免疫耐受期HBV感染者抗病毒治疗的临床争议
2024-06-06胡林慧王艳
胡林慧 王艳

摘要: 为实现 “2030年消除病毒性肝炎作为公共卫生危害” 的目标, 目前针对慢性HBV感染提倡更广泛筛查、 更积极预防和抗病毒治疗。但 “慢性HBV感染免疫耐受期患者是否启动抗病毒治疗” 尚无统一观点。部分专家认为免疫耐受期患者肝脏免疫微环境稳定, 疾病进展可能小, 且治疗效果不佳, 不建议启动抗病毒治疗; 而另有多项研究提示免疫耐受期患者肝脏仍存在炎症损伤, 有疾病进展风险, 接受抗病毒治疗后成本效益高, 因此部分专家建议对免疫耐受期患者应积极启动抗病毒治疗。本文对慢性HBV感染者免疫耐受期的定义、 抗病毒治疗的利弊进行文献综述, 并基于既往文献进行初步的系统分析, 以增加对慢性HBV感染免疫耐受期是否抗病毒治疗的证据积累, 为未来免疫耐受期患者的规范临床诊疗奠定基础。
关键词: 乙型肝炎病毒; 免疫耐受; 治疗学
Clinical controversies over antiviral therapy for patients in the immune-tolerant phase of hepatitis B virus infection
HU Linhui, WANG Yan. (Department of Infectious Diseases, Peking University First Hospital, Beijing 100034, China)
Corresponding author: WANG Yan, wangyanwang@bjmu.edu.cn (ORCID: 0000-0002-8577-0527)
Abstract:
To achieve the goal of “eliminating viral hepatitis as a public health hazard by 2030”, extensive screening, active prevention, and antiviral therapy are currently recommended for chronic hepatitis B virus (HBV) infection; however, no consensus has been reached on whether to initiate antiviral therapy for patients in the immune-tolerant phase of chronic HBV infection. Some experts believe that patients in the immune-tolerant phase tend to have a stable liver immune microenvironment, with a low risk of disease progression and poor response to treatment, and thus it is not recommended to initiate antiviral therapy. However, various other studies have shown that patients in the immune-tolerant phase still have inflammatory damage in the liver, with a risk of disease progression and a high level of cost effectiveness, and therefore, some experts suggest that antiviral therapy should be actively initiated for patients in the immune-tolerant phase. This article performs a literature review of the definition of patients in the immune-tolerant phase of chronic HBV infection and the advantages and disadvantages of antiviral therapy and conducts a preliminary analysis based on previous studies, in order to accumulate the evidence for whether to initiate antiviral therapy in the immune-tolerant phase of chronic HBV infection and lay a foundation for standardized clinical diagnosis and treatment of patients in the immune-tolerant phase.
Key words: Hepatitis B virus; Immune Tolerance; Therapeutics
我國免疫耐受 (immune tolerance, IT) 期患者基数较大, 约1 584万例[1] 。“慢性HBV感染免疫耐受期患者是否启动抗病毒治疗” 尚无统一观点。关于IT期的定义、诊断和治疗尚有很多亟待解决的问题。部分专家认为免疫耐受期患者肝脏免疫微环境稳定, 疾病进展可能小, 且治疗效果不佳, 对延长生存期未见明显获益, 不建议启动抗病毒治疗; 而另有学者认为IT期患者肝脏仍存在炎症损伤, 有疾病进展风险, 接受抗病毒治疗后对于减缓疾病进展、 减少感染患者数量、 抑制HBV的全球传播有很大获益。鉴于目前仍存争议, 本文就现有研究对CHB患者IT期的定义、 IT期治疗的不同观点作汇总对比, 并基于既往文献进行初步系统分析。
1 IT期定义
鉴于对IT期免疫应答状态的解读不同, 各地区关于IT期的定义尚不统一 (表1)。
我国 《慢性乙型肝炎防治指南 (2022年版)》6将慢性HBV感染自然史分期分为: IT期、 免疫活动期、 免疫控制期、 再活动期。但仍有部分CHB患者并不符合通常定义的任何一种免疫状态分期, 处于 “灰区”(grey zone,GZ)。2018年版AASLD慢性乙型肝炎指南[2] 将CHB “灰区” 分为以下4组: GZ-A: HBeAg阳性, ALT水平正常, 血清HBV DNA≤106 IU/mL; GZ-B: HBeAg阳性, ALT水平升高, 血清HBV DNA≤2×104 IU/mL; GZ-C: HBeAg阴性, ALT水平正常, 血清HBV DNA≥2×103 IU/mL; GZ-D: HBeAg阴性, ALT水平升高, 血清HBV DNA≤2×103 IU/mL。
2 IT期免疫应答状态
HBV导致的肝细胞损伤通过免疫介导, 其严重程度取决于HBV与宿主免疫系统的相互抗衡[7] 。HBV感染慢性化的原因之一是不能有效激活过继免疫、 病毒特异性CD8+T淋巴细胞功能耗竭以及特异性B淋巴细胞功能障碍、 数量减少[8-10] 。参与抗HBV免疫反应的大多数细胞因子及细胞毒性颗粒 (如穿孔素、 颗粒酶B等) 进而在HBV感染慢性化过程中发挥作用[11]。同时, HBsAg、HBeAg、 HBcAg可进一步抑制免疫细胞功能, 诱导免疫耐受, 临床表现为ALT水平正常, HBeAg阳性, HBV DNA水平显著升高[12] 。根据2018年版AASLD慢性乙型肝炎指南[2] , 免疫耐受是指免疫系统在接受特定抗原刺激后无特异性免疫应答产生。然而, 有学者[13] 认为HBV慢性感染的自然病程中不存在绝对免疫耐受状态 (即使在IT期)。Bertoletti等[14]研究证实CHB患者IT期可以产生完全正常的T淋巴细胞应答; Traum等[9] 和Liang等[15] 发现T淋巴细胞程序性死亡受体1表达在IT期并没有显著增加; Kennedy等[16] 研究结果提示在IT期存在HBV特异性T淋巴细胞应答; 甚至有研究[17-19] 发现IT期患者外周血辅助性T淋巴细胞9、 CD4+CD25+Foxp3+ T淋巴细胞以及调节性T淋巴细胞的频率升高。但IT期免疫应答相关研究结果并不统一。徐清浪等[20] 通过对55例IT期慢性HBV感染者的随访研究发现, IT组CD3+T淋巴细胞、CD4+T淋巴细胞绝对值及比例、 CD4+/CD8+T淋巴细胞比值均低于非IT组, 差异有统计学意义。另有研究[21] 发现对于病毒载量>107IU /mL的患者, 几乎检测不到CD8+T淋巴细胞, 其缺失机制尚不清楚, 有报道称可能部分与CD8+T淋巴细胞凋亡增加有关。
3 IT期抗病毒治疗的利弊
3. 1 IT期患者抗病毒可减轻肝损伤, 降低疾病进展风险, 成本效益高 有研究[22] 报道, ALT水平正常且HBV DNA高水平的慢性HBV感染者中有很大一部分存在肝脏炎症。随访12个月, 有60%高病毒载量且血清ALT水平正常或轻微升高的患者出现明显纤维化[23] 。一个队列研究[24] 对IT期患者进行肝组织活检, 结果显示32. 7%的IT期患者肝脏存在F≥2肝纤维化; 按AASLD定义的乙型肝炎IT期 (ALT界值: 男性<35 U/L, 女性<25 U/L) 再次界定患者, 仍有22. 7% IT期患者的肝脏存在F≥2肝纤维化, 队列中年轻的IT期患者肝纤维化的比例甚至更高, 结果显示<30岁的IT期患者有36. 0%存在F≥2肝纤维化。Li等[25] 发现IT期患者接受抗病毒治疗后, 产生IFN-γ的恒定自然殺伤T淋巴细胞的百分比增加, 而产生IL-4的恒定自然杀伤T淋巴细胞的百分比减少, 提示抗病毒治疗可促进HBV清除, 缓解肝细胞免疫介导的损伤。
尽管HBeAg阳性的慢性HBV感染者发展为肝细胞癌 (HCC) 的概率很低, 但随着感染持续、 年龄增长、 并发症出现, HCC累积发生风险会逐年增高。抗病毒治疗可以将HCC 10年累积发病率降低至1. 7%以下[26] 。IT期患者进行早期抗病毒干预, 可通过抑制HBV DNA复制,从而减轻免疫介导的肝脏炎症程度、 缩短炎症持续时间, 进而减少肝细胞再生、 降低肝细胞克隆扩增的选择压力。Lai等[27] 随访26例IT期并接受抗病毒治疗的患者, 结果显示抗病毒治疗可阻止疾病进展、 降低HBV相关病死率。
国内外均有研究[28-29] 通过建模比较IT期患者即刻启动抗病毒治疗与延迟至活动期再开启抗病毒治疗的成本效益, 比较预期成本和调整寿命年, 结果显示在IT期即刻开始抗病毒治疗可改善生活质量, 减少过早死亡, 防止并发症带来的昂贵费用, 更具有成本效益。
3. 2 IT期肝损伤轻微, 疾病进展可能性小, 抗病毒疗效不佳, 停药容易复发 IT期的临床特征是高病毒复制、HBeAg阳性以及血清ALT水平正常、 无明显肝损伤。部分学者[17]认为IT期肝脏炎症坏死或纤维化很少或没有, 因此疾病进展的总体风险极低。Hui等[30] 对57例IT期成年患者随访5年, 发现84%患者仍处于IT期且肝纤维化程度无进展。Chen等[31] 研究结果显示HBV DNA水平持续高于2×106 IU/mL且ALT水平正常的患者中, 高病毒载量与预期的高肝癌风险并不相关。全敏团队[32] 前期研究发现不同水平的HBV DNA与HCC发生的关系并不呈线性相关。
在IT期进行抗病毒治疗, HBeAg血清转换率低。Feld等[33] 研究结果显示28例IT期患者抗病毒治疗48周后, 只有1例 (3. 6%) 达到HBeAg阴转。Wu等[34] 观察发现年龄<20岁、 HBV DNA>108 IU/mL、 ALT水平正常的患者抗病毒治疗后病毒下降不理想, 部分患者抗病毒治疗2年后HBV DNA仍>104 IU/mL。HBsAg清除或血清学转化被视为 “功能性治愈” 和治疗终点。然而, IT期患者进行抗病毒治疗时 HBsAg的阴转率很低。Kim 等[35]对5 409例接受核苷 (酸) 类似物治疗的CHB患者随访6年后发现仅有110例患者实现了HBsAg阴转 (年血清清除率为0. 33%)。
李彥霖等[36]通过检索PubMed、 EMBASE、 Cochrane library、 中国知网、 万方数据库自数据库建立至2020年9月CHB患者IT期抗病毒药物治疗的临床试验, 共纳入821例患者, 结果发现抗病毒治疗结束后病毒学复发常见。Feld等[33] 亦发现, IT期患者在停止治疗后HBV DNA可反弹至基线水平。既往研究[2-4] 结果提示IT期患者抗病毒治疗后不仅HBV DNA得不到有效抑制, 还可能诱发HBV耐药突变。
4 IT期是否启动抗病毒治疗
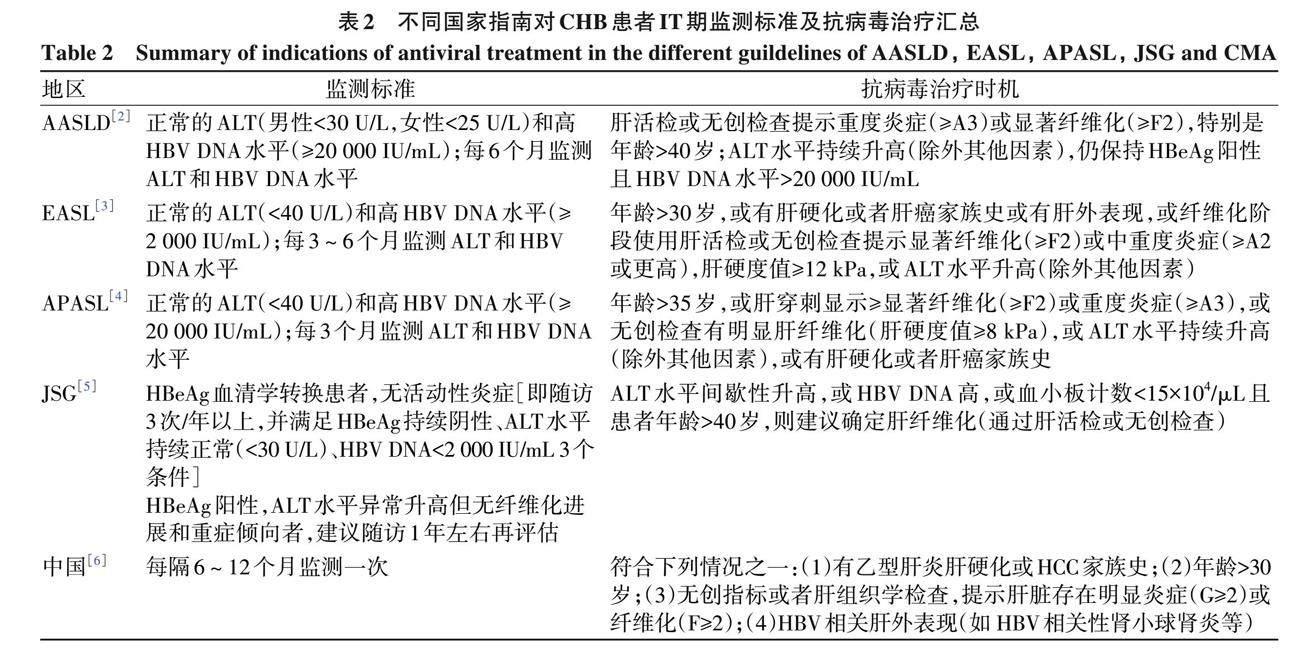
针对CHB患者IT期的监测标准以及何时是启动抗病毒治疗的最佳时机, 各地区诊疗指南均根据年龄、 是否有HCC或肝硬化的家族史以及是否发生肝纤维化给予临床推荐意见 (表2)。
基于既往数据结论和观点尚不统一, 笔者检索了PubMed、 Web of Science、 EMBASE、 Cochrane library、 中国知网自数据库建立至2024年2月慢性HBV感染者IT期抗病毒治疗的相关文献, 以HCC发生率与病毒学应答指标为结局指标进行文献汇总及分析。尝试系统评价IT期患者启动抗病毒治疗的疗效和价值。以HCC发生率为结局指标, 共检索445篇文献, 最终纳入4项研究[37-40] 1 660例IT期患者, 分析结果显示未抗病毒治疗患者HCC发生率为5% (95%CI: 0. 00~0. 10), 10年发病率为6% (95%CI:?0. 01~0. 12)。以HBV DNA阴转率、 HBeAg阴转率、HBsAg阴转率为结局指标, 共检索535篇文献, 最终纳入4项研究[41-44] 230例患者, 发现HBV DNA阴转率可达40% (95%CI: 0. 08~0. 72); 其中3项研究发现HBeAg阴转率为15% (95%CI: ?0. 01~0. 31); 其中2项研究分析发现HBsAg阴转率8% (95%CI: ?0. 03~0. 20)。从最大化抑制病毒的角度, IT期患者接受抗病毒治疗可部分获益。由于纳入分析的研究较少, Galbraith图提示存在发表偏倚, 漏斗图明显不对称。且由于各个研究间的差异, 无法进一步定量分析, 亟待开展更多具有肝组织学随访的高质量临床研究以进一步明确CHB患者IT期抗病毒治疗是否可以获益。
5 讨论
关于CHB患者IT期定义、 诊治观点不一。目前临床亟待解决的问题是:(1) 如何从ALT水平正常人群中准确识别真正的IT期患者, 对此尚缺乏确凿的诊断依据和指标。(2) 现有的抗病毒药物治疗IT期患者很难实现病毒清除, cccDNA的清除及HBV DNA的整合问题仍是难题。(3) 参与抗HBV免疫反应的大多数细胞因子及细胞毒性颗粒 (如穿孔素、 颗粒酶B等) 在HBV感染慢性化过程中发挥作用, 以穿孔素、 颗粒酶为治疗靶点延缓病情进展的应用前景如何?对于CHB患者IT期是否即刻启动抗病毒治疗仍需要更多高质量大样本的前瞻性研究来明确, 为临床诊疗指明方向。
利益冲突声明: 本文不存在任何利益冲突。
作者贡献声明: 胡慧林负责文章撰写; 王艳负责结构框
架设计并最后定稿。
参考文献:
[1] ZHUANG H. Correction note on the estimated number of patients in the immune-tolerant phase of hepatitis B virus infection in China and globally[J]. J Clin Hepatol, 2021, 37(4): 785-786. DOI: 10.3969/j.issn.1001-5256.2021.04.012.
庄辉. 全球和我国HBV感染免疫耐受期患者人数估计更正说明[J]. 临床肝胆病杂志, 2021, 37(4): 785-786. DOI: 10.3969/j.issn.1001-5256.2021.04.012.
[2] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599. DOI: 10.1002/hep.29800.
[3] European Association for the Study of the Liver. EASL 2017 Clinical Prac?tice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398. DOI: 10.1016/j.jhep.2017.03.021.
[4] SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10(1): 1-98. DOI: 10.1007/s12072-015-9675-4.
[5] Drafting Committee for Hepatitis Management Guidelines, the Ja?pan Society of Hepatology. Japan Society of Hepatology guidelines for the management of hepatitis C virus infection: 2019 update[J]. Hepatol Res, 2020, 50(7): 791-816. DOI: 10.1111/hepr.13503.
[6] Chinese Society of Hepatology, Chinese Medical Association, Chi?nese Society of Infectious Diseases, Chinese Medical Association.Guidelines for the prevention and treatment of chronic hepatitis B (2022 version)[J]. Chin J Infect Dis, 2023, 41(1): 3-28. DOI: 10.3760/cma.j.cn311365-20230220-00050.
中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华传染病杂志, 2023, 41(1): 3-28. DOI: 10.3760/cma.j.cn311365-20230220-00050.
[7] ZHENG PY, DOU YQ, WANG QY. Immune response and treatment targets of chronic hepatitis B virus infection: Innate and adaptive immu?nity[J]. Front Cell Infect Microbiol, 2023, 13: 1206720. DOI: 10.3389/fcimb.2023.1206720.
[8] ZHENG JR, WANG ZL, FENG B. Hepatitis B functional cure and im?mune response[J]. Front Immunol, 2022, 13: 1075916. DOI: 10.3389/fimmu.2022.1075916.
[9] TRAUM D, WANG YJ, SCHWARZ KB, et al. Highly multiplexed 2-di?mensional imaging mass cytometry analysis of HBV-infected liver[J]. JCI Insight, 2021, 6(7): e146883. DOI: 10.1172/jci.insight.146883.
[10] LIU Y, WANG GY, CHEN YX, et al. HBcAg-induced upregulated 4-1BB ligand on B cells contributes to B-cell hyperactivation during chronic hepatitis B infection[J]. J Med Virol, 2019, 91(5): 781-790. DOI: 10.1002/jmv.25377.
[11] HU LH, CHENG H, YAO TT, et al. Role of perforin and granzyme B in different immune status of hepatitis B[J]. Infect Dis Inf, 2023, 36(5): 458-462, 468. DOI: 10.3969/j.issn.1007-8134.2023.05.014.
胡林慧, 程浩, 姚甜甜, 等. 穿孔素与颗粒酶B在乙型肝炎不同免疫状态中的作用[J]. 传染病信息, 2023, 36(5): 458-462, 468. DOI: 10.3969/j.issn.1007-8134.2023.05.014.
[12] Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Diseases, Chinese Medical Association; Chi?nese Society of Hepatology, Chinese Medical Association. Manage?ment algorithm for interrupting mother-to-child transmission of hepa?titis B[J]. J Clin Hepatol, 2017, 33(7): 1214-1217. DOI: 10.3969/j.issn.1001-5256.2017.07.003.
中国肝炎防治基金会, 中华医学会感染病学分会, 中华医学会肝病学分会. 乙型肝炎母婴阻断临床管理流程[J]. 临床肝胆病杂志, 2017, 33(7): 1214-1217. DOI: 10.3969/j.issn.1001-5256.2017.07.003.
[13] ZHOU J, WANG FD, WANG ML, et al. Antiviral therapy for chronic HBV infection with persistently normal alanine aminotransferase: Contro?versy and consensus[J]. Front Med (Lausanne), 2021, 8: 717125. DOI: 10.3389/fmed.2021.717125.
[14] BERTOLETTI A, KENNEDY PT. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept[J]. Cell Mol Immunol, 2015, 12(3): 258-263. DOI: 10.1038/cmi.2014.79.
[15] LIANG XS, ZHOU Y, LI CZ, et al. Natural course of chronic hepatitis B is characterized by changing patterns of programmed death type-1 of CD8-positive T cells[J]. World J Gastroenterol, 2010, 16(5): 618-624. DOI: 10.3748/wjg.v16.i5.618.
[16] KENNEDY PTF, SANDALOVA E, JO J, et al. Preserved T-cell func?tion in children and young adults with immune-tolerant chronic hepa?titis B[J]. Gastroenterology, 2012, 143(3): 637-645. DOI: 10.1053/j.gastro.2012.06.009.
[17] WEI JG, SHI TD, ZOU LY. The role of Th9 cells in different stages of chronic hepatitis B virus infection[J]. Immunol J, 2019, 35(10): 879-884. DOI: 10.13431/j.cnki.immunol.j.20190137.
魏金刚, 石统东, 邹丽云. Th9细胞在慢性乙型肝炎病毒感染不同阶段中的作用[J]. 免疫学杂志, 2019, 35(10): 879-884. DOI: 10.13431/j.cnki.immunol.j.20190137.
[18] DENG M, LI MH, LIU SN, et al. Studies about the level of CD4+ CD25+ regulatory T cells and relation between expression of Foxp3 and CD127 in peripheral blood of chronic HBV infection[J]. Chin J Exp Clin Virol, 2010, 24(1): 21-23. DOI: 10.3760/cma. j. issn. 1003-9279.2010.01.008.
邓敏, 李明慧, 刘顺爱, 等. 慢性HBV感染者调节性T细胞水平及Foxp3与CD127表达关系的研究[J]. 中华实验和临床病毒学杂志, 2010, 24(1): 21-23. DOI: 10.3760/cma.j.issn.1003-9279.2010.01.008.
[19] BERTOLINI TB, BISWAS M, TERHORST C, et al. Role of orally in?duced regulatory T cells in immunotherapy and tolerance[J]. Cell Immunol, 2021, 359: 104251. DOI: 10.1016/j.cellimm.2020.104251.
[20] XU QL, HUANG JS, FU JW, et al. Characteristics and clinical signifi?cance of T lymphocyte subsets in peripheral blood during immune tol?erance period of chronic HBV infection[J/CD]. Electron J Emerg In?fect Dis, 2022, 7(4): 6-11, 2. DOI: 10.19871/j.cnki.xfcrbzz.2022.04.002.
徐清浪, 黄建生, 付吉伟, 等. 慢性HBV感染免疫耐受期外周血T淋巴细胞亚群变化特征及临床意义[J/CD]. 新发传染病电子杂志, 2022, 7(4): 6-11, 2. DOI: 10.19871/j.cnki.xfcrbzz.2022.04.002.
[21] LOPES AR, KELLAM P, DAS A, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection[J]. J Clin Invest, 2008, 118(5): 1835-1845. DOI: 10.1172/JCI33402.
[22] CHAO DT, LIM JK, AYOUB WS, et al. Systematic review with meta-analysis: The proportion of chronic hepatitis B patients with normal ala?nine transaminase≤40 IU/L and significant hepatic fibrosis[J]. Aliment Pharmacol Ther, 2014, 39(4): 349-358. DOI: 10.1111/apt.12590.
[23] PARK JY, PARK YN, KIM DY, et al. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with geno?type C and high serum HBV DNA levels[J]. J Viral Hepat, 2008, 15(8): 615-621. DOI: 10.1111/j.1365-2893.2008.00989.x.
[24] LIN X. Correlation between HBsAg and Liver Fibrosis in Immune-tol?erant Hepatitis B Patients[D]. Fuzhou: Fujian Medical University, 2020.
林欣. HBsAg与乙肝免疫耐受期患者肝纤维化的关系研究[D]. 福州: 福建医科大学, 2020.
[25] LI M, ZHOU ZH, SUN XH, et al. The dynamic changes of circulating invariant natural killer T cells during chronic hepatitis B virus infec?tion[J]. Hepatol Int, 2016, 10(4): 594-601. DOI: 10.1007/s12072-015-9650-0.
[26] KOFFAS A, MAK LY, GILL US, et al. Early treatment consideration in patients with hepatitis B ‘e antigen-positive chronic infection: Is it time for a paradigm shift?[J]. Viruses, 2022, 14(5): 900. DOI: 10.3390/v14050900.
[27] LAI M, HYATT BJ, NASSER I, et al. The clinical significance of per?sistently normal ALT in chronic hepatitis B infection[J]. J Hepatol, 2007, 47(6): 760-767. DOI: 10.1016/j.jhep.2007.07.022.
[28] KIM HL, KIM GA, PARK JA, et al. Cost-effectiveness of antiviral treat?ment in adult patients with immune-tolerant phase chronic hepatitis B[J]. Gut, 2021, 70(11): 2172-2182. DOI: 10.1136/gutjnl-2020-321309.
[29] LI RQ, LIN X, WANG JY, et al. Cost-effectiveness of combination anti?viral treatment with extended duration for hepatitis B e antigen (HBeAg)-negative chronic hepatitis B in China[J]. Ann Transl Med, 2021, 9(17): 1365. DOI: 10.21037/atm-21-1666.
[30] HUI CK, LEUNG N, YUEN ST, et al. Natural history and disease progres?sion in Chinese chronic hepatitis B patients in immune-tolerant phase[J]. Hepatology, 2007, 46(2): 395-401. DOI: 10.1002/hep.21724.
[31] CHEN CF, LEE WC, YANG HI, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma[J]. Gastroenterology, 2011, 141(4): 1240-1248, 1248. e1-1248. e2. DOI: 10.1053/j.gastro.2011.06.036.
[32] QUAN M, XING HC. Antiviral therapy is not recommended in im?mune-tolerant phase chronic hepatitis B patients without complica?tions[J]. J Clin Hepatol, 2021, 37(6): 1279-1280. DOI: 10.3969/j.issn.1001-5256.2021.06.011.
全敏, 邢卉春. 无合并症的真实慢性乙型肝炎免疫耐受期患者不建议抗病毒治疗[J]. 临床肝胆病杂志, 2021, 37(6): 1279-1280. DOI: 10.3969/j.issn.1001-5256.2021.06.011.
[33] FELD JJ, TERRAULT NA, LIN HH S, et al. Entecavir and peginter?feron alfa-2a in adults with hepatitis B e antigen-positive immune-tol?erant chronic hepatitis B virus infection[J]. Hepatology, 2019, 69(6): 2338-2348. DOI: 10.1002/hep.30417.
[34] WU ZX, CHEN FS, ZHOU XL, et al. Tenofovir and telbivudine combi?nation therapy rapidly decreases viral loads in immune-tolerant chronic hepatitis B patients awaiting assisted reproduction: An open-label, randomized, controlled study[J]. Eur J Gastroenterol Hepatol, 2019, 31(7): 832-835. DOI: 10.1097/MEG.0000000000001345.
[35] KIM GA, LIM YS, AN J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: Clinical outcomes and durability[J]. Gut, 2014, 63(8): 1325-1332. DOI: 10.1136/gutjnl-2013-305517.
[36] LI YL, WANG Y, ZHOU YQ, et al. Antiviral therapy for patients with chronic hepatitis B in the immune-tolerant phase: A systematic re?view[J]. J Clin Hepatol, 2021, 37(6): 1282-1287. DOI: 10.3969/j.issn.1001-5256.2021.06.013.
李彥霖, 王妍, 周颖琼, 等. 慢性乙型肝炎免疫耐受期的抗病毒治疗: 系统综述[J]. 临床肝胆病杂志, 2021, 37(6): 1282-1287. DOI: 10.3969/j.issn.1001-5256.2021.06.013.
[37] JEON MY, KIM BK, LEE JS, et al. Negligible risks of hepatocellular carcinoma during biomarker-defined immune-tolerant phase for pa?tients with chronic hepatitis B[J]. Clin Mol Hepatol, 2021, 27(2): 295-304. DOI: 10.3350/cmh.2020.0216.
[38] LEE HA, LEE HW, KIM IH, et al. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in im?mune-tolerant phase[J]. Aliment Pharmacol Ther, 2020, 52(1): 196-204. DOI: 10.1111/apt.15741.
[39] LEE HW, KIM SU, BAATARKHUU O, et al. Comparison between chronic hepatitis B patients with untreated immune-tolerant phase vs. those with virological response by antivirals[J]. Sci Rep, 2019, 9(1): 2508. DOI: 10.1038/s41598-019-39043-2.
[40] KIM GA, LIM YS, HAN S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B[J]. Gut, 2018, 67(5): 945-952. DOI: 10.1136/gutjnl-2017-314904.
[41] WANG FD, ZHOU J, ZHANG DM, et al. A study of the effectiveness of nucleos(t)ide analogues in the treatment of HBeAg-positive chronic hepatitis B with normal alanine aminotransferase and high level of HBV DNA[J]. Chin J Hepatol, 2022, 30(4): 389-394. DOI: 10.3760/cma.j.cn501113-20210705-00318.
王发达, 周静, 张冬梅, 等. 核苷(酸)类似物治疗丙氨酸转氨酶正常HBeAg阳性且HBV DNA高水平慢性乙型肝炎的有效性研究[J]. 中华肝脏病杂志, 2022, 30(4): 389-394. DOI: 10.3760/cma. j.cn501113-20210705-00318.
[42] ROSENTHAL P, LING SC, BELLE SH, et al. Combination of entecavir/peginterferon alfa-2a in children with hepatitis B e antigen-positive im?mune tolerant chronic hepatitis B virus infection[J]. Hepatology, 2019, 69(6): 2326-2337. DOI: 10.1002/hep.30312.
[43] PERRILLO R, LIN HH S, SCHWARZ KB, et al. Changes in serum hepatitis B surface and e antigen, interferon-inducible protein 10, and aminotransferase levels during combination therapy of immune-tolerant chronic hepatitis B[J]. Hepatology, 2022, 76(3): 775-787. DOI: 10.1002/hep.32400.
[44] ZHU SS, ZHANG HF, DONG Y, et al. Antiviral therapy in hepatitis B vi?rus-infected children with immune-tolerant characteristics: A pilot open-label randomized study[J]. J Hepatol, 2018, 68(6): 1123-1128. DOI: 10.1016/j.jhep.2018.01.037.
收稿日期:2024-03-03; 录用日期:2024-04-10
本文编辑:王莹
