Different bactericidal abilities of plasmaactivated saline with various reactive species prepared by surface plasmaactivated air and plasma jet combinations
2024-03-18YikangJIA贾怡康TianhuiLI李甜会RuiZHANG张瑞PengyuZHAO赵鹏瑜ZifengWANG王子丰MinCHEN陈旻LiGUO郭莉andDingxinLIU刘定新
Yikang JIA (贾怡康) ,Tianhui LI (李甜会) ,Rui ZHANG (张瑞) ,Pengyu ZHAO (赵鹏瑜) ,Zifeng WANG (王子丰) ,Min CHEN (陈旻) ,Li GUO (郭莉),* and Dingxin LIU (刘定新)
1 State Key Laboratory of Electrical Insulation and Power Equipment,Center for Plasma Biomedicine,Xi’an Jiaotong University,Xi’an 710049,People’s Republic of China
2 School of Life Science and Technology,Xi’an Jiaotong University,Xi’an 710049,People’s Republic of China
Abstract Plasma-activated water (PAW),as an extended form of cold atmospheric-pressure plasma,greatly expands the application of plasma-based technology.The biological effects of PAW are closely related to the aqueous reactive species,which can be regulated by the activation process.In this study,surface plasma-activated air (SAA) and a He+O2 plasma jet (Jet) were parallelly combined (the SAA+Jet combination) or sequentially combined (the SAA → Jet combination and the Jet → SAA combination) to prepare plasma-activated saline (PAS).The PAS activated by the combinations exhibited stronger bactericidal effects than that activated by the SAA or the Jet alone.The concentrations of H2O2 and NO-2 were higher in the PAS activated by the Jet →SAA combination,while ONOO- concentrations were close in the three kinds of PAS and 1O2 concentrations were higher in the PAS activated by the SAA+Jet combination.The analysis of scavengers also demonstrated that H2O2,1O2,and ONOO- in the PAS activated by the SAA +Jet combination,and 1O2 in the PAS activated by the Jet → SAA combination played critical roles in bactericidal effects.Further,the effective placement time of the three PAS varied,and the PAS activated by the Jet → SAA combination could also inactivate 2.6-log10 of MRSA cells after placement for more than 60 min.The regulation of reactive species in plasma-activated water via different combinations of plasma devices could improve the directional application of plasma-activated water in the biomedical field.
Keywords: plasma-activated water,surface plasma-activated air,plasma jet,bactericidal effect,reactive species
1.Introduction
Plasma-activated water (PAW),as an expanding form of cold atmospheric-pressure plasma,has been widely studied for applications in the biomedical and agricultural fields,such as disinfection and preservation of fruit and vegetables[1-8].PAW has the advantages of storing reactive species and precisely controlling their dosage and uniformity [9-11].Compared with direct treatment with cold atmospheric-pressure plasma,PAW is free from the limitations of the device and can be used for spray and washing,which significantly expands the application of plasma technology [9,12].
In previous studies,PAW was commonly prepared by a single plasma device,an atmospheric-pressure plasma jet(APPJ) or a surface dielectric barrier discharge (SDBD) [1,11,13].The preparation time for the PAW activated by the plasma jet was long,and the bactericidal effects varied with the working gas [13-15].The PAW prepared by a plasma jet with helium or argon for 50 min inhibited approximately 40%and 45% Staphylococcus aureus (S.aureus),respectively,while that prepared by a plasma jet with air for 30 min inactivated approximately 4.4-log10Escherichia coli (E.coli) [13,14].There are two ways to prepare PAW using surface discharge plasma: in the first method,the reactive species generated in surface plasma diffuses into the water,and in the other,the gas treated by surface plasma with the reactive species is blown into the water [1,3,16,17].The bactericidal effects of PAW prepared by surface plasma vary with the material of the dielectric material layer [1,18,19].The PAW activated by surface plasma with a dielectric layer of polytetrafluoroethylene (PTFE) exhibited strong bactericidal effects,and the PAW activated for 2 min could inactivate approximately 4.4-log10S.aureus [18].However,the PAW activated by surface plasma with a dielectric layer of quartz exhibited weak bactericidal effects,and the PAW activated by surface plasma with the quartz dielectric layer for 3 h inactivated approximately 5-log10E.coli [1].The effects of PAW activated by surface plasma with a ceramic dielectric layer are less known.
Plasma-activated saline (PAS) is one of the PAWs that is commonly used in biomedical applications to eliminate the impact of osmotic pressure [19,20].Recently,our group demonstrated PAS that was prepared by a serial combination of surface discharge plasma under different discharge modes and a plasma jet with a working gas of helium [21].The PAS,activated by the combination of the surface plasma under nitrogen oxide mode and the plasma jet for 5 min,inactivated 5.5-log10E.coli [21].The study proposed a combination strategy of different plasma devices for PAW preparation to promote the biological effects of PAW.
The PAW produced by a single plasma device often exhibits limitations,such as low sterilization efficiency and instability;therefore,both surface discharge plasma and plasma jets have been used in collaboration for a strong bactericidal effect.Wang et al developed a novel approach for the preparation of PAW by combining a gliding arc with dielectric barrier discharge.The resulting PAW was highly effective in the inactivation of methicillin-resistant Staphylococcus aureus (MRSA) with a reduction of more than 6.0-log10within 10 min [22].However,the study of PAW produced via the combination of different plasma devices is less reported,which could potentially offer significant benefits for sterilization applications.Therefore,new parallel or sequential combinations of the air activated by surface discharge plasma under nitrogen oxide mode and a plasma jet with the working gas of helium with 1% oxygen (He+1%O2) were used to prepare the PAS in this study.MRSA was used as a model to evaluate the bactericidal effects of the three kinds of PAS.The aqueous reactive species in PAS were determined,and the critical reactive species in PAS for the bactericidal effects were investigated.The effective placement times of the three kinds of PAS were compared,and the changes in reactive species in PAS with the placement time were also detected.This study provides a strategy for regulating the preparation of plasma-activated water through different combinations of plasma devices.
2.Experimental method
2.1.Experimental set-up
The devices and the different combinations of the surface discharge plasma and plasma jets used to prepare PAS are illustrated in figure 1.The surface discharge plasma device with a “sandwich” structure consisted of a high-voltage(HV) copper electrode with a size of 60 mm × 60 mm,a dielectric layer of alumina ceramic with a thickness of 1.5 mm,and a hexagon-shaped steel grounded mesh electrode.The discharge area of the surface plasma was placed into a gas chamber with a size of 70 mm × 70 mm × 30 mm.The synthetic air (0.05 l min-1) passed through the surface plasma chamber and the surface plasma-activated air was introduced into the saline.The plasma jet device with a needle-ring structure consisted of an HV stainless steel rod placed in a quartz tube with a coaxial structure and a copperfoil grounded ring electrode wrapped around the outer surface of the quartz tube.The needle tip of the HV electrode was flush with the lower edge of the grounded electrode,and the distance from the quartz tube nozzle was approximately 5 mm.The outer and inner diameters of the quartz tube nozzle were 6 mm and 3 mm,respectively.The plasma was discharged with the working gas of helium(99%,2.97 l min-1) and 1% oxygen (1%,0.03 l min-1) to activate the saline,and the distance between the nozzle and the surface of the saline was approximately 10 mm.Two sinusoidal power supplies (Suman Inc.,CTP-2000 K,500 W)with a constant frequency of 20 kHz were separately connected to the surface plasma and the plasma jet devices.Oscilloscopes (Tektronix,MDO3054) with high-voltage probes (Tektronix,P6015A) and current probes (Pearson 2877) were employed to monitor the waveforms of the discharge voltages and currents,and the discharge powers were obtained by integrating the product of the voltage and current.
2.2.Preparation of PAS
The PAS was prepared via the different combinations of the surface plasma-activated air (SAA) and the He+1%O2plasma jet (Jet),including PAS that was parallelly activated by the surface plasma-activated air and the plasma jet(abbreviated as ‘SAA+Jet combination’,figure 1(a)),PAS activated by the surface plasma-activated air followed by the plasma jet (abbreviated as ‘SAA → Jet combination’,figure 1(b)),and PAS activated by the plasma jet followed by the surface plasma-activated air (abbreviated as ‘Jet →SAA combination’,figure 1(c)).PAS that was activated by the SAA or the Jet alone was also prepared and used for comparative analysis.
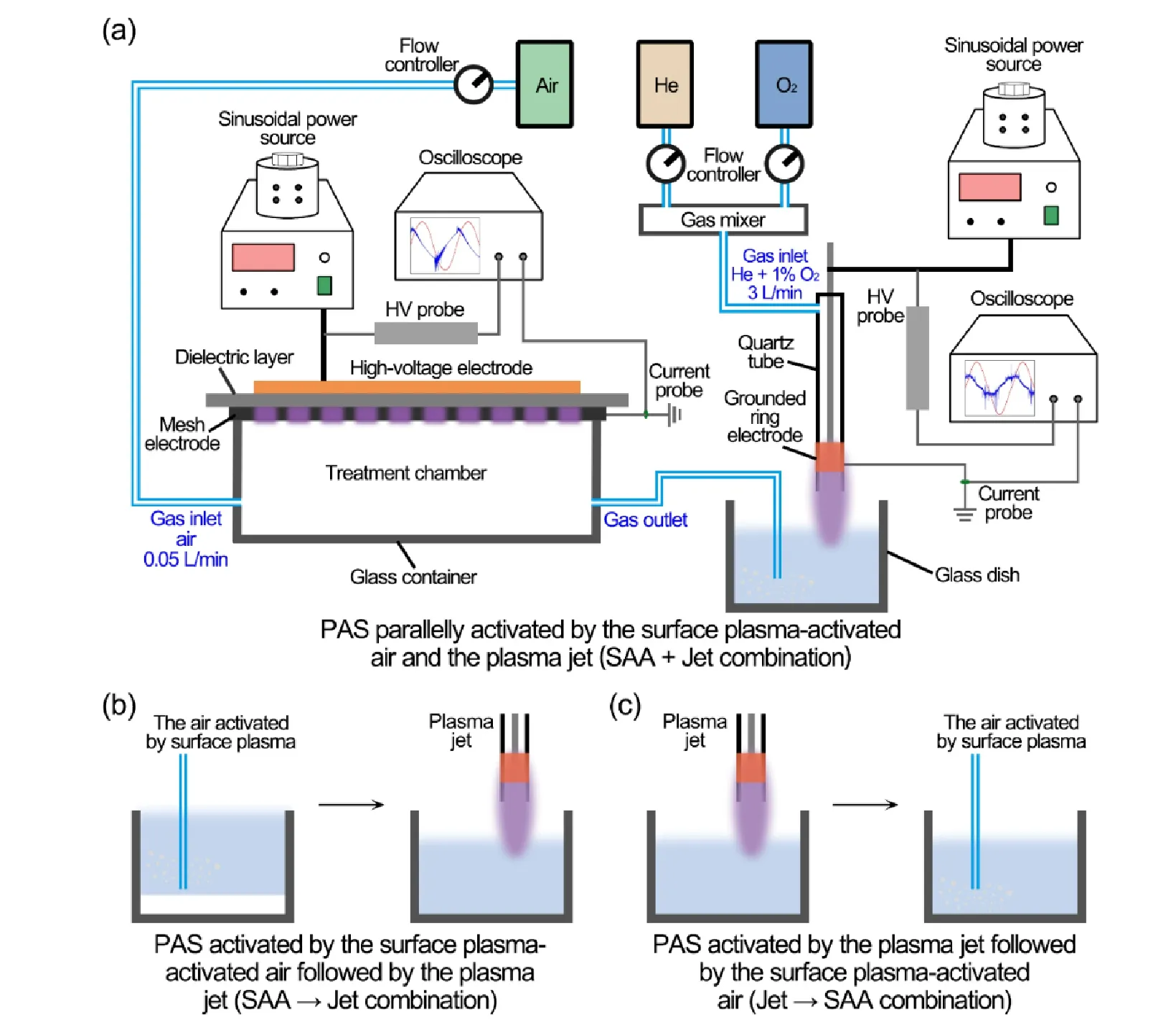
Figure 1.A schematic diagram of the PAS preparation via the different combinations.(a) PAS parallelly activated by the surface plasmaactivated air and the plasma jet (SAA+Jet combination).(b) The PAS activated by the surface plasma-activated air followed by the plasma jet (SAA → Jet combination).(c) The PAS activated by the plasma jet followed by the surface plasma-activated air (Jet → SAA combination).
2.3.Determination of the gaseous reactive species
The gaseous species in the surface plasma-activated air were analyzed using Fourier transform infrared (FTIR) spectroscopy (Bruker,Tensor II) with a wavenumber range of 700-3200 cm-1.The radiative species in the plume of the plasma jet were diagnosed using optical emission spectrometry(OES,Andor,SR750) at the quartz nozzle with a spectral range between 200 and 800 nm.
2.4.Bactericidal assay
A single methicillin-resistant S.aureus colony was grown in 4 ml tryptic soy broth (TSB,Oxoid) overnight at 37 °C with shaking at 250 r min-1.Then,the culture was transferred into a fresh TSB medium with a ratio of 1:100 and cultured for approximately 3 h to reach the exponential phase.Next,the bacterial culture was harvested by centrifugation at 4000 r min-1for 3 min and washed once with saline.The pellet was then resuspended with saline to an optical density value at 600 nm of 2,approximately 108cells ml-1.The preparation of the E.coli suspension was similar to that described previously [21].The bacterial suspensions (50 μl)were incubated with the different PAS (450 μl) and incubated for 30 min at room temperature.Then,the bacteria samples were serially ten-fold diluted using phosphatebuffered saline (PBS),and 10 μl of each dilution was spotted on TSB plates.The TSB plates were cultured at 37 °C for 24 h and the numbers of colony-forming units (c.f.u.) were calculated.The detected limitation for the drop plate method was 102cells ml-1.
2.5.Determination of aqueous reactive species
The aqueous long-lived reactive species were quantitatively measured,and short-lived species were detected using a microplate reader (Thermo Scientific Varioskan®Flash Reader).The concentrations of the aqueous long-lived reactive species hydrogen peroxide and nitrate/nitrite were measured using a hydrogen peroxide assay kit (Beyotime Biotechnology) and a nitrate/nitrite colorimetric assay kit(Beyotime Biotechnology),respectively.Three fluorescent probes,including disodium terephthalate (TPT,Aladdin,3 mM),trans-1-(2′-methoxyphenyl) pyrene (tMVP,J&K Scientific,10 μM),and coumarin boronic acid pinacolate ester(CBA,Cayman,20 μM),were used to detect short-lived·OH,1O2,and ONOO-,respectively.After incubation for 15 min in the dark at room temperature,the fluorescence intensities were measured at the corresponding excitation and emission wavelengths (310/425 nm for TPT,405/460 nm for tMVP,and 332/470 nm for CBA).
2.6.Scavenger assay
The analysis of the scavengers was performed as described previously [21].The scavengers,which included sodium pyruvate (Sigma-Aldrich,St.Louis,MO,10 mM),mannitol(Sigma-Aldrich,St.Louis,MO,100 mM),tiron(Sigma-Aldrich,St.Louis,MO,10 mM),sodium azide(Sigma-Aldrich,10 mM),L-histidine (Beijing Biodee Biotechnology Co.,Ltd.,10 mM),carboxy-PTIO(Sigma-Aldrich,St.Louis,MO,100 μM),and uric acid(Sigma-Aldrich,St.Louis,MO,100 μM),were mixed with the MRSA suspension first;then the PAS was added into the mixture with the scavengers at the final concentrations.After incubation for 30 min at room temperature,the samples were diluted and cultured.Then,the surviving bacteria were calculated,as described above.
3.Results
3.1.PAS prepared by different combinations of surface plasma and plasma jet
In this study,the surface discharge plasma and the plasma jet were applied by sinusoidal power supplies with a constant frequency of 20 kHz and maintained at constant discharge powers by controlling the applied voltages.When the discharge power of the surface plasma was maintained at approximately 10.8 W with a peak-to-peak voltage and current of approximately 4.8 kV and 160.7 mA,respectively,stable and uniform surface plasmas were generated in hexagonal meshes from the grounded electrode with a discharged area of approximately 36 cm2(figure S1(a)).A steady plasma plume with a length of~13 mm was generated when the discharged power of the plasma jet was maintained at approximately 1.5 W with a peak-to-peak voltage and current of approximately 10.5 kV and 18.4 mA,respectively(figure S1(b)).
3.2.Bactericidal effects of the PAS prepared by different combinations
First,the bactericidal effects of the PAS prepared by the three different combinations were compared and were also compared with those of the PAS activated by the SAA or the Jet alone.The PAS activated by the SAA+Jet combination for 3 min and 5 min reduced 1.0-log10and 5.3-log10MRSA cells,respectively.Meanwhile,the PAS activated by the SAA → Jet combination for 3 min and 5 min reduced 0.3-log10and 4.1-log10MRSA cells,respectively,and the PAS activated by the Jet → SAA combination for 3 min and 5 min inactivated 1.6-log10and 4.8-log10,respectively (figure 2).However,the PAS activated by the SAA or the Jet alone for 10 min inactivated only 1.1-log10and 1.9-log10MRSA cells,respectively,and was much weaker than the PAS prepared by the combinations (figure 2).These results demonstrated that the PAS prepared by the three different combinations all exhibited strong bactericidal effects.
3.3.Analysis of the gaseous reactive species in the surface plasma-activated air and the He+O2 plasma jet
The gaseous reactive species in the surface plasma-activated air were detected using FTIR spectroscopy,and the peaks of N2O5,N2O,NO2,and NO were diagnosed (figure 3(a)).The radiative species generated in the plasma plume by the plasma jet were determined using OES (figure 3(b)).The main identified spectrum lines included·OH(A) at 309 nm;N2(C3Πu→B3Πg) at 316 nm,337 nm,353 nm,357 nm,375.5 nm,380 nm,394.5 nm,400 nm,and 405.7 nm;at 391.4 nm,427.5 nm,and 470.9 nm;metastable He at 447.2 nm,492.2 nm,501.6 nm,587.7 nm,667.9 nm,706.7 nm,and 728.2 nm;and the O atom at 777.5 nm.The reactive species mentioned above could initiate a large number of plasma chemical reactions,resulting in the production of multitudinous primary and secondary reactive species.
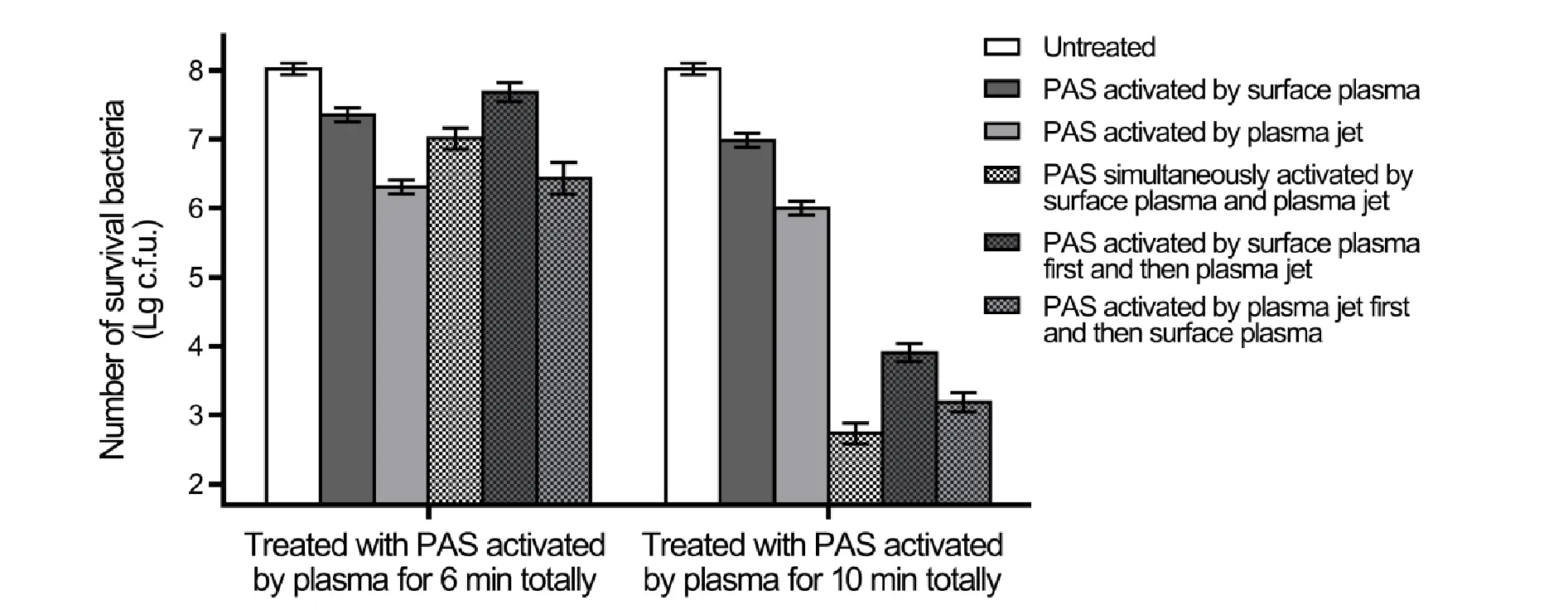
Figure 2.The bactericidal effects on MRSA of the different PAS.The PAS activated by the SAA,the Jet,or the three different combinations of the SAA and the Jet were added into the MRSA suspensions and incubated at room temperature for 30 min.Serial dilutions of each sample were performed,and 10 μl of each dilution was spotted onto TSB plates and incubated overnight at 37 °C.The resulting colony-forming units were calculated and analyzed.

Figure 3.Determination of gaseous reactive species.(a) The gaseous reactive species in the surface plasma-activated air were analyzed using FTIR spectroscopy.(b) Optical emission spectra of the He+1%O2 plasma jet.
3.4.Comparison of the aqueous reactive species in the PAS
The gaseous reactive species dissolved into the saline and reacted to produce the aqueous reactive species in PAS,which contributed to the biological effects of the PAS.Then,the aqueous reactive species in PAS activated by the three combinations and by the SAA or the Jet alone for 3 min and 5 min were determined and compared.First,the concentration of long-lived H2O2in the PAS activated by the Jet →SAA combination was the highest (321.3 μM),while those in PAS activated by the SAA+Jet combination (197.9 μM)and in PAS activated by the SAA → Jet combination (178.4 μM)were slightly higher than those in PAS activated by the SAA or the Jet alone (86.3 μM and 125.3 μM,respectively)(figure 4(a)).The concentrations of long-livedandin PAS activated by SAA alone for 5 min were 3207.6 μM and 760.7 μM,respectively,while those in the PAS activated by the Jet alone for 5 min were 11.6 μM and 8.9 μM,respectively (figure 4(a)).The concentrations ofin the PAS activated by the SAA+Jet,SAA → Jet,and Jet → SAA combinations for 5 min were 1852.2 μM,452.1 μM,and 3602.7 μM,respectively,while the concentrations ofin the three kinds of PAS were at a similar level(1319.7 μM,1717.4 μM,and 1560.4 μM) (figure 4(a)).
The short-lived species in the PAS were compared using the fluorescent probes that included TPT for·OH,tMVP for1O2,and CBA for ONOO-.The fluorescence intensities of TPT in PAS activated by the three combinations for both 3 min and 5 min were significantly higher than those in PAS activated by the SAA or the Jet alone (figure 4(b)).The fluorescence intensity of tMVP in the PAS activated by the SAA alone for 3 min was the highest,while those in the other PAS activated for 3 min were similar.The fluorescence intensities of tMVP in PAS activated for 5 min were ordered as follows: PAS activated by the SAA+Jet combination > PAS activated by the Jet → SAA combination > PAS activated by the SAA → Jet combination > PAS activated by the SAA alone > PAS activated by the Jet alone.The fluorescence intensities of CBA in PAS activated by the SAA alone for 3 min and 5 min were 6.9 and 3.3,respectively,while those in PAS activated by the Jet alone and by the three combinations for 3 min were between 58.5 and 144.8 and,for 5 min,were between 114.2 and 185.9 (figure 4(b)).These results indicated that the PAS containing different aqueous reactive species could be achieved by activation via the different combinations of the SAA and the Jet.
3.5.Effects of the scavengers on the bactericidal effects of the PAS
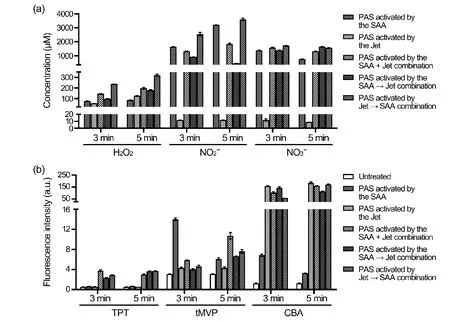
Figure 4.Measurement of the aqueous reactive species in different PAS.(a) The concentrations of three long-lived H2O2,,andin PAS activated by the SAA,the Jet,or the three different combinations of the SAA and the Jet.(b) The fluorescence intensities of three probes with PAS activated by the SAA,the Jet,or the three different combinations of the SAA and the Jet.Three probes (TPT,tMVP,and CBA) were incubated with the PAS or saline,and incubated at room temperature for 15 min.Then,the fluorescence intensities were measured.
When the role of the long-lived species in PAS was determined,the mixture of long-lived species of H2O2(320 μM),(3500 μM),and(1500 μM) exhibited few bactericidal effects,as demonstrated in previous studies (figure S2)[21].The results suggested that the three long-lived species in PAS did not play crucial roles in the biological application.To further investigate the critical reactive species in the PAS prepared by the different combinations in the bacteria inactivation,seven scavengers,including sodium pyruvate for H2O2,mannitol for·OH,tiron for,sodium azide and L-histidine for1O2,carboxy-PTIO for NO,and uric acid for ONOO-,were used.For PAS activated by the SAA+Jet combination,sodium pyruvate,sodium azide,and L-histidine almost eliminated the bactericidal effects,while uric acid was slightly eliminated (figure 5).For the PAS activated by the SAA → Jet combination,sodium pyruvate,sodium azide,L-histidine,and uric acid entirely reversed the bactericidal effects,while tiron and carboxy-PTIO partially reversed them (figure 5).For the PAS activated by the Jet →SAA combination,only sodium azide and L-histidine eliminated the bactericidal effects (figure 5).The results indicated that H2O2,1O2,and ONOO-in the PAS activated by the SAA+Jet combination played key roles,and1O2in the PAS activated by the Jet → SAA combination played the critical role.Meanwhile,H2O2,1O2,ONOO-,,and NO in the PAS activated by the SAA → Jet combination played roles together in the bacteria inactivation.The results demonstrated that the functional reactive species in the PAS prepared by the different combinations were different.
3.6.Changes in the bactericidal effects of the PAS and aqueous reactive species in the PAS with the placement
Previous studies demonstrated that the biological effects of plasma-activated water gradually became weak with the placement time [13,21].The bactericidal effects of the PAS prepared by the three combinations after placement at room temperature for different timescales were examined.The PAS activated by the SAA+Jet combination barely inactivated approximately 2.2-log10after placement for 1 min and almost lost bactericidal ability after placement for 5 min(figure 6(a)).The bactericidal effects of PAS prepared by the SAA → Jet combination inactivated less than 2.0-log10after placement for 1-3 min and it almost lost its bactericidal ability after placement for 5 min (figure 6(a)).The PAS activated by the Jet → SAA combination could inactivate approximately 3.3-log10after placement for 10 min,and could inactivate approximately 0.9-log10after placement for 90 min (figure 6(a)).
Meanwhile,the changes in the reactive species in the PAS with placement time were also compared.The concentration of H2O2in PAS activated by the SAA+Jet combination decreased approximately 52.6% after placement for 5 min,that in the PAS activated by the SAA → Jet combination exhibited slight changes after placement for 5 min,and that in the PAS activated by the Jet → SAA combination decreased approximately 52.8% after placement for 90 min(figures 6(b)-(d)).The·OH detected by the fluorescence intensities of TPT in the PAS activated by the SAA+Jet combination and PAS activated by the SAA → Jet combination decreased with the placement time,while those in the PAS activated by the Jet → SAA combination increased after placement for 10 min and then decreased (figures 6(b)-(d)).The1O2and ONOO-detected by the fluorescence intensities of tMVP and CBA in the PAS activated by the different combinations all decreased,and1O2in the PAS activated by the Jet → SAA combination decreased slowly(figures 6(b)-(d)).These results provided clues that the bactericidal effects of the PAS were attributed to the existence of short-lived species.
4.Discussion
In this study,the reactive species generated by surface plasma with a working gas of air and a plasma jet with a working gas of helium and 1% oxygen were introduced into saline via different combinations to prepare three kinds of PAS.Based on the FTIR spectroscopy results,reactive nitrogen species were the main components in the surface plasma.Nitrogen in the air reacted with oxygen to be oxidized to nitric oxide (R1),and then the nitric oxide and oxygen atoms were able to form nitrogen dioxide (R2)[16,23-25].The oxygen atom interacted with oxygen to generate ozone,which oxidized nitrogen dioxide to produce nitrogen trioxide (R3) [16,24-26].Nitrogen dioxide could react with nitrogen trioxide to form nitrogen pentoxide (R4)[24,25].Except for the fact that nitrous oxide did not react with any aqueous reactive species,the nitrogen oxides generated by the surface plasma mentioned above could be transferred from the gaseous phase into saline [24]:
In the He+1%O2plasma jet,the majority of the reactive species were comprised of reactive oxygen species.During the plasma generation processes,the electrons reacted with oxygen molecules to generate a variety of reactive oxygen species,including singlet oxygen,oxygen atoms,and superoxide anions (R5-R7) [25,27,28].Additionally,the oxygen atom could react with another oxygen molecule to form ozone (R8) [25,28]:
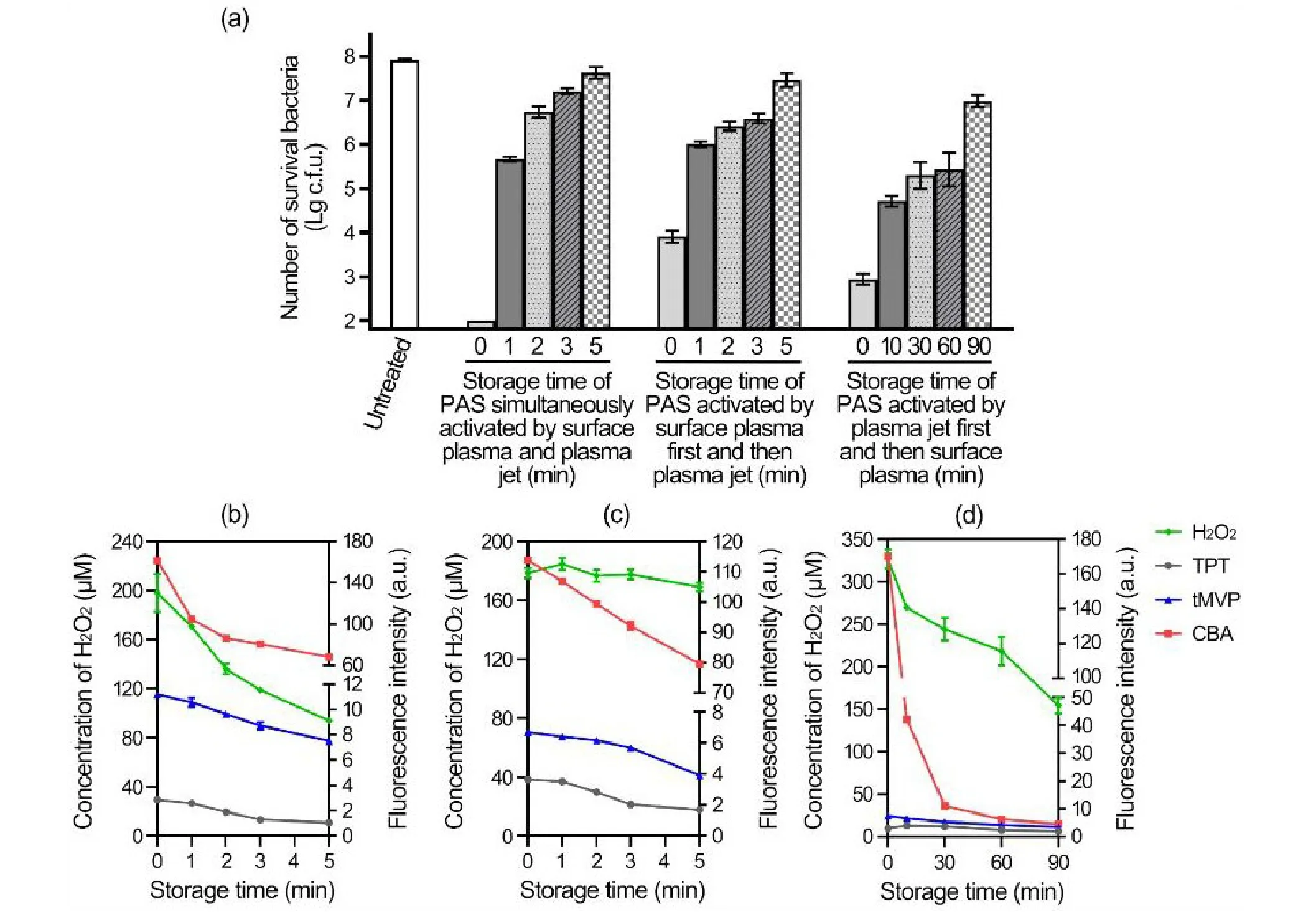
Figure 6.Storage times for the bactericidal effects of PAS.(a) The decrease in the bactericidal effects of PAS activated by the different combinations with the storage time.The PAS activated by the different combinations was stored at room temperature for the indicated time and incubated with the MRSA suspensions for 30 min at room temperature.Serial dilutions of each sample were performed,and 10 μl of each dilution was spotted onto TSB plates and incubated overnight at 37 °C.The resulting colony-forming units were calculated and analyzed.(b) The change in the reactive species in PAS activated by the different combinations with the storage time.The PAS was simultaneously activated by surface plasma and the plasma jet,and activated by surface plasma first.Then,the plasma jet was incubated at room temperature for 5 min.Meanwhile,that activated by the plasma jet first and then surface plasma was incubated at room temperature for 90 min.The inactivation effects of the PAS and the reactive species in the PAS stored for different time periods were compared.
The gaseous reactive species in the surface plasma-activated air and He+1% O2plasma jet were parallelly or sequentially introduced into saline,and the reactive species dissolved in the saline and then reacted with each other or with the water to produce the aqueous reactive species in different PAS.In the PAS activated by the SAA+Jet combination (figure 7(a)),the electrons generated by the Jet reacted with water to form hydroxyl groups,which could react with each other to form hydrogen peroxide (R9,R10)[24,29,30].Nitrite and peroxynitrous acid reacted with hydrogen peroxide to form peroxynitrous acid and peroxynitric acid,respectively (R11,R12) [31-34].Peroxynitric acid peroxide could be decomposed into singlet oxygen and nitrite (R13) [35]:
For the PAS activated by the SAA → Jet combination(figure 7(b)),after the saline was introduced with SAA,the saline mainly contained superoxide hydrogen,nitrite,nitrate,singlet oxygen,and low levels of peroxynitrous acid,and the reactive species could react and form other species,such as peroxynitric acid.Next,the saline was reactivated by the Jet,the reactive species generated by the Jet dissolved into the saline,and the oxygen atoms reacted with water to form superoxide hydrogen and then further reacted to generate hydrogen dioxide,which could be decomposed into superoxide ions (R14,R15) [13,25].Superoxide ions reacted with hydrogen ions in the saline activated by SAA to form singlet oxygen and superoxide hydrogen (R16) [35]:

Figure 7.The chemical processes of the gaseous and aqueous reactive species in the different combinations.
For the PAS activated by the Jet → SAA combination(figure 7(c)),after the saline was treated with the Jet,the saline mainly contained superoxide hydrogen,low levels of nitrite and nitrate,singlet oxygen,and peroxynitrous acid.Next,the saline was treated by SAA,the reactive species of NOxgenerated by surface plasma dissolved into the saline,and the nitric oxide and nitrogen dioxide reacted with the water to form nitrous acid (R17) [13].Then,the nitrite reacted with superoxide hydrogen to form peroxynitrous acid and peroxynitric acid (R11,R12) [13,34].Both peroxynitrous acid and peroxynitric acid could be dissociated (R18,R19) [32-34]:
The concentrations of nitrite,nitrate,and the fluorescence intensity of tMVP were found to be high in PAS prepared by SAA,while the fluorescence intensity of CBA was high in the PAS prepared by the plasma jet.After activation with the combination of SAA and Jet,the reactive species in the PAS exhibited a significant increase.The main differences in the concentrations of reactive species in the PAS prepared by three different combinations were singlet oxygen and nitrite.The content of singlet oxygen in the SAA → Jet and Jet → SAA treatments was lower than that of the SAA+Jet treatment,which may result from the loss of singlet oxygen at the interval switching between the two treatments.Furthermore,the order of nitrite concentration was Jet → SAA > SAA+Jet > SAA → Jet,which suggested that more nitrite in SAA+Jet and SAA → Jet was oxidized to higher-valence nitrogen oxides.
In the previous study,the surface plasma-activated air was mixed with helium in the proportion of 2% as the working gas of the plasma jet [21].Then,the plasma jet was used to activate saline to prepare the PAS,and the PAS activated for 5 min could inactivate approximately 5.8-log10E.coli [21].In this study,the PAS activated by three different combinations for 2 min inactivated more than 5.8-log10E.coli (figure S3).Therefore,the PAS prepared by the parallel and sequential combination of the SAA and the Jet can promote the bactericidal ability of the PAS.
The PAS prepared by the different combinations exhibited various bactericidal effects,and the key reactive species in the three kinds of PAS were also different based on the analysis of the scavengers.In PAS activated by the SAA +Jet combination,hydrogen peroxide,singlet oxygen,and peroxynitrous acid played dominant roles in bacterial inactivation.The presence of hydrogen peroxide in a solution probably reduced bacterial tolerance,collaborated with other reactive species to inactivate MRSA cells,and also contributed to the formation of peroxynitrite and peroxynitrite (R11,R12) [31,33,34].Singlet oxygen can cause substantial oxidative damage to MRSA cells,and high concentrations of singlet oxygen were detected in PAW [13].Peroxynitrous acid could induce nitrosylation in both proteins and nucleic acids,leading to protein inactivation and aggregation,and interference with the replication and transcription processes [35].Peroxynitrous acid could also promote the generation of singlet oxygen (R12,R13) [34,35].The bactericidal effects of PAS activated by the SAA → Jet combination were relatively weak,and the hydrogen peroxide,superoxide,singlet oxygen,nitric oxide,and peroxynitrous acid probably all contributed.Superoxide could have strong oxidative destructive effects on bacteria,while nitric oxide could also induce protein damage,such as oligomerization and nitration [36].Furthermore,superoxide and nitric oxide could react and form peroxynitrite (R20) [13,37].For the PAS activated by the Jet → SAA combination,only singlet oxygen played a crucial role in inactivation:
When the PAS was added to the bacterial suspensions,the reactive species in PAS entered the bacteria cells and reacted with the biomacromolecules.Different reactive species react with amino acids or DNA at different rates and form various products,which lead to different types of damage [38-40].When the damage cannot be timely repaired,it will lead to the death of bacteria.
Interestingly,the placement time of the three kinds of PAS prepared by the different combinations was also varied.The PAS activated by the SAA+Jet and activated by the SAA → Jet lost their bactericidal ability after placement for 5 min,while the PAS activated by the Jet → SAA almost lost its bactericidal ability after placement for 90 min(figure 6).In particular,in the PAS activated by the Jet →SAA combination,the hydroxyl radicals exhibited a process of first rising and then falling after placement for 30 min.The change in hydroxyl radicals reflected the sustained chemical reactions that existed in the PAS,which could generate various kinds of reactive species.
5.Conclusion
In this study,PAS was prepared via parallel or sequential activation of surface plasma-activated air and a He+1%O2plasma jet,including PAS parallelly activated by the SAA and the Jet (SAA+Jet combination),PAS activated by the SAA followed by the Jet (SAA → Jet combination),and PAS activated by the Jet followed by the SAA (Jet → SAA combination).These were compared and analyzed.The PAS prepared by the three combinations exhibited various bactericidal effects,and all were stronger than those prepared by the SAA or the Jet alone.The same gaseous reactive species in the SAA and the Jet prepared the three kinds of PAS with different aqueous reactive species via parallelly or sequentially combined introduction into saline.The concentrations of H2O2andwere higher in the PAS activated by the Jet → SAA combination,while the levels of1O2were higher in the PAS activated by the SAA+Jet combination.The analysis of scavengers also demonstrated that the key reactive species in PAS prepared by different combinations were diverse.The effective placement time of the PAS activated by the Jet → SAA combination was significantly longer than those of the other two PAS,which was attributed to the changes in the reactive species in the PAS.The PAS prepared by the different combinations of plasma devices exhibited different aqueous reactive species,bactericidal abilities,and effective placement times.This provides new strategies for regulation of the reactive species in plasmaactivated water to improve the directional application of plasma-activated water in the biomedical field.
Supplementary material
See the supplementary material for the waveforms of the applied voltage and discharge current,the bactericidal effects of the mixed solutions of H2O2,,and,and the bactericidal effects of the different PAS on E.coli.
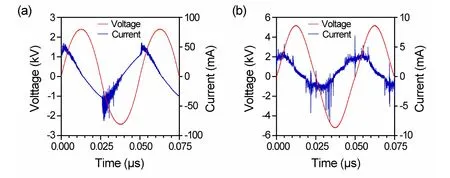
Figure S1.The waveforms of the applied voltage and discharge current.(a) The sinusoidal voltage waveform and the current waveform of surface discharge plasma.(b) The sinusoidal voltage waveform and the current waveform of the He+1% O2 plasma jet.

Figure S2.Bactericidal effects of the mixed solutions of H2O2,,and .The bactericidal effects of the mixedsolutions.H2O2(320 μM), (3500 μM),and (1500μM) alone or combined were incubated with MRSA suspensions for 30 minat room temperature.Serial dilutions of each sample were performed,and 10 μl of each dilution was spotted onto the TSB plates and incubated overnight at 37 °C.The resulting colony-forming units were calculated and analyzed.
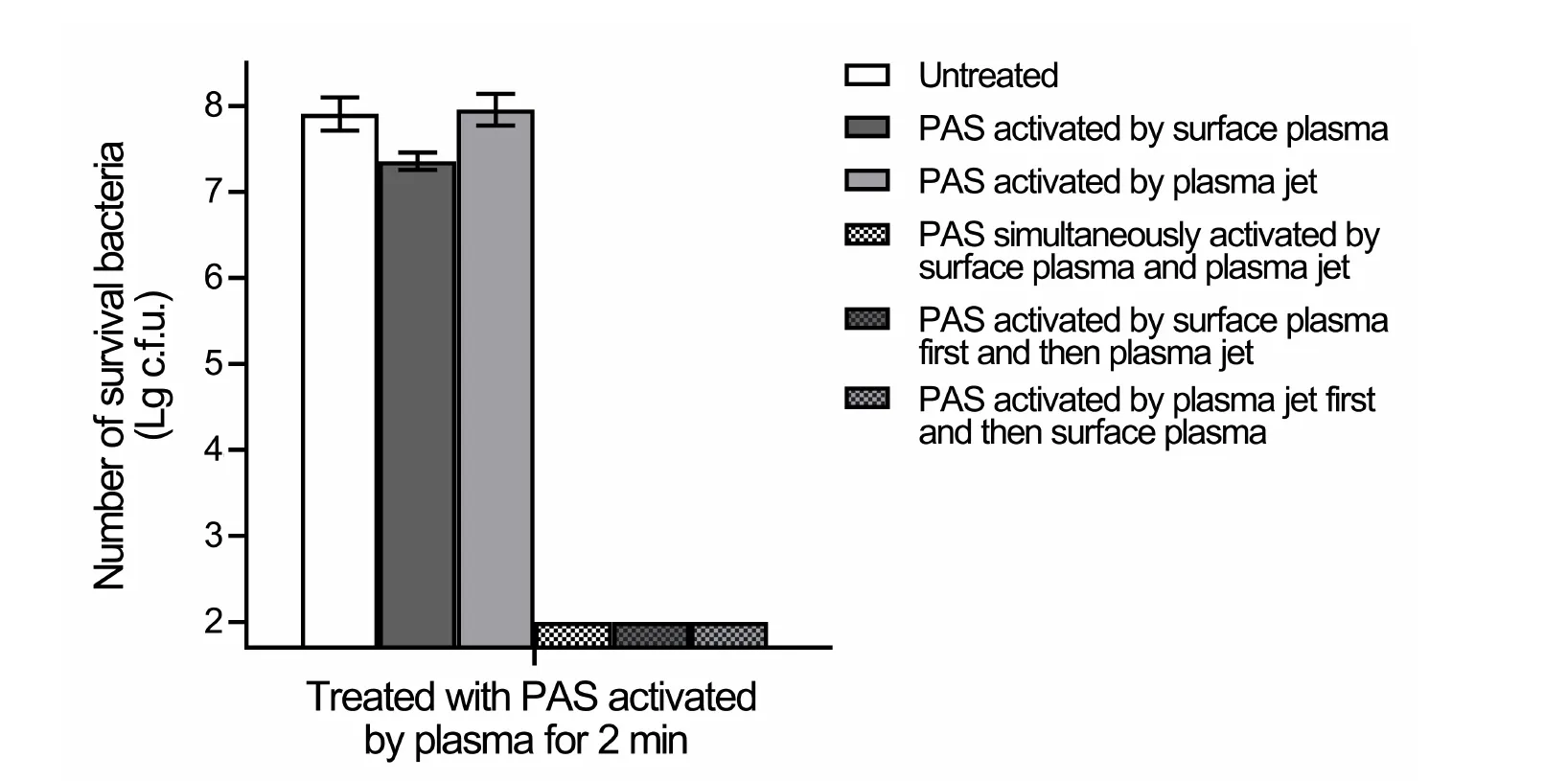
Figure S3.Bactericidal effects of the different PAS on E.coli.The PAS activated by the SAA,the Jet,or the three different combinations of the SAA and the Jet were incubated with E.coli suspensions for 30 min at room temperature.Serial dilutions of each sample were performed,and 10 μl of each dilution was spotted onto LB plates and incubated overnight at 37 °C.The resulting colony-forming units were calculated and analyzed.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No.51977174).
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Preliminary electromagnetic analysis of the COOL blanket for CFETR
- Laser-induced plasma formation in water with up to 400 mJ double-pulse LIBS
- Plasma nitrogen fixation system with dual-loop enhancement for improved energy efficiency and its efficacy for lettuce cultivation
- Influence of the position relationship between the cathode and magnetic separatrix on the discharge process of a Hall thruster
- A spatiotemporal evolution model of a short-circuit arc to a secondary arc based on the improved charge simulation method
- Modification of streamer-to-leader transition model based on radial thermal expansion in the sphere-plane gap discharge at high altitude
