Plasma nitrogen fixation system with dual-loop enhancement for improved energy efficiency and its efficacy for lettuce cultivation
2024-03-18ZeyangHAN韩泽阳MengxueZHANG张梦雪DiZHANG张頔XinHE何欣TianjunJING井天军ZhixuanGE葛知轩YugeLI李玉鸽TongZHU朱童YunhongREN任云鸿ChongshanZHONG仲崇山andFangJI季方
Zeyang HAN (韩泽阳) ,Mengxue ZHANG (张梦雪) ,Di ZHANG (张頔) ,Xin HE (何欣) ,Tianjun JING (井天军) ,Zhixuan GE (葛知轩) ,Yuge LI (李玉鸽),Tong ZHU (朱童),Yunhong REN (任云鸿),Chongshan ZHONG (仲崇山),* and Fang JI (季方),*
1 College of Information and Electrical Engineering,China Agricultural University,Beijing 100083,People’s Republic of China
2 College of Water Resources and Civil Engineering,China Agricultural University,Beijing 100083,People’s Republic of China
3 Electric Power Research Institute State Grid Gansu,Electric Power Company Gansu,Lanzhou 730030,People’s Republic of China
Abstract Plasma nitrogen fixation (PNF) has been emerging as a promising technology for greenhouse gasfree and renewable energy-based agriculture.Yet,most PNF studies seldom address practical application-specific issues.In this work,we present the development of a compact and automatic PNF system for on-site agricultural applications.The system utilized a gliding-arc discharge as the plasma source and employed a dual-loop design to generate from air and water under atmospheric conditions.Experimental results showed that the system with a dualloop design performs well in terms of energy costs and production rates.Optimal operational parameters for the system were determined through experimentation,resulting in an energy cost of 13.9 MJ mol-1 and an energy efficiency of 16 g kWh-1 for production,respectively.Moreover,the concentration of exhausted NOx was below the emission standards.Soilless lettuce cultivation experiments demonstrated that produced by the PNF system could serve as liquid nitrate nitrogen fertilizer.Overall,our work demonstrates the potential of the developed PNF system for on-site application in the production of green-leaf vegetables.
Keywords: plasma nitrogen fixation,gliding arc,soilless cultivation,lettuce
1.Introduction
The Haber-Bosch (HB) synthesis is a catalytic process that converts N2and H2into NH3with an energy cost of 0.48 MJ mol-1[1,2].Over the past century,it has played a vital role in feeding the growing global population.However,the side effects of the Haber-Bosch process cannot be ignored,such as considerable emission of greenhouse gases and environmental pollution [3].There has been a quest for alternative nitrogen fixation technologies to achieve sustainable development.Furthermore,there is potential that renewable energy can be applied as the primary power input for the nitrogen fixation process [4,5].
Plasma nitrogen fixation (PNF) has gained attention from researchers worldwide due to its environmental advantages[6].PNF technology takes advantage of on-site resources such as air,water,and electricity without requiring extremely high pressure or temperature.The theoretical energy consumption of PNF is more than 2.5 times lower than that of the HB process [7].
Currently,PNF technology can be broadly classified into two categories.The first route is a catalytic reaction,in which the catalyst reduces NOxto NH3[8] or plasmacatalytic NH3synthesis by N2and H2[9-11].The second route involves the production of nitrateor nitritein liquid by gas-liquid hybrid discharge [12-16] or absorbing NOxgenerated by gaseous discharge [17,18].
Plasma ammonia synthesis technology has excellent potential for industrial-scale nitrogen fixation processes.However,the current world fertilizer production is mainly based on ammoniacal nitrogen,with fewer nitrate-based fertilizers [19].Increasing the production and application of nitrate-based fertilizers is of significant importance for optimizing agricultural fertilization structure and improving fertilizer utilization efficiency.Nitrate-based fertilizers also have environmental benefits that reduce ammonia volatilization [19].As a result,the second route may be more suitable for on-site nitrogen fertilizer supply near various crops with high demand for nitrate nitrogen,such as wheat [20],tomato[21],and lettuce [22].
Several methods have been used to produce NOxgas at low temperatures,including corona,glow discharge,dielectric barrier discharge,microwave plasma,gliding-arc discharge,and plasma jet [23-28].Among them,Kelly and Bogaerts reported the lowest reported energy cost(2 MJ mol-1) using an atmospheric pressure microwave plasma [27].Gliding-arc discharge,due to its high energy density,enables selective chemical processes with high production rates [29].Fridman et al studied the influence of airflow on gliding-arc discharge characteristics by applying a high-voltage power supply to knife blade electrodes [30].The advantage of using gliding-arc plasma is that it can be operated under atmospheric pressure and uses an economical power supply (e.g.,high-frequency and high-voltage source) for on-site nitrogen fixation systems.However,NOxemission from the PNF system should also be considered a vital air pollutant [31-34],and thus its emission must be controlled in applications.
Meanwhile,there have been significant breakthroughs in the study of the mechanism and numerical simulation of the PNF process.For instance,Shu et al investigated atmospheric-pressure warm air glow discharge and implemented a numerical modeling employing laser spectroscopic diagnostics [35].Severe disproportionality and high energy cost of NO production in the positive column zone were attributed to the processes of vibrational excitation or dissociation of N2and O2molecules [36].For gliding arc discharge,Wang et al developed a detailed chemical kinetics model and investigated the reaction pathways and dominant production process of NOx[37].Patil studied and discussed the relationship between NOxproduction and electrical or process parameters like the frequency and amplitude of input voltage,feed mixture,and flow rate,etc [38].
NOx,when absorbed by water,can be fixed into an aqueous solution that can be utilized as nitrogen-rich irrigation water.Sivachandiran and Khacef [39] conducted experiments on the growth of radishes,tomatoes,and sweet peppers under laboratory conditions using plasma-activated water (PAW).The results showed that stem length increased by approximately 60% when plants were watered with PAW treated for 15 min,compared to controls.Another study by Rashid [40] utilized PAW prepared by an underwater air discharge plasma jet as a foliar spray on rice plants,resulting in an increase in rice yield by approximately 14%compared to the control.
From an agricultural production perspective,PNF technology shows the potential to be integrated into drip irrigation systems on farms or plant plants in the future [17].Hence,it offers the possibility of supplying fertilizer by directly converting atmospheric air into liquid nitrate fertilizer.Moreover,the nitrogen fixation process can be powered entirely by renewable energy sources without generating greenhouse gas emissions.
The literature has demonstrated the effectiveness of gliding arc plasma technology for nitrogen fixation,but the investigations reported thus far have been limited to laboratory settings.Yet,most PNF studies seldom address practical application-specific issues.It would be beneficial to develop a system that can be used to study real-world agricultural scenarios to make progress toward practical agricultural applications.Accordingly,this study has created an onsite PNF system with low energy costs and NOxemissions,which can operate fully automatically.Lettuce was chosen as the experimental subject for soilless cultivation,and this system provided all of its nitrate nutrients.
2.Principle of the system and experimental method
2.1.Components of the PNF system and its working principle
The PNF system was comprised of five components,as illustrated in figure 1.
(1) A previous study demonstrated that utilizing a highfrequency power source yields higher efficiency than a lowfrequency power source.Meanwhile,the frequency range of 5 -80 kHz was found to have no significant impact on the nitrogen fixation outcomes [17].Therefore,a cost-effective and space-saving high-voltage power supply was designed to produce 9 kV (peak-to-peak) at a frequency of 7 kHz,using an input source of 220 V (AC).
(2) The gliding-arc plasma reactor,which was made of cylindrical quartz glass,housed a pair of stainless-steel electrodes.A gliding arc was ignited between the electrodes’2 mm gap and was propelled upwards by an airflow of 15 L min-1.The reactor had a volume of approximately 1.5 L.
(3) The gas mixing chamber,which was sealed,had two purposes: to store NOxgas from the gliding-arc discharge and to allow sufficient time for NO to be oxidized into NO2,which is more soluble in water.Its volume was about 6 L.
(4) A tightly sealed plastic bucket served as the NOxabsorption vessel,where NOxwas converted into liquid nitrate.In this experiment,the water level was maintained at 23 cm using a water level sensor,and the quantity of water used was roughly 8 L.To increase NOxabsorption in water,gas was pulverized into a multitude of tiny bubbles using six or more air stones.The gas paths of the gas mixing chamber and NOxabsorption vessel were interconnected,allowing NOxto be absorbed during multiple loops.
(5) The control board,with a microcontroller(STC8H1k17,STC Co.,China) as its core,was developed to manage the system’s automatic operations.
It should be noted that the PNF system employed a dualloop operation mode,as depicted in figure 1.In the first loop(which lasted for t2),NOxgas was generated via gliding-arc discharge and accumulated in the reactor and gas mixing chamber.During this process,all valves and pumps,except for pump P3,were closed.Once this step was completed,the system transitioned to the second loop.Subsequently,pump P4 was activated.Throughout t3,the air-NOxmixture was looped through the water several times until the NOxconcentration had fallen below the required emission standard.A water vapor separator was incorporated into the gas pathway to avert the corrosion risks associated with electrode exposure to excessive water vapor within the gliding arc reactor.
After the completion of the second loop,fresh air was replenished in the gas mixing chamber.This process lasted for a duration of t1,during which P2,V1,and V2 were turned on while P2 and P4 were turned off.This sequence of three stages was repeated multiple times to produce a nitrogen-rich aqueous solution.The resulting liquid product was pumped out for lettuce cultivation using pump P6.Finally,fresh tap water was injected using pump P5.
2.2.Experiments to determine optimal operating parameters
An optimal combination of time parameters for the three stages (t1,t2,and t3) was identified.To determine the most effective operation parameters,experiments were conducted using ten different sets of values,as outlined in table 1.The primary optimization goals were to achieve the lowest energy cost and the highest production rate of.
Considering the volume of the gas mixing chamber (6 L)and the flow rate of the gas pump (15 L min-1),it was determined that the chamber could be filled with fresh air within 30 s.
The impact of repetition number (m) on the production rate and energy cost ofproduction was investigated experimentally for the three stages (t1,t2,and t3).
A digital power monitor (P06S-10A,PUUCAI,China)was used to measure the input power of the PNF system.Gas sensors (JXM-NO and JXM-NO2,Jingxunchangtong Electronic Technology Co.,Ltd) shown in figure 1 were used to measure the concentration of NOxat the outlet of the PNF system.The concentration of aqueousandwere also essential indices for the PNF system and were measuredusing a UV-Vis spectrophotometer (LH-T725,Zhejiang Lohand Environment Technology Co.,Ltd.) [41,42].Since tap water was used as the feed liquid in the experiments,the influence of initialin tap water needed to be removed.
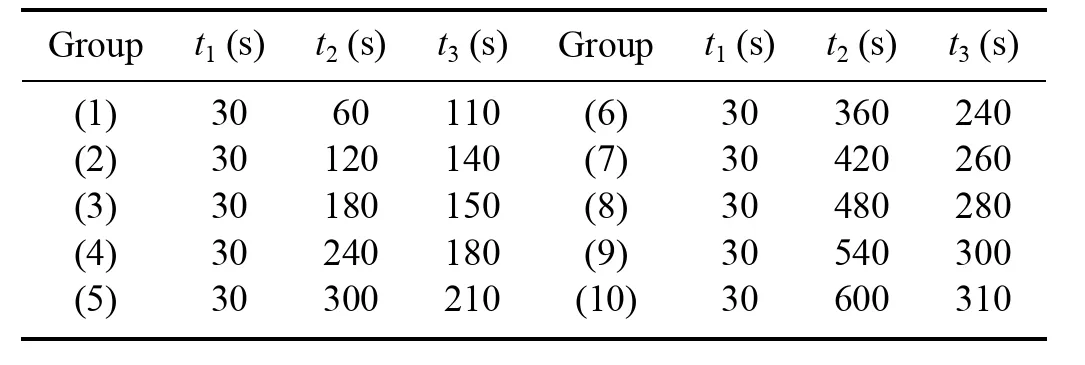
Table 1.Time parameters of operation stages.
In practical applications,exhaust gas must meet the emission standard for NOx.Therefore,the concentration of NOxemission was an important index for assessment.Different standards,such as nitric acid production [31],waste incineration plants [32,33],and boiler air pollutants [34],have set various limits for NOxemissions worldwide.This work implemented a relatively strict real-time emission limit of 117.6 ppm to assess the NOxemissions of the PNF system.
2.3.Calculation of energy cost,energy efficiency,and production rate
The energy cost was defined as the amount of energy required to produce one mole of a specific species.To determine the energy cost of producing gaseous NOx(EC(g)) and aqueous nitrogen (EC(aq)),equations (1) and (2) were used,respectively.
where the variables tn(s) and Pn(W) represent the durations and power input of the three stages of the dual-loop mentioned above (where n=1,2,3).The variable m denotes the repetition number,while cv(ppm) stands for the volume concentration of gaseous species.Q (L min-1) refers to the gas flow rate of the pump,and Vm(L mol-1) represents the molar volume of gas species.The typical values of Pnare presented in table 2.
The quantity of a specific aqueous species,denoted as n(aq)(mol),was determined using equation (3),while the increase in mass concentration of the same aqueous species,represented by Δcm,was calculated using equation (4).

Table 2.Input power of each working stage.
where Vlrepresents the volume of the aqueous solution,which was 8 L.M (g mol-1) refers to the relative molecular mass of a specific aqueous species.cm1(mg L-1) indicates the mass concentration of this aqueous species after treatment,while cm0(mg L-1) is the initial mass concentration.As tap water was used as the feed liquid in the experiments,the initial nitrogen concentration in tap water was excluded here.
The average power input (W) of the PNF system was calculated using equation (5).
The energy efficiency refers to the quality of certain species produced per kilowatt hour (kWh).Specifically,the energy efficiency ofproduction,denoted ascan be calculated using equation (6).
The production rate is a measure of the amount of a particular substance that is generated per hour.In this case,the production rate of aqueous nitrogen (PR(aq)) was calculated using equation (7).
2.4.Experiments to investigate the effect of dual-loop design
To decrease the energy cost of the PNF system,a dual-loop design has been implemented in this study as shown in figure 2.Three experiments were conducted to examine the impact of the dual-loop design (in figure 2(b)) on the energy cost ofproduction.Figure 2(a) depicts the one-loop design,which only contained a loop during the gas discharge phase,while figure 2(c) displays the typical mode of operation with no loop process at all.The duration of discharge and absorption for all three experiments was set at 180 s.
2.5.Effects of PNF system products on lettuce cultivation
Lettuce,as a leafy vegetable possessing high market recognition and representative characteristics,is a suitable experimental subject.Lettuce cultivation (specifically,little butter lettuce from Beijing Vertex Modern Agricultural Development Co.,Ltd.) was selected to determine whether the products of the PNF system could enhance plant growth.The experiments were conducted in a plant factory equipped with artificial light,which enabled precise control of the cultivation environment.
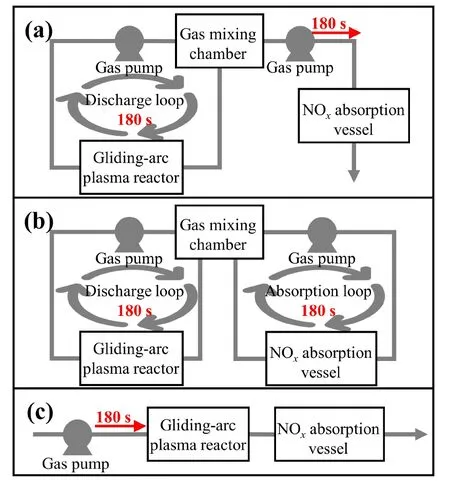
Figure 2.The configuration of groups (a),(b),and (c) in the comparison experiment.
The cultivation environment was established as follows:the light/dark loop was 16/8 h;the light intensity was 250 μmol m-2s-1;the R:B ratio of white LED lamps was 2.7;the photoperiod was 16 h per day.The temperature was maintained at (22±1) °C during the light period and (18±1) °C during the dark period,while the relative humidity was kept at 75%±5%.Figure 3 provides an illustration of the actual planting environment for the cultivation experiments.
Thirty-two lettuce seedlings with similar conditions were selected and randomly divided into four experimental groups(as shown in table 3).They were then transplanted into plant pots on cultivation beds (8 pots per bed,as depicted in figure 3)for 24 days.The pots were filled with a substrate consisting of peat,perlite,and vermiculite in a 1:1:1 volume ratio.All groups were irrigated using the bottom self-priming method,with 1.5 L of irrigation solution administered every two days.The irrigation solutions used are listed in table 3.

Figure 3.Schematic diagram of cultivation experiment.
Four types of irrigation solutions were used for treatment: tap water (TW),tap water treated by PNF system (TWPNF),Yamazaki lettuce nutrient solution (YN),and PNFtreated water with other nutrients except for nitrogen (YNPNF),as shown in table 3.The TW-PNF was transformed into a-rich solution and then adjusted to a pH range of 7-7.5 using a 10% NaOH solution.The formula for the Yamazaki lettuce nutrient solution is listed in table 4.The YN-PNF was treated with the PNF system,and additional nutrients were added according to the Yamazaki formula.The pH was adjusted to 6-6.5 using 10% NaOH.The tap water was used as a control,which contained a small amount ofCa2+,Mg2+,and Fe2+.The concentration ofin TW-PNF,YN,and YN-PNF was 372 mg L-1.Thein TW-PNF and YN-PNF were supplied by the PNF system,and thein YN was supplied by KNO3and Ca(NO3)·4H2O.
The cultivation experiments were statistically analyzed and tested using independent sample T-tests to verify significance using Microsoft Excel 2021 and SPSS Statistics 20.The results were presented as mean ± standard deviation.** represents a statistically significant difference (p < 0.01),while NS denotes no significant difference.All experiments were performed with three replicates.
3.Results
3.1.Effect of dual-loop design onproduction and energy cost of nitrogen fixation
In non-loop design,the concentration of NO and NO2was approximately 1471 ppm and 728 ppm during a 10 min discharge,respectively.The energy cost of NOxproduction was calculated to be approximately 5.1 MJ mol-1(using equation (2)),which is within the average range according to previous studies [29,43].
Figure 4 illustrates that the dual-loop design yielded the highest concentration ofat 23 mg L-1,which was 1.76 times and 4.62 times higher than that of the one-loop and non-loop designs,respectively.Furthermore,the dualloop design had the lowest energy cost at 33.9 MJ mol-1,which was 1.79 times and 4.06 times lower than that of the one-loop and non-loop designs,respectively.These results indicate that the dual-loop design utilized in this study demonstrated the advantages of highyield and low energy cost.

Table 3.The irrigation solutions of cultivation experiment.
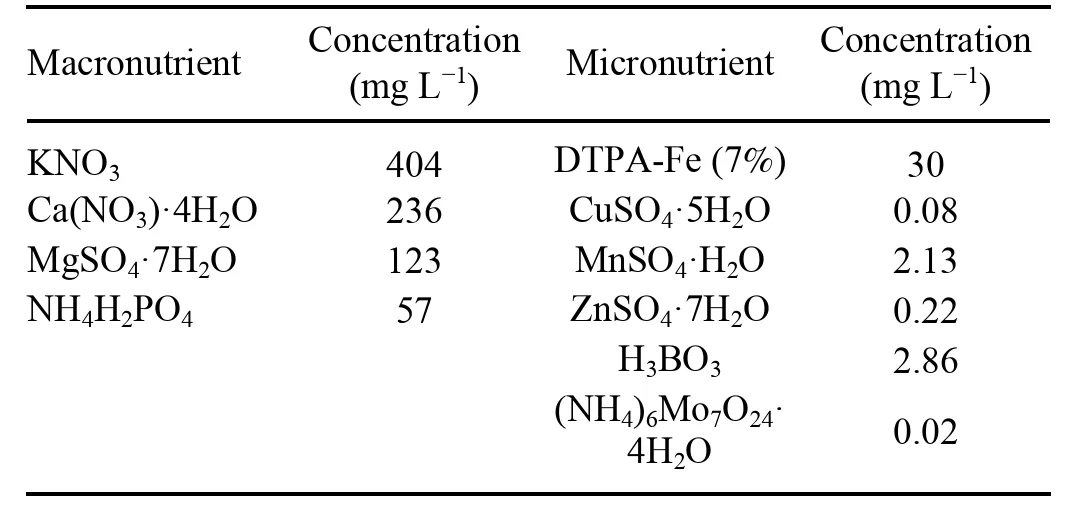
Table 4.The Yamazaki lettuce nutrient solution formula.
3.2.Effect of absorption time on NOx emission
Figure 5 shows the correlation between absorption time (t3in equation (2)) and NOxemissions.The initial concentration of NO and NO2before absorption (after 60 s of discharge in the loop) was 1887 ppm and 1654 ppm,respectively.It was observed that the concentration of NOxemissions decreased exponentially with an increase in absorption time.Within the first 30 s,nearly 70% of the NOxwas absorbed by the water,while the absorption rate decreased significantly thereafter.By 110 s,the NOxconcentration had dropped to 104 ppm,which was below the emission standard.At this point,97%of the NOxhad been absorbed,demonstrating the PNF system’s effective capacity to absorb gaseous NOxand meet the emission standard (117.6 ppm) given a sufficient duration (t3).

Figure 4.Effect of dual-loop design.(a) Dual-loop design,(b) oneloop design during gas discharge,(c) non-loop design.
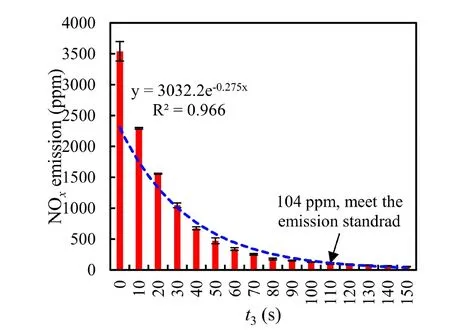
Figure 5.The relationship between absorption time and NOx emission.
3.3.Optimal operation-time parameters of the PNF system
Figure 6 demonstrates the dependency of the NOxproduction rate and energy cost on the specific combination of operation time.Among the combinations listed in table 1,the third combination produced the highest nitrogen oxides per unit of time (as shown in figure 6(a)) while simultaneously consuming the least energy for producing one mole of(as shown in figure 6(b)).Therefore,it can be concluded that the optimal operating time parameters for the PNF system are as follows: the gas renewal time should be 30 s,the gas discharge time should be 180 s,and the gas absorption time should be 150 s.Moreover,it should be noted that the maximum emission of NOxreached 112.6 ppm when the PNF system operated under the optimal operation-time parameters.
3.4.pH andproduct values in relation to repetition number
The term “repetition number” (m) in this study referred to the number of times that the optimal operation time was repeated (i.e.,t1=30 s,t2=180 s,t3=150 s).

Figure 6.Production rate of and energy cost of produc-tion under different operation-time combinations.
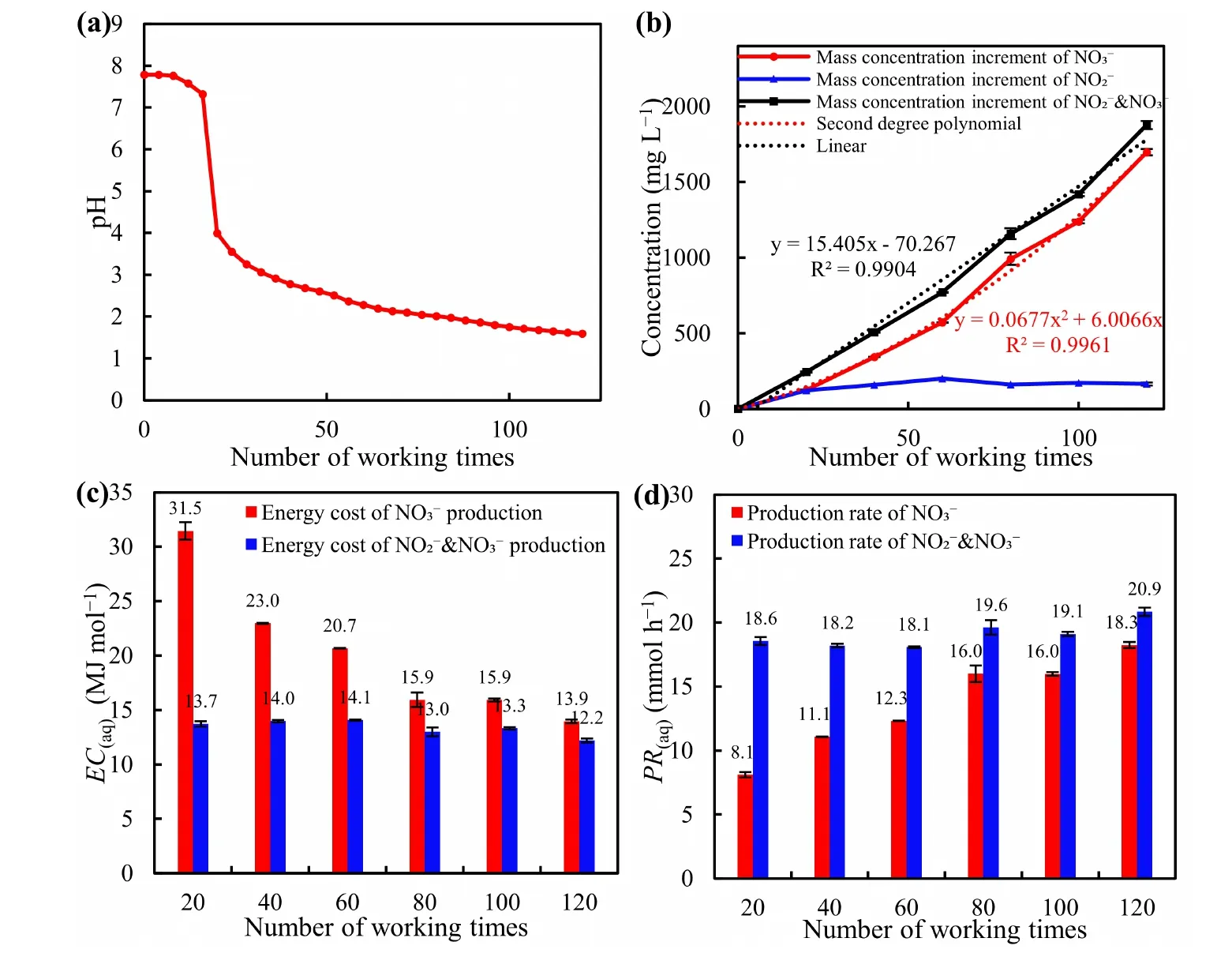
Figure 7.The results of the 120th of treatment based on the optimal operation-time parameters.(a)pH,(b)theconcentrationofand,(c)the energy cost of and NO-x production,(d) the production rate of and NO-x .
As depicted in figure 7(a),the pH value decreased nonlinearly with increasing repetition number.Initially,it decreased slowly for the first 16 repetitions,possibly due to the presence of alkaline substances in the tap water [44].Afterwards,before the 20th repetition,it sharply declined and continued to decrease gradually.After 120 repetitions,the pH had dropped to 1.6.
Figure 7(b) demonstrates that the concentration ofincreased linearly with the repetition number (R2> 0.99),while theconcentration followed a quadratic function trend (R2> 0.99),which accounted for the majority of.The absorption vessel also detected simultaneous generation ofand.Theconcentration was stable at around 3.6-4.3 mol L-1,and its formation was rapid in the early repetition numbers.Furthermore,the proportion ofinincreased with the increasing repetition number,possibly becausewas unstable in liquid and quickly converted into.As a result,the energy cost ofproduction declined,and the production rate increased with the repetition number.Sincewas the sum ofand,its energy cost and production rate changed less.After the 120th treatment,the final EC(aq)() was as low as 13.9 MJ mol-1,while the EC(aq)() was 12.2 MJ mol-1.
Moreover,with the optimal operation parameters of the system,the PNF system can achieve an energy efficiency ofproduction (EE()) of 16 g kWh-1(calculated by equation (6)).
3.5.Effect of PNF products on the growth of lettuce
Table 5,along with figures 8 and 9 show the effect of different irrigation solutions on the morphology of lettuce.It was observed that TW-PNF significantly promoted the morphology and biomass of lettuce compared to TW.Specifically,the shoot fresh weight in TW-PNF was 7.95 times heavier in TW-PNF than that in TW.These findings suggested thatin TW-PNF played a vital role in lettuce growth.In contrast,there were no significant differences in leaf length,stem thickness,shoot fresh weight,and shoot dry weight in YN and YN-PNF.
Lettuce irrigated with TW ceased growth due to the lack of nitrogen fertilizer.In contrast,lettuce irrigated with watertreated by the PNF system thrived.Both YN and YN-PNF showed insignificant growth differences,indicating that the liquid products of the PNF system had the same effect as the nitrate component of traditional nutrient solution.Furthermore,the PNF system product could replacein the Yamazaki nutrient solution.
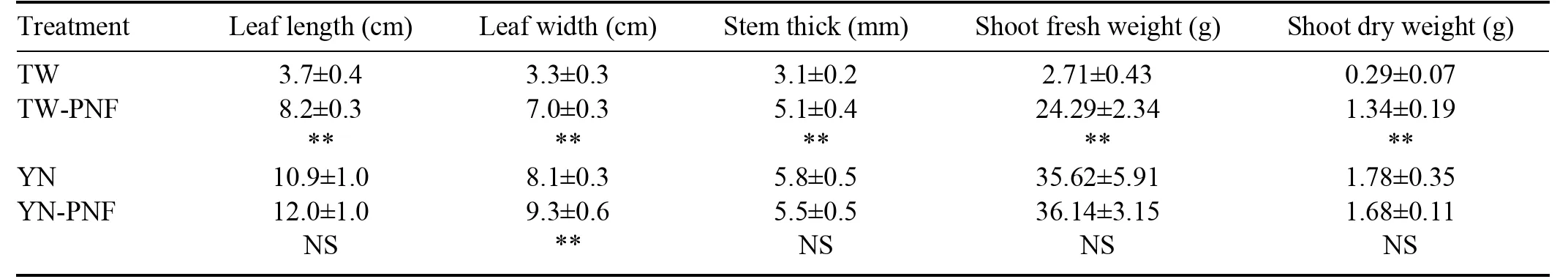
Table 5.Lettuce leaf morphology and biomass accumulation in four experimental groups.
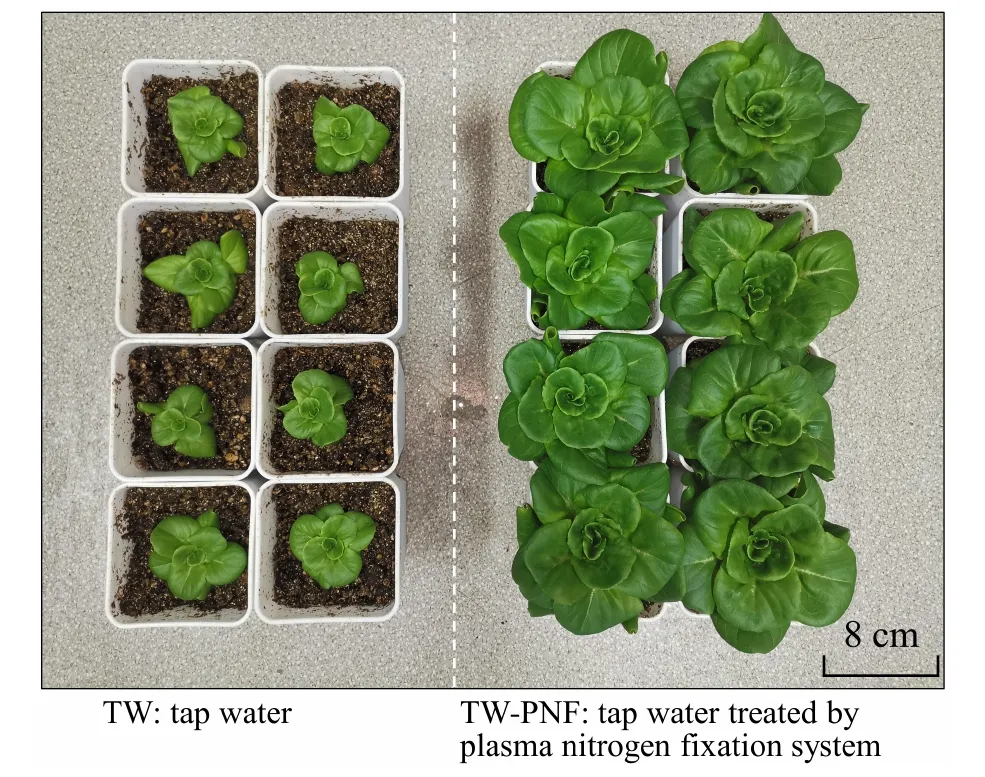
Figure 8.Growth characteristics of lettuces from the TW and TWPNF after 24-day cultivation.

Figure 9.Growth characteristics of lettuces with different treatments after 24-day cultivation.
However,it is worth mentioning that the lettuce in TWPNF grew slower than that in YN and YN-PNF,probably because the irrigation solution of TW-PNF lacked other nutrients,such as phosphorus and potassium,aside from nitrate.
4.Discussion
4.1.Advantages of the dual-loop design in this work
This study investigated the reactions involved in the formation of gliding-arc plasma in the gas phase and the absorption of gaseous NOxin the liquid phase.The reactions of PNF are detailed in equations (8)-(15),including the production of a mixture of NO and NO2via the reactions of energetic electrons with gaseous N2and O2[45] (shown in equations (8)-(11)).While NO2readily dissolves in water,NO exhibits solubility and cannot be converted intodirectly.Moreover,new NO is produced during the absorption of NO2in water (see equations (12) and (15)),making it necessary to promote the proportion of NO2in a gaseous mixture of NOxfor accelerating the formation of aqueous.
The oxidation of NO in equation (11) is the most important net formation process of NO2and can be quantitatively analyzed by kinetic equations [37,38].The reaction is a third-order gas phase reaction,and the reaction rate of NO(d[NO]/dt) can be expressed by equation (16) [46]:
where [NO] and [O2] were the concentrations of gaseous NO and O2(mol L-1),respectively.t is the reaction time.kgis the rate constant of gaseous species (L2mol-2s-1).Nishimura et al reported that the kgis equal to 7.3×103L2mol-2s-1in the mechanical ventilation setting [47].
Since O2concentration (2×105ppm) is markedly larger (≥1 0-fold) than that of NO,it can be assumed that [O2] remains unchanged [48].Consequently,the reaction in equation (11)can be described using pseudo-second-order kinetics.Integrating equation (16) can obtain equation (17) [46,48].
where [NO]trepresents NO concentration after the reaction time t and [NO]0is the initial NO concentration.
f was defined as the fraction of NO that has been oxidized in time t in equation (18).The equation (17) can be transformed into equation (19).
It is evident from equation (19) that the time required for a certain fraction of NO that has been oxidized is inversely proportional to the initial NO concentration [NO]0and the rate constant kg.
The dual-loop design has improved the initial NO concentration and provided ample time for the oxidation of NO.For group (1) mentioned in table 1 (t2=60 s,t3=110 s),the [NO]0before absorption was 1887 ppm,which was 1.3 times that of the non-loop design (1471 ppm).The [NO]t=110scould be calculated by equation (17) that after 110 s of absorption in the loop,56.7% of NO (1070 ppm) was theoretically converted into NO2.The initial NO concentration can be significantly increased by extending the discharge time (t2),and if the [NO]0increases to 5000 ppm,up to 81.4%of NO would be oxidized after 110 s.Besides,as there was only 104 ppm of NOxemission after 110 s in figure 5,the actual rate constant kgin the absorption loop was more significant than the value from reference [47].The phenomenon had also been reported in the study [49] that the existence of water vapor could increase the rate constant kg.
The PNF system used air as feed gas,avoiding additional costs and making it more suitable for on-site nitrogen fixation.The dual-loop design was found to increase the percentage of NO2due to two loop processes: (1) during the discharge loop,the initial NO concentration was significantly increased,and NO had ample time to be oxidized;(2) in the loop of absorption,a little energy consumption(14.6 W of power) supported relatively long time for the NO oxidation.Meanwhile,the newly produced NO in equation(15) could also be converted to NO2during this time.The results in figure 4 demonstrate the advantages of the dualloop design,which not only helped to raise the concentration ofbut also reduced the energy cost of nitrogen fixation.
4.2.Energy cost of production
In this study,the optimal energy cost ofproduction was 13.9 MJ mol-1while still meeting the required concentration of NOxemissions.The energy cost was defined as the total energy input required to produce each mole of,including power input from the high-voltage source,control system,pumps,valves,control circuits,and power loss.Therefore,the energy cost analysis in this study was more comprehensive than previous studies that only considered power consumption in the plasma reactor.
Table 6 displays the energy cost ofproduction from various methods and conditions reported in previous studies.The results show that the energy cost in this study was relatively low.Among studies that used pure air as the feed gas,this study has achieved the lowest energy cost forproduction.Since air served as a cost-free raw material,this could potentially reduce the overall cost of the PNF systemin the future.

Table 6.Nitrogen fixation to reactive nitrogen species using plasma technologies.
The combination of the dual-loop mode and optimization of runtime parameters were the primary factors contributing to the low energy cost achieved in this study.Additionally,gas renewal was crucial for providing adequate air for nitrogen oxidation.During the discharge loop,NOxwas consistently formed and steadily accumulated.
In terms of generalizability,it is worth noting that other low-temperature plasma discharge sources could also be employed in our system to achieve betterproduction performance.In summary,this study provides a potential method for converting gaseous NOxinto the liquid phase with reduced energy costs while demonstrating how to determine optimal operation-time parameters for the nitrogen fixation system.
4.3.Influence of on the liquid product
Nitrogen is a crucial nutrient for plant growth,and in the agricultural ecosystem,plants can directly absorb two inorganic nitrogen sources:and[50].As shown in figure 7(b),nitrogen fixation water contained a significant amount ofand a small amount of.
4.4.Prospects for on-site application
The PNF system developed in this study has several advantages,including low energy costs,automatic operation,and compliance with NOxemissions standards.Furthermore,it featured a compact structure and low input power,averaging around 70 W (as calculated by equation (5)),making it well-suited for modular application and on-site nitrogen fertilizer production [52].
For example,a single PNF module can be powered by solar panels generating over 200 W.This allows nitrogen fertilizer production using only air,water,and renewable energy,thereby adapting to various application scenarios,such as vegetable cultivation on islands,ships,deserts,and plant factories.In contrast,the Haber-Bosch method requires large fertilizer factories,and the ammonia nitrogen fertilizer must be transported to the cultivation site.
Lettuce,being a leafy-green vegetable,highly relies on nitrogen fertilizer for optimal growth.The PNF system can effectively promote lettuce growth,making it a potential nitrate fertilizer supplier for leafy-green cultivation.For instance,fresh leafy greens are often in short supply on long sea voyages,as they have a short storage life of only a few days.By using the PNF system,it becomes feasible to cultivate leafy greens during the voyage,providing the ship's crew with essential vitamins and nutrients.
Nonetheless,further efforts should focus on enhancing the efficiency and reliability of the PNF system in the future.The raw nitrate solution produced is weakly acidic and requires treatment before irrigating plants.Additionally,it is essential to incorporate other essential elements to improve both the yield and quality of leafy greens.
5.Conclusions
An automatic PNF system based on gliding-arc discharge has been developed and utilized for lettuce cultivation.The experimental results show that the dual-loop design is effective in reducing energy costs and increasing the production rate of.Optimal operation parameters were identified experimentally for this PNF system,including t1=30 s,t2=180 s,t3=150 s,and m=120.Under these parameters,the energy cost ofproduction was 13.9 MJ mol-1,and energy efficiency was 16 g kWh-1,respectively.Moreover,the concentration of exhausted NOxmet the emission standards.The experimental results demonstrated that theproduced by the PNF system could be used as a liquid nitrate nitrogen fertilizer for lettuce cultivation.This study suggests that in the future,(1) PNF systems can be developed as modules for on-site applications and powered by renewable energy sources,and (2) the product of the PNF system could serve as an effective fertilizer for leafy green vegetables.
Acknowledgments
This work is supported by the Science and Technology Project of State Grid Corporation of China (No.5400-202133157A-0-0-00).The authors were partially supported by the State Grid Gansu Electric Power Company,China.Many suggestions from colleagues are also highly appreciated.
杂志排行
Plasma Science and Technology的其它文章
- Ion-acoustic waves with non-planar wavefronts
- Gyro-Landau-fluid simulations of impurity effects on ion temperature gradient driven turbulence transport
- Investigation on the roles of equilibrium toroidal rotation during edge-localized mode mitigated by resonant magnetic perturbations
- Gyrokinetic simulation of magnetic-islandinduced electric potential vortex mode
- Modification of streamer-to-leader transition model based on radial thermal expansion in the sphere-plane gap discharge at high altitude
- Different bactericidal abilities of plasmaactivated saline with various reactive species prepared by surface plasmaactivated air and plasma jet combinations
