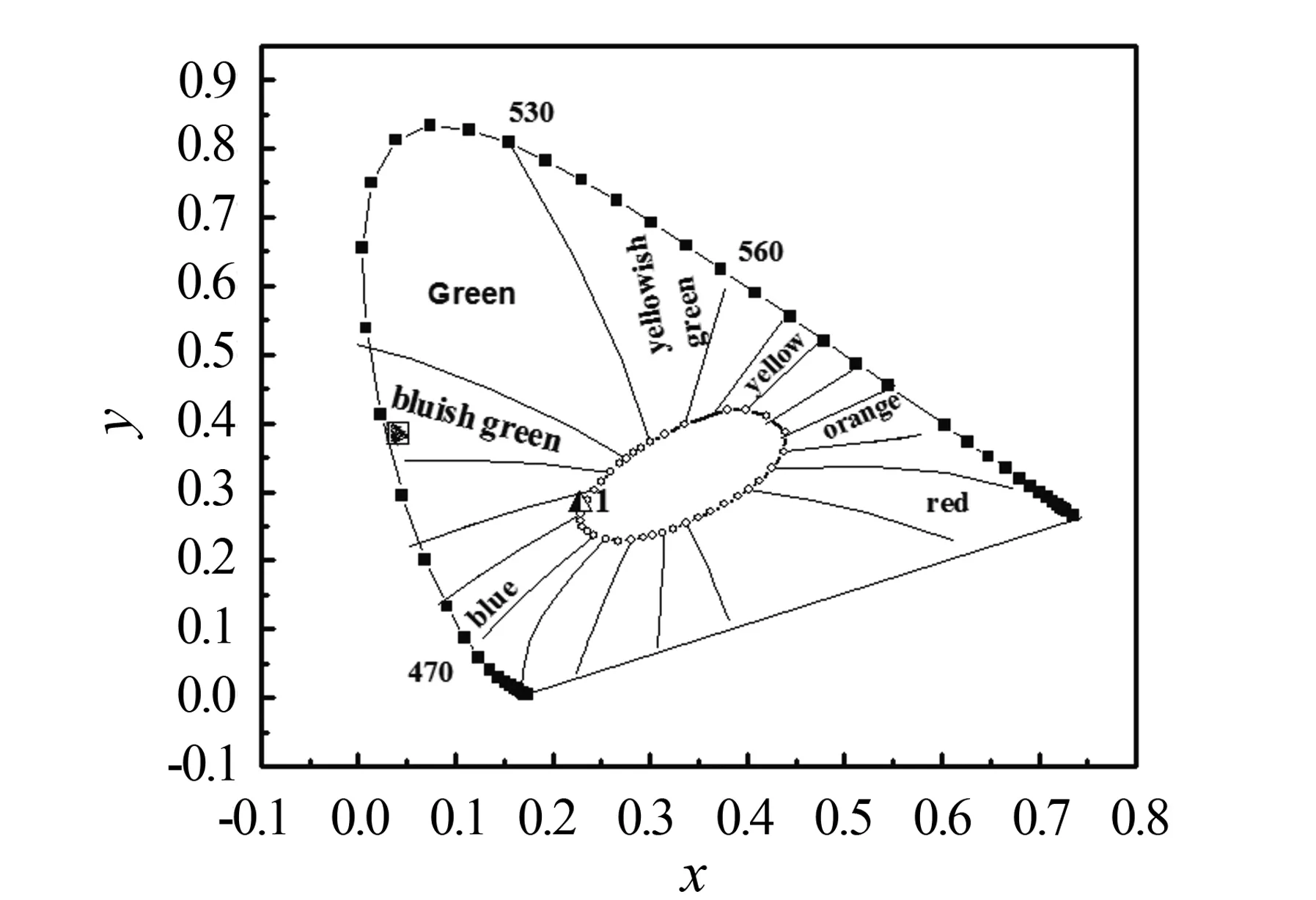
图5 Li6SrLa2-xNb2O12:Pr3+(0.5 mol%)的色度图
3 结论
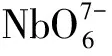
参考文献:
[1] 杨深,石挺,唐效峰,等.无汞光源研究进展:上[J].中国照明电器,2010(1):1-5.
[2] 王海鸥,译.无汞高强度放电灯[J].中国照明电器,2004(11):32-33.
[3] 刘洋,陈育明,龙奇,等.无汞光源的调研和探讨[J].中国照明电器,2005(12):14-17.
[4] Wu Y F, Yin X F, Zhang Q J, et al. The recycling of rare earths from waste tricolor phosphors in fluorescent lamps:a review of processes and technologies[J]. Resources, Conservation and Recycling, 2014,88:21-31.
[5] Yamamoto H. White LED phosphors:the next step[J]. Proc of SPIE, 2010,7598:75908-1-10.
[6] Zhu X W, Masubuchi Y, Motohashi T, et al. Thezvalue dependence of photoluminescence in Eu2+-dopedβ-SiAlON (Si6-zAlzOzN8-z) with 1≤z≤4[J]. J Alloys Compd, 2010,489(1):157-161.
[7] Yin J, Zou Z G, Ye J H. Photophysical and photocatalytic properties of MIn0.5Nb0.5O3(M=Ca,Sr and Ba)[J]. J Phys Chem B, 2003,107(12):61-65.
[8] Hsiao Y J, Fang T H, Ji L W. Photoluminescence and preparation of ZnNb2O6doped with Eu3+and Tm3+nanocrystals for solar cell[J]. Mater Chem Phys, 2011,130(3):1187-1190.
[9] Fang T H, Hsiao Y J, Chang Y S, et al. Photoluminescent characterization of KNbO3:Eu3+[J]. Mater Chem Phys, 2006,100(3):418-422.
[10] Cavalli E, Belletti A, Mahiou R, et al. Luminescence properties of Ba2NaNb5O15crystals activated with Sm3+, Eu3+, Tb3+or Dy3+ions[J]. J Lumin, 2010,130(4):733-736.
[11] Blasse G. Structure and Bonding 42[M]. Berlin:Springer-Verlag Press, 1980:25.
[12] Xiao X Z, Yan B. Synthesis and luminescent properties of novel RENbO4:Ln3+(RE=Y, Gd, Lu; Ln=Eu, Tb)[J]. J Non-cryst Solids, 2005,351(46-48):3634-3639.
[13] Shionoya S, Yen W M. Phosphor Handbook[M]. Boca Raton:CRC Press, 1999:187.
[14] Tian L H, Mho S I, Jin Z. Luminescence properties of red-emitting praseodymium-activated BaTi4O9phosphor[J]. J Lumin, 2009,129(8):797-800.
[15] Diallo P T, Boutinaud P, Mahiou R, et al. Red luminescence in Pr3+-doped calcium titanates[J]. Phys Status Solidi A, 1997,160(1):255-263.
[16] Hoefdraad H E, Blasse G. Green emitting praseodymium in calcium zirconate[J]. Phys Status Solidi A, 1975,29(1):K95-K97.
[17] 沈雷军,赵增祺,韩莉,等.Zn,Cd对CaTiO3:Pr3+发光性质的影响[J].发光学报,2007,28(1):74-78.
[18] Venkataraman T, Werner W. Li6ALa2Nb2O12(A=Ca, Sr, Ba):a new class of fast lithium ion conductors with garnet-like structure[J]. J Am Ceram Soc, 2005,88(2):411-418.
[19] 俞淳善,田莲花.Ca4LaNbMo4O20:Pr3+荧光粉的发光性质[J].发光学报,2012,33(5):499-503.
[20] 杨志平,郭智,王文杰,等.Pr3+摩尔浓度对CaTiO3:Pr3+红色长余辉材料的影响[J].功能材料与器件学报,2003,9(4):473-476.
[21] Liu F X, Fang Y Z, Hou J S, et al. Garnet-based red emitting phosphors Li6MLa2Nb2O12:Eu3+(M=Ca, Sr, Ba):photoluminescence improvement by changing crystal lattice[J]. Ceram Int, 2014,40(2):3237-3241.
[22] Geng J, Chen Y H, Gu G R, et al. Tunable white-light-emitting Sr2-xCaxNb2O7:Pr3+phosphor by adjusting the concentration of Ca2+ion[J]. Opt Mater, 2014,36(7):1093-1096.
