姜烯衍生物的合成及其与吲哚类化合物的Friedel-Crafts反应*
2013-03-26潘博文刘雄伟王华林
潘博文,石 洋,刘雄伟,曹 煜,王华林,周 英
(1.贵州大学生命科学学院贵州省中药民族药创制工程中心,贵州贵阳 550025;2.贵阳医学院附属医院,贵州贵阳 550025)
姜黄作为传统中药,具有破血行气、通经止痛的功能,现代医学研究表明,姜黄具有抗炎、抗氧化、抗肝毒、抗微生物、抗肿瘤及清除自由基等作用[1],近年来被用于治疗高血脂症。姜黄属植物主要包含挥发油和姜黄素类[2,3],姜黄素类具有抗肿瘤、抗突变、抗诱变、抗人类免疫缺陷病毒、抗氧化、降血脂和抗动脉粥样硬化的作用;姜黄挥发油中含有芳姜黄酮、姜烯等化合物[7,8],它们不仅有抑制肿瘤、抗癌、止咳平喘的作用,还能增强免疫功能[9]。
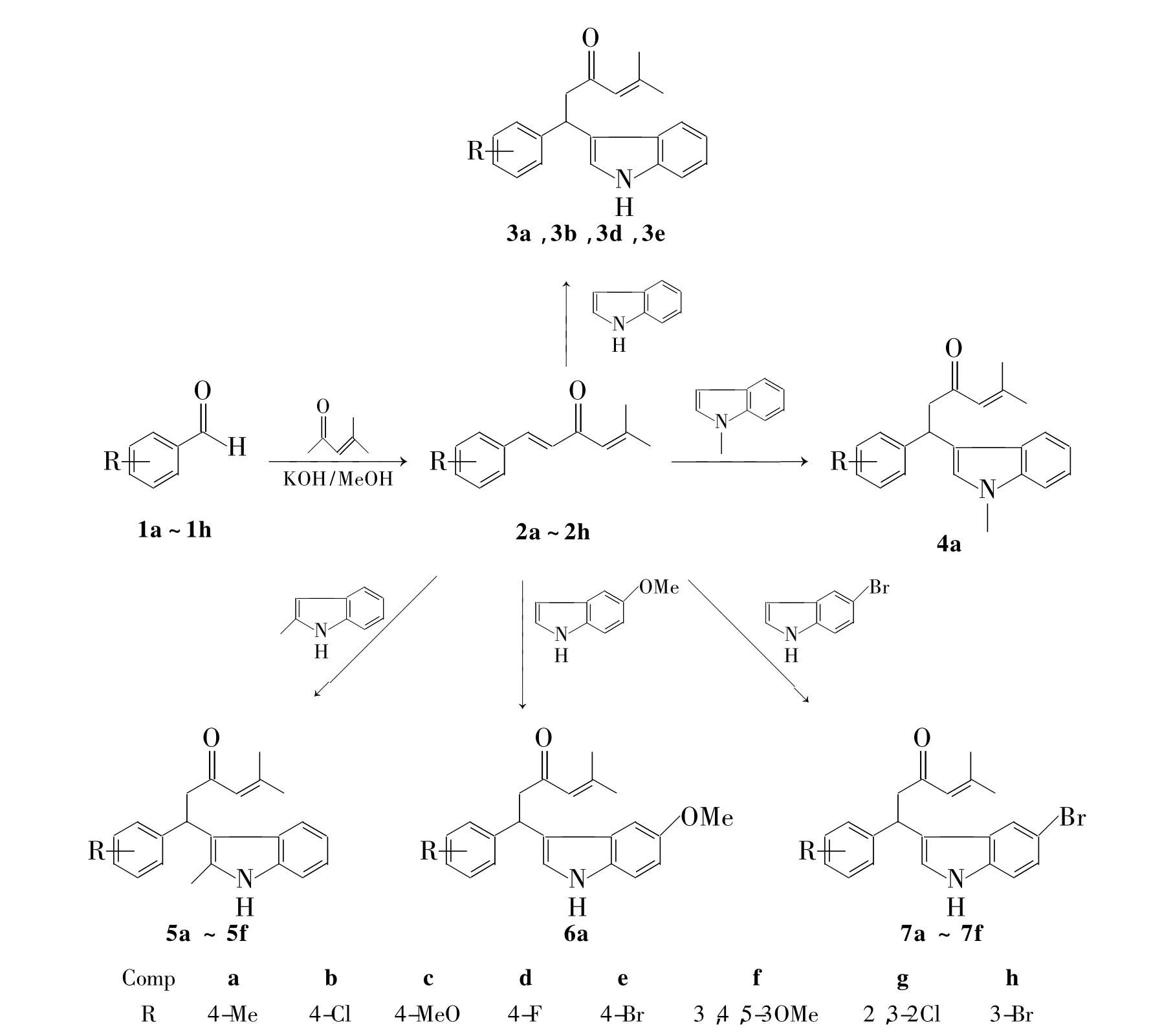
Scheme 1
为了满足生物活性和新药研究开发的需要,姜黄挥发油中一种活性成分芳姜黄酮类似物的结构衍生化反应已有相关文献报道[10~14]。本文根据药物设计原理,以姜黄挥发油中另一种活性成分姜烯为先导化合物,对其进行结构修饰,以期寻找新型生物活性物质。以芳基甲醛(1a~1h)和异丙叉丙酮为原料,经羟醛缩合反应合成了姜烯(2a)及其一系列衍生物(2b~2h);2a~2f与吲哚类化合物经Friedel-Crafts反应合成了18个新型的包含姜烯骨架和吲哚骨架的拼接衍生物(3~7,Scheme 1),其结构经1H NMR 和13C NMR表征。
1 实验部分
1.1 仪器与试剂
ZF-20D型三用紫外分析仪;Bruker-DRX500型和Bruker-AV400型核磁共振仪(CDCl3为溶剂,TMS为内标)。
所用试剂均为分析纯;所需无水溶剂均按标准程序处理。
1.2 合成
(1)2a~2h的合成[以5-甲基-1-(4-甲基苯基)-1,4-二己烯-3-酮(2a)为例]
在圆底烧瓶中依次加入对甲基苯甲醛(1a)6.00 g(50 mmol),异丙叉丙酮 14.70 g(150 mmol),甲醇15 mL和15%KOH 溶液1 mL,搅拌下于室温反应5 h。加入乙酸1 mL中和,用乙酸乙酯(3×60 mL)萃取,合并萃取液,用无水Mg-SO4干燥。减压蒸馏,收集第二个馏分(130℃ ~132℃/0.55 kPa),浓缩后用石油醚重结晶得淡黄色固体 2a 5 g,收率 59.0%;1H NMR δ:1.96(d,J=0.8 Hz,3H),2.21(d,J=0.8 Hz,3H),2.37(s,3H),6.34(s,1H),6.74(d,J=16.0 Hz,1H),7.19(d,J=8.0 Hz,2H),7.45(d,J=8.0 Hz,2H),7.54(d,J=16.0 Hz,1H);13C NMR δ:21.0,21.5,27.8,76.7,76.9,77.0,77.1,77.2,77.4,123.7,127.4,128.2,129.6,132.3,140.5,142.0,155.8,190.3。
用类似方法合成2b~2h,其表征数据[16]与Scheme 1预期结构吻合。
(2)3~7的合成(以3a为例)
在反应管中加入碘8 mg,于室温反应24 h[TLC跟踪,展开剂:A=V(石油醚)∶V(乙酸乙酯)=4∶1],旋蒸脱溶后经柱层析(CH2Cl2溶解上样,洗脱剂:A=8∶1)纯化得2-甲基-6-(4-甲基苯基)-6-吲哚基-2-己烯-4-酮(3a)。
用类似方法合成3~7。
3a:黄色液体,收率 90.0%;1H NMR δ:1.86(s,3H),2.09(s,3H),2.31(s,3H),3.13~3.18(m,1H),3.24 ~ 3.30(m,1H),4.88(t,J=7.4 Hz,1H),6.13(s,1H),6.97(s,1H),7.02 ~7.17(m,4H),7.22 ~7.31(m,3H),7.46(d,J=8.0 Hz,1H),8.09(br s,1H);13C NMR δ:20.8,21.0,27.7,38.0,50.9,76.8,77.1,77.4,111.1,119.2,119.3,119.5,121.4,121.9,123.8,126.7,127.6,129.1,135.5,136.6,141.4,155.6,199.5。
1-(3-吲哚基)-5-甲基-1-(4-氯苯基)-4-己烯-3-酮(3b):褐色液体,收率 94.2%;1H NMR δ:1.86(s,3H),2.08(d,J=8.0 Hz,3H),3.09 ~3.15(m,1H),3.22 ~3.28(m,1H),4.85(d,J=7.6 Hz,1H),6.09(s,1H),6.97 ~7.04(m,2H),7.14 ~7.26(m,5H),7.32(d,J=8.0 Hz,1H),7.38(d,J=8.0 Hz,1H),8.11(br s,1H);13C NMR δ:21.3,28.1,38.2,51.0,77.2,77.5,77.8,111.6,119.2,119.8,121.8,122.6,124.2,126.9,128.9,129.1,129.2,129.6,132.2,137.0,143.4,156.6,199.3。
1-(3-吲哚基)-5-甲基-1-(4-氟苯基)-4-己烯-3-酮(3d):红棕色液体,收率 95.0%;1H NMR δ:1.85(s,3H),2.08(s,3H),3.09 ~ 3.15(m,1H),3.22 ~3.28(m,1H),4.87(t,J=7.2 Hz,1H),6.09(s,1H),6.91 ~ 7.02(m,4H),7.14~7.18(m,1H),7.25 ~ 7.40(m,4H),8.10(br s,1H);13C NMR δ:21.2,28.1,38.1,51.2,77.2,77.5,77.8,111.6,115.4,115.6,119.5,119.8,119.9,121.8,122.6,124.2,126.9,129.6,129.7,137.0,140.5,140.6,156.4,160.5,162.9,199.5。
1-(3-吲哚基)-5-甲基-1-(4-溴苯基)-4-己烯-3-酮(3e):棕色液体,收率 85.6%;1H NMR δ:1.86(s,3H),2.09(s,3H),3.09 ~ 3.15(m,1H),3.22 ~3.27(m,1H),4.85(t,J=7.2 Hz,1H),6.09(s,1H),6.96(s,1H),7.01 ~7.05(m,1H),7.14 ~7.20(m,3H),7.31 ~7.39(m,4H),8.12(br s ,1H);13C NMR δ:20.8,27.7,37.7,50.4,76.7,77.1,77.4,111.2,118.6,119.4,119.9,121.4,122.2,123.7,126.4,129.6,131.4,136.6,143.5,156.3,198.8。
1-(3-N-甲基吲哚基)-5-甲基-1-(4-甲基苯基)-4-己烯-3-酮(4a):褐色液体,收率 48.8%;1H NMR δ:1.85(s,3H),2.07(d,J=2.8 Hz,3H),2.30(s,3H),3.12 ~ 3.17(m,1H),3.22 ~3.28(m,1H),3.71(t,J=5.8 Hz,3H),4.12 ~4.17(m,1H),6.11(s,1H),6.84(s,1H),7.03 ~7.09(m,3H),7.17 ~7.27(m,4H),7.46(d,J=8 Hz,1H);13C NMR δ:20.7,21.0,27.7,32.7,37.9,51.0,76.8,77.1,77.4,109.1,118.0,119.6,121.5,123.8,126.2,127.0,127.6,127.7,129.0,135.5,137.3,141.5,155.4,199.3。
1-(3-2-甲基吲哚基)-5-甲基-1-(4-甲基苯基)-4-己烯-3-酮(5a):土黄色固体,收率88.0%;1H NMR δ:1.68(s,3H),1.94(s,3H),2.18(s,3H),2.28(d,J=2.4 Hz,3H),3.24 ~3.27(m,2H),4.79(s,1H),5.91(t,J=2.4 Hz,1H),6.90 ~6.98(m,4H),7.10 ~7.16(m,3H),7.37(d,J=7.6 Hz,1H),7.71(br s,1H);13C NMR δ:12.2,20.7,20.9,27.6,36.4,49.1,76.7,77.1,77.4,110.3,113.9,119.0,119.2,120.6,124.3,127.4,127.7,128.9,131.5,135.2,135.5,141.5,154.8,199.6。
1-(3-2-甲基吲哚基)-5-甲基-1-(4-氯苯基)-4-己烯-3-酮(5b):黄绿色液体,收率 71.7%;1H NMR δ:1.70(s,3H),1.96(s,3H),2.28(s,3H),3.18 ~3.27(m,2H),4.80(t,J=7.2 Hz,1H),5.92(s,1H),6.90 ~7.00(m,2H),7.08 ~ 7.16(m,5H),7.31(d,J=8.0 Hz,1H),7.76(br s,1H);13C NMR δ:11.1,19.7,26.6,35.1,47.8,75.7,76.0,76.3,109.4,112.3,117.9,118.1,119.8,123.0,126.4,127.2,127.9,130.4,130.6,134.4,142.0,154.5,198.1。
1-(3-2-甲基吲哚基)-5-甲基-1-(4-甲氧基苯基)-4-己烯-3-酮(5c):红色液体,收率 72.4%;1H NMR δ:1.77(d,J=0.8 Hz,3H),2.03(d,J=0.8 Hz,3H),2.39(s,3H),3.26 ~3.36(m,2H),3.74(s,3H),4.85(t,J=7.2 Hz,1H),5.98(t,J=1.2 Hz,1H),6.76 ~6.78(m,2H),6.96 ~ 7.00(m,1H),7.03 ~ 7.07(m,1H),7.20 ~7.25(m,3H),7.44(d,J=7.6 Hz,1H),7.76(br s,1H);13C NMR δ:12.2,20.7,27.5,36.0,49.2,55.2,76.7,77.0,77.3,110.3,113.6,119.0,119.2,120.6,124.2,127.6,128.4,130.1,131.4,135.4,136.6,154.8,157.6,199.6。
1-(3-2-甲基吲哚基)-5-甲基-1-(4-氟苯基)-4-己烯-3-酮(5d):棕色液体,收率 76.5%;1H NMR δ:1.78(s,3H),2.04(s,3H),2.37(s,3H),3.25 ~3.38(m,2H),4.88(t,J=7.2 Hz,1H),5.99(d,J=0.8 Hz,1H),6.89(t,J=8.8 Hz,2H),6.97 ~7.01(m,1H),7.06(t,J=7.4 Hz,1H),7.21 ~7.26(m,3H),7.40(d,J=8.0 Hz,1H),7.80(br s,1H);13C NMR δ:12.2,20.7,27.6,36.0,49.1,76.7,77.0,77.4,110.4,113.7,114.8,115.0,119.1,119.2,120.8,124.1,127.5,128.9,129.0,131.5,135.5,140.2,155.3,159.9,162.3,199.3。
1-(3-2-甲基吲哚基)-5-甲基-1-(4-溴苯基)-4-己烯-3-酮(5e):淡黄色液体,收率 50.8%;1H NMR δ:1.77(s,3H),2.02(s,3H),2.36(s,3H),3.24 ~3.43(m,2H),4.83(t,J=7.2 Hz,1H),5.98(s,1H),6.95 ~6.98(m,1H),7.03 ~7.06(m,1H),7.19 ~ 7.26(m,1H),7.30 ~ 7.38(m,1H);13C NMR δ:12.4,21.1,28.0,36.6,49.1,77.2,77.5,77.8,110.9,119.3,119.4,119.5,119.6,119.9,121.0,121.1,124.4,127.7,129.7,131.6,144.0,156.2,199.9。
1-(3-2-甲基吲哚基)-5-甲基-1-(3,4,5-三甲氧基苯基)-4-己烯-3-酮(5f):淡黄色液体,82.4%;1H NMR δ:1.79(s,3H),2.05(s,3H),2.39(s,3H),3.32(d,J=7.2 Hz,2H),3.73(s,6H),3.79(s,3H),4.87(t,J=7.2 Hz,1H),6.00(s,1H),6.57(s,2H),7.02 ~7.07(m,2H),7.25(t,J=6.6 Hz,1H),7.54(d,J=7.6 Hz,1H),8.00(br s,1H);13C NMR δ:12.6,21.1,28.0,37.6,50.0,56.4,61.2,77.2,77.5,77.8,105.1,105.6,110.9,113.7,119.4,119.6,121.1,124.6,128.0,132.1,135.9,140.9,153.3,155.7,199.9。
1-(3-5-甲氧基吲哚基)-5-甲基-1-(4-甲基苯基)-4-己烯-3-酮(6a):深黄色液体,收率75.8%;1H NMR δ:1.83(s,3H),2.05(d,J=4.4 Hz,3H),2.28(d,J=8.8 Hz,3H),3.08 ~3.14(m,1H),3.18 ~3.24(m,1H),3.74(d,J=3.6 Hz,3H),4.76 ~ 4.80(m,1H),6.08(s,1H),6.77 ~6.80(m,1H),6.85(s,1H),6.94(s,1H),7.04 ~7.08(m,2H),7.16 ~7.25(m,3H),7.90(br s,1H);13C NMR δ:20.8,21.0,27.7,38.0,50.8,55.8,76.7,77.0,77.4,101.7,111.7,112.0,119.3,122.1,123.9,127.2,127.3,127.6,129.1,129.3,131.8,135.5,141.4,153.7,155.4,199.3。
1-(3-5-溴代吲哚基)-5-甲基-1-(4-甲基苯基)-4-己烯-3-酮(7a):土黄色液体,收率57.2%;1H NMR δ:1.84(s,3H),2.04(s,3H),2.28(s,3H),3.06 ~3.21(m,2H),4.76(t,J=7.4 Hz,1H),6.08(s,1H),6.97(d,J=2.0 Hz,1H),7.06(d,J=8.0 Hz,2H),7.13 ~7.21(m,4H),7.54(d,J=1.2 Hz,1H),8.06(br s,1H);13C NMR δ:20.8,21.0,27.7,37.8,50.9,76.7,77.0,77.4,112.5,112,6,119.2,122.1,122.6,123.7,124,9,127.5,128.5,129.2,135.2,135.8,141.0,155.9,199.0。
1-(3-5-溴代吲哚基)-5-甲基-1-(4-氯苯基)-4-己烯-3-酮(7b):棕色液体,收率 74.2%;1H NMR δ:1.86(s,3H),2.07(s,3H),3.06 ~3.12(m,1H),3.16 ~ 3.22(m,1H),4.78(t,J=7.0 Hz,1H),6.09(s,1H),6.95(s,1H),7.15 ~7.26(m,6H),7.50(s,1H),8.20(br s,1H);13C NMR δ:20.9,27.7,37.4,50.5,76.7,77.0,77.3,112.6,112.7,118.4,121.8,122.6,123.6,125.1,128.2,128.6,129.0,132.0,135.2,142.5,156.6,198.6。
1-(3-5-溴代吲哚基)-5-甲基-1-(4-甲氧基苯基)-4-己烯-3-酮(7c):棕色液体,收率 67.5%;1H NMR δ:1.85(s,3H),2.06(s,3H),3.06 ~3.11(m,1H),3.15 ~ 3.21(m,1H),3.76(s,3H),4.75(t,J=7.4 Hz,1H),6.10(s,1H),6.80(d,J=8.0 Hz,2H),6.96(s,1H),7.13 ~7.19(m,4H),7.53(s,1H),8.19(br s,1H);13C NMR δ:20.8,27.7,37.4,50.9,55.2,76.7,77.0,77.3,112.5,113.8,119.2,122.0,122.6,123.7,124.8,128.4,128.6,135.2,136.1,156.0,157.9,199.2。
1-(3-5-溴代吲哚基)-5-甲基-1-(4-氟苯基)-4-己烯-3-酮(7d):深棕色液体,收率 72.1%;1H NMR δ:1.86(s,3H),2.07(s,3H),3.06 ~3.12(m,1H),3.16 ~ 3.22(m,1H),4.79(t,J=7.2 Hz,1H),6.10(s,1H),6.95(d,J=7.6 Hz,3H),7.16 ~7.24(m,4H),7.49(s,1H),8.28(br s,1H);13C NMR δ:20.8,27.7,37.4,50.7,76.7,77.0,77.4,112.6,112.7,115.1,115.3,118.6,121.8,122.6,123.6,125.0,128.2,129.0,129.1,135.2,139.7,156.5,160.1,162.6,198.9。
1-(3-5-溴代吲哚基)-5-甲基-1-(4-溴苯基)-4-己烯-3-酮 (7e):红色液体,收率 74.3%;1H NMR δ:1.86(s,3H),2.07(s,3H),3.06 ~3.12(m,1H),3.16 ~ 3.22(m,1H),4.77(t,J=7.2 Hz,1H),6.09(s,1H),6.94(s,1H),7.13 ~7.22(m,4H),7.37(d,J=8.0 Hz,2H),7.50(s,1H),8.24(br s,1H);13C NMR δ:20.9,27.8,37.5,50.4,76.7,77.0,77.4,112.7,118.3,120.1,121.8,122.6,123.6,125.1,126.2,129.4,131.5,135.2,143.0,156.6,198.6。
1-(3-5-溴代吲哚基)-5-甲基-1-(3,4,5-三甲氧基苯基)-4-己烯-3-酮(7f):棕色液体,收率61.8%;1H NMR δ:1.85(s,3H),2.07(s,3H),3.07 ~ 3.12(m,1H),3.16 ~ 3.22(m,1H),3.77(s,5H),3.80(s,4H),4.75(t,J=7.2 Hz,1H),6.10(s,1H),6.50(s,2H),6.98(s,1H),7.15 ~7.22(m,2H),7.59(s,1H),8.40(br s,1H);13C NMR δ:20.8,27.7,38.5,50.8,56.0,60.8,104.7,112.5,112.7,118.6,122.0,122.8,123.7,124.9,128.3,135.2,136.2,139.9,153.0,156.2,198.9。
2 结果与讨论
以芳基甲醛和异丙叉丙酮为原料,合成了姜烯2a及其衍生物2b~2h。2a~2f与吲哚类化合物进行Friedel-Crafts反应,合成了18个含姜烯骨架和吲哚骨架的拼接衍生物,此拼接衍生物和拼接方法均未见文献报道。这26个化合物的抑制肿瘤及抗癌活性研究正在进行中。
2a在与吲哚进行Friedel-Crafts反应中进行了催化剂的筛选,分别使用了10 mol%磷钼酸、硝酸铋、碘、氯化亚铜、脯氨酸和硫酸铁铵,其中以碘为催化剂时收率最高(90%)。在最优的催化条件下进行了底物的扩展,在3~7的合成中,先以2a分别与吲哚类化合物进行反应,然后又选择具有供电子取代基的2-甲基吲哚和具有吸电子取代基的5-溴代吲哚分别与姜烯衍生物进行反应,结果显示供电子取代基使反应活性提高,反应时间缩短。
[1]汤敏燕,汪洪武,孙凌峰.中药姜黄挥发油化学成分研究[J].江西师范大学学报(自然科学版),2000,24(8):274 -277.
[2]李时珍.本草纲目(校点本)第2册[M].北京:人民卫生出版社,1979.
[3]陈铁晖,等.姜黄的化学成分及抗肿瘤作用研究进展[J].海峡预防医学志,2004,10(6):23 -25.
[4]杨模坤,黄晓萍,唐耀书.姜黄化学成分的研究[J].植物学通报,1984,(21):53 -54.
[5]方红钜,等.五种姜黄属要用根茎挥发油化学成分的比较[J].药学通报,1982,17(6):441.
[6]陈毓亨,等.我国姜黄属植物的研究Ⅲ.姜黄Curcuma longa根茎和块根挥发油和酚性成分的比较[J].中药通报,1983,8(1):27 -29.
[7]侯卫,韩素丽,王鸿梅.姜黄挥发油化学成分的分析[J].中草药,1999,1:15 -16.
[8]孙秀燕,李秀琴,王金辉等.姜黄挥发油抗癌活性成分研究[J].中草药,2006,37(7):982 -98.
[9]石雪蓉,顾健,谭睿.姜黄挥发油抗肿瘤作用机制研究[J].中药药理与临床,2003,19(6):15 -16.
[10]〛赵泽贞,温登瑰,魏丽珍等.姜黄油抗突变作用机理进一步试验研究[J].癌变.畸变.突变,1999,11(2):75-77.
[11]Sundberg R J.The reaction of vinyl grignard reagents with 2-substituted nitroarenes:A new approach to the synthesis of 7-substituted indoles[C].The Chemistry of Indoles,Academic Press:New York,1970.
[12]Gribble G W.Recent developments in indole ring synthesis-methodology and applications[J].J Chem Soc,Perkin Trans Ⅰ,2000,7:1045 -1075.
[13]Park O S.Synthetic studies on the sesquiterpenoids in nature:ar-Turmerone,a-Curcumene,Nuciferal[J].Synth Commun,1977,7:345.
[14]耿娓琴,等.芳姜黄酮和姜烯衍生物的合成及抗生育活性初探[J].华东化工学院学报,1990,16:61.
[15]Grieco P A,Frinkelhor R S.Dianions of beta-ketophosphonates.Two-step synthesis of(+)-ar-turmerone[J].J Org chem,1973,38:2909 -2910.
[16]5-甲基-1-(4-氯苯基)-1,4-二己烯-3-酮[2b,b.p.(146~148)℃/0.55 kPa]:淡黄色固体,收率51.5%;1H NMR δ:1.99(s,3H),2.24(s,3H),6.35(s,1H),6.73(d,J=16.0 Hz,1H),7.07 ~7.11(m,2H),7.53 ~7.57(m,3H);13C NMR δ:21.0,25.1,76.8,77.0,77.3,123.5,128.7,129.1,129.3,133.5,135.9,140.4,189.7.5-甲基-1-(4-甲氧基苯基)-1,4-二己烯-3-酮[2c,b.p.(162~164)℃/0.55 KPa]:黄绿色固体,收率46.2%;1H NMR δ:1.97(s,3H),2.21(s,3H),3.84(s,3H),6.34(s,1H),6.68(d,J=16.0 Hz,1H),6.91(d,J=8.5 Hz,2H),7.53(t,J=13.5 Hz,3H);13C NMR δ:20.9,25.1,55.3,114.3,123.7,126.1,127.6,129.8,130.1,141.9,161.3,190.3.5-甲基-1-(4-氟苯基)-1,4-二己烯-3-酮[2d,b.p.(136 ~138)℃ /0.55 kPa]:淡黄色固体,收率 48.0%;1H NMR δ:1.99(s,3H),2.24(s,3H),6.35(s,1H),6.73(d,J=16.0 Hz,1H),7.09(t,J=8.8 Hz,2H),7.53 ~7.57(m,3H);13C NMR δ:21.1,25.1,76.8,77.0,77.3,115.9,116.1,123.5,127.9,130.0,130.1,131.2,140.6,162.8,164.8,189.9.5-甲基-1-(4-溴 苯 基)-1,4-二 己 烯-3-酮[2e,b.p.(148~150)℃/0.55 kPa]:淡黄色固体,收率45.4%;1H NMR δ:1.97(d,J=1.2 Hz,3H),2.22(d,J=0.8 Hz,3H),6.33(t,J=1.2 Hz,1H),6.76(d,J=16.0 Hz,1H),7.40 ~ 7.42(m,2H),7.49 ~ 7.52(m,3H);13C NMR δ:21.1,27.9,76.7,77.0,77.4,123.5,124.3,128.7,129.5,132.1,133.9,140.5,157.0,189.7.5-甲基-1-(3,4,5-三甲氧基苯基)-1,4-二己烯-3-酮[2f,b.p.(174 ~176)℃ /0.55 kPa,无水乙醇重结晶]:淡黄色固体,收率 46.8%;1H NMR δ:2.27(d,J=4.8 Hz,3H),2.38(d,J=3.6 Hz,3H),3.87 ~3.92(m,9H),6.30(s,1H),6.71(d,J=4.0 Hz,2H),6.79(d,J=4.0 Hz,1H),6.91 ~ 6.96(m,1H);13C NMR δ:14.0,32.2,56.1,61.0,104.2,105.3,127.0,131.7,132.0,135.4,150.3,153.5,199.0.5-甲基-1-(2,3-二氯苯基)-1,4-二己烯-3-酮[2g,b.p.(141~143)℃/0.55 kPa]:黄色固体,收率47.5%;1H NMR δ:2.28(s,3H),2.39(s,3H),6.31(s,1H),7.18 ~ 7.22(m,1H),7.26(s,1H),7.37 ~ 7.42(m,2H),7.49 ~ 7.51(m,1H);13C NMR δ:14.1,32.2,124.9,127.3,128.4,130.0,131.2,132.0,133.8,136.0,137.0,149.6,199.0.5-甲基-1-(3-溴苯基)-1,4-二己烯-3-酮[2h,b.p.(145 ~147)℃ /0.55 kPa]:黄绿色固体,收率 45.6%;1H NMR δ:2.27(s,3H),2.35(d,J=1.2 Hz,3H),6.29(s,1H),6.76(d,J=16.0 Hz,1H),6.90(d,J=16.0 Hz,1H),7.21 ~ 7.26(m,1H),7.36 ~ 7.43(m,2H),7.63(t,J=1.6 Hz,1H);13C NMR δ:14.0,32.2,76.7,77.0,77.3,123.0,125.7,127.9,129.7,130.3,131.4,133.7,138.6,149.7,199.1.
