腺病毒介导的肝细胞生长因子/血管内皮生长因子165对大鼠超比例随意皮瓣成活的影响及机制研究
2024-03-04赵毅闫洪伟王玉琦陈丽娟洪城田诗政
赵毅 闫洪伟 王玉琦 陈丽娟 洪城 田诗政

[摘要]目的:觀察术前注射腺病毒介导的肝细胞生长因子/血管内皮生长因子165(Ad-HGF/VEGF165)对大鼠超比例随意皮瓣成活的影响和其机制的初步研究。方法:大鼠背部设计超比例随意皮瓣模型,将其随机分为Ad-HGF/VEGF165组、Ad-VEGF165组和对照组。术前7 d,Ad-HGF/VEGF165组和Ad-VEGF165组分别于大鼠皮瓣及创缘内多点注射Ad-HGF/VEGF165及Ad-VEGF165,对照组注射等容量0.9%氯化钠注射液。分别于术后7 d、14 d观测皮瓣成活面积,切取皮瓣组织标本HE染色、免疫组化观察微血管密度及组织间VEGF阳性率等指标,Western Blot检测目的蛋白表达情况。结果:术后7 d、14 d,Ad-HGF/VEGF165组、Ad-VEGF165组皮瓣存活面积、组织间微血管数目、VEGF及细胞外调节蛋白激酶(ERK1/2)蛋白表达情况均高于对照组(P<0.001),且Ad-HGF/VEGF165组明显高于Ad-VEGF165组(P<0.05)。结论:Ad-HGF/VEGF165具有协同作用,能显著促进VEGF和ERK1/2信号因子表达,短期内促进血管网重建,改善皮瓣血液灌注,提高大鼠超比例随意皮瓣成活面积。
[关键词]超比例随意皮瓣;基因治疗;腺病毒;血管新生;肝细胞生长因子;血管内皮生长因子
[中图分类号]R622 [文献标志码]A [文章编号]1008-6455(2024)02-0060-05
Experimental Study on the Influence of Adenovirus-mediated Hepatocyte Growth Factor/vascular Endothelial Growth Factor 165 on the Survival and Mechanism of Hyper-proportion Random Flap in Rats
ZHAO Yi1,2,YAN Hongwei2,WANG Yuqi1,CHEN Lijuan2,HONG Cheng2,TIAN Shizheng2
(1.Jinzhou Medical University,Jinzhou 121000,Liaoning,China; 2.Department of Burn Plastic Surgery,Shiyan People's Hospital/ Affiliated People's Hospital of Hubei University of Medicine,Shiyan 442000,Hubei,China)
Abstract: Objective To observe the effect of adenovirus-mediated hepatocyte growth factor / vascular endothelial growth factor 165 (Ad-HGF/VEGF165) injection before flap operation on the survival and Mechanism of Hyper-proportion random flap in rats. Methods The model of Hyper-proportion random flap was designed on the back of rats and randomly divided into Ad-HGF/VEGF165 group, Ad-HGF/VEGF165 group and control group. Seven days before operation, Ad-HGF/VEGF165 group and Ad-HGF/VEGF165 group were separate multipoint injection Ad-HGF/VEGF165 and Ad-VEGF165 into the rat skin flap at multiple points in the experimental group, while 0.9% sodium chloride injection was injected in the control group. The survival area of the flap was observed on the 7 day and 14 day after operation, and the tissue samples of the flap were obtained for HE staining, the microvessel density and VEGF expression were observed by immunohistochemistry, and Western blot for detection of protein of target protein. Results On the 7 day and 14 day after operation, skin flap survival area,the number of microvessels, VEGF and the expression of extracellular regulated protein kinase (ERK1/2) protein in the Ad-HGF/VEGF165 group and Ad-HGF/VEGF165 group were higher than those in the control group(P<0.001), and all indexes were significantly higher in the Ad-HGF/VEGF165 group than in the Ad-VEGF165 group (P<0.05). Conclusion Ad-HGF/VEGF165 has a synergistic effect, which can significantly promote the expression of VEGF and ERK1/2 signal factors, increase the survival area of Hyper-proportion random flap, promote angiogenesis and improve the blood circulation of the flap.
Key words: hyper-proportion random flap; gene-therapy; adenovirus; revascularization; hepatocyte growth factor; vascular endothelial growth factor
头颈部肿瘤、急性外伤、重度烧伤及糖尿病等基础疾病导致的皮肤创面缺损是临床皮瓣修复手术中常见的疾病。随意皮瓣移植因不受蒂部限制,设计和操作简便、可重复性好,在临床上得到了广泛的应用[1],但是,由于皮瓣远端延迟或不完全的血管網重建,限制了随意皮瓣术后的存活率。为了拓宽皮瓣的应用范围,提高随意皮瓣的存活面积,研究者通过不同的载体系统将多种类的外源性基因注射至受区,通过直接或旁分泌途径提高生长因子浓度,从而诱导微血管形成[2-4]。但已有数据表明,仅靠一种生长因子并不能在皮瓣移植术后早期提供足够的血运灌注和稳定的血管网络。将多种基因联合应用后,发现可在同时期内建立更密集的小动脉分支[5-7]。此外,还应选择适当的注射时机,否则在外源性基因高表达前,皮瓣可能因血液供应不足而出现缺血性坏死。相关文献显示,与术后即刻注射相比,术前注射腺病毒介导的外源性基因在改善皮瓣存活率和血液循环方面效果显著[8-9]。为了优化生长因子潜在的疗效,并确保在有限的时间内发挥其生物活性,本研究结合以往理论基础,采用大鼠背部超比例随意皮瓣模型,以腺病毒为载体,术前7 d于皮瓣远端及创缘处局部注射Ad-HGF/VEGF165,观察其对皮瓣成活的影响,同时测定与分析组织内微血管数目、生长因子的变化情况,现报道如下。
1 材料和方法
1.1 实验主要仪器与试剂:Ad-HGF/VEGF165、Ad-VEGF165为湖北医药学院附属人民医院临床研究所王家宁博士惠赠,VEGF兔多克隆抗体(Immunoway)、CD31羊多克隆抗体(R&D)、ERK1/2兔单克隆抗体(Cell signaling Technology)。普通/荧光显微镜(Nikon公司)、凝胶成像仪(BIO-RAD)、电泳仪及转膜仪(BIO-RAD)、石蜡切片机(Lecia德国)。
1.2 实验动物和分组:选用12周龄SD雄性大鼠30只(湖南斯莱克景达公司),体重250~320 g,适应性饲养1周。按照随机数字表法分为Ad-HGF/VEGF165组、Ad-VEGF165组和对照组,每组10只。动物实验经伦理委员会审批通过。
1.3 皮瓣模型制备及Ad-HGF/VEGF165运用:以大鼠背部中线为皮瓣纵轴,肩胛下缘为皮瓣蒂部,设计8 cm×2 cm超比例随意皮瓣。术前7 d,Ad-HGF/VEGF165组和Ad-VEGF165组分别在皮瓣的中远端和边缘处注射共计1 ml含有2×108滴度单位的Ad-HGF/VEGF165和Ad-VEGF165,每点注入0.05 ml,等距注射至深筋膜层,共20个点。对照组注入等容量的0.9%氯化钠注射液。异氟烷气体麻醉大鼠,俯卧位固定于操作台,切开皮瓣皮肤,于深筋膜浅层将皮瓣自远端向蒂部掀起,皮瓣掀起后,确保破坏创面床与创缘吻合的血管网,将皮瓣原位缝合。
1.4 观察指标
1.4.1 皮瓣大体观察及存活率:术后定期监测大鼠皮瓣血运情况,测量皮瓣存活区域面积,计算各组皮瓣的成活面积,并求取其皮瓣平均存活率。 。
1.4.2 病理学观察:三组大鼠分别于术后7 d切取距存活与坏死组织交界处0.5 cm的近端皮瓣组织标本,10%多聚甲醛固定后包埋切片,二甲苯脱蜡处理,光镜下进行病理学观察。
1.4.3 免疫组化:采用SABC法,抗原修复后滴加山羊血清封闭50 min,滴加一抗,VEGF、CD31抗体浓度比分别为1∶200与1∶100。放于4℃冰箱孵育过夜,磷酸盐缓冲液(PBS)充分冲洗;二抗孵育1 h,苏木素染色。检测不同时期CD31标记皮瓣远端存活区的微血管生成数目,400倍光学显微镜下,随机求取5个不同视场的血管横截面数目,求得各组皮瓣的新生血管数目,观测VEGF在组织中阳性表达。
1.4.4 Western Blot:将皮瓣组织放入液氮进行研磨,匀浆放入EP管,离心后取上清液计算蛋白浓度。根据标准程序,进行SDS电泳,将制备胶转移至PVDF膜上,放入5%脱脂牛奶置于摇床封闭30 min,随后将一抗VEGF165、ERK1/2和P-ERK1/2放于4℃冰箱孵育过夜,浓度比1∶1 000。TBS-T中冲洗3遍,室温下二抗孵育2 h。TBS-T中冲洗3遍,每遍15 min。最后在凝胶成像仪上对蛋白表达量进行检测。
1.5 统计学分析:使用SPSS 26.00软件进行统计学分析,计量资料均采用(x¯±s)表示,三组间比较行单因素方差分析,通过t检验确定两组间差异,采用Image-Pro-Plus6.0检测VEGF的IOD值,P<0.05为差异有统计学意义。
2 结果
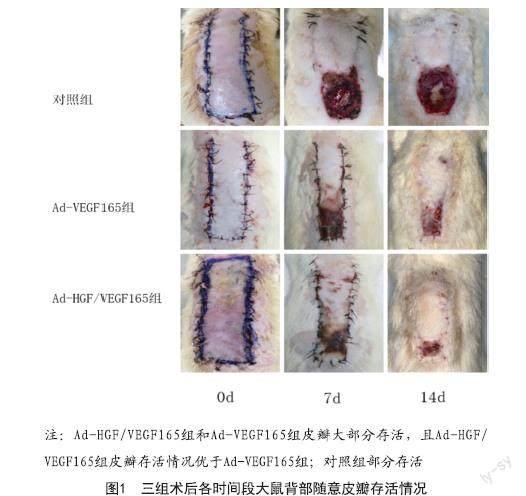
2.1 皮瓣大体观察:术后12 h,皮瓣远端区域皮温稍凉,呈淡紫色。术后7 d,饮食及二便正常,Ad-HGF/VEGF165组和Ad-VEGF165组皮瓣远端缺血区域皮肤皱缩,继而发黑干性坏死,部分创面破溃。对照组皮瓣坏死区域有少量脓性分泌物,与Ad-HGF/VEGF165组和Ad-VEGF165组相比坏死面积增大,存活与坏死区域界限清楚。术后14 d,对照组清除硬痂及坏死组织后基底欠新鲜,Ad-HGF/VEGF165组皮瓣末端发生血运障碍区域较另两组明显减少,坏死区痂皮溶脱,显露基底新鲜肉芽组织。见图1。
2.2 三组大鼠皮瓣成活率比较:术后7、14 d,Ad-HGF/VEGF165组和Ad-VEGF165组大鼠背部皮瓣存活面积均高于对照组,且Ad-HGF/VEGF165组高于Ad-VEGF165组(P<0.05)。采用LSD法两两比较,组间差异有统计学意义,且三组数据行单因素方差分析得出P<0.001。见表1。
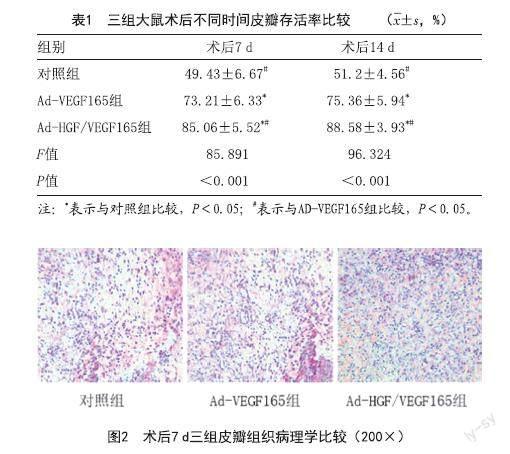
2.3 组织学检测:光镜下观察,术后7 d对照组皮瓣坏死区域急性炎症反应加重,Ad-HGF/VEGF165组和Ad-VEGF165组皮瓣成活区表现出较厚的真皮层,可见大量成纤维细胞增生,周围新生血管丰富,血管壁薄。对照组表皮层较薄,血管生成少见。见图2。
2.4 三组VEGF阳性表达情况及微血管密度比较:术后7 d,Ad-HGF/VEGF165组血管周围呈棕黄色染色呈团簇分布,表明有大量VEGF的表达,Ad-VEGF165组中阳性表达有所欠缺,对照组皮瓣成活区周围组织少见。术后7 d、术后14 d,Ad-HGF/VEGF165组和Ad-VEGF165组皮瓣微血管密度均高于对照组,Ad-HGF/VEGF165组新生微血管管腔结构良好,管壁薄。与Ad-VEGF165组相比,同时期内建立的小血管分支更密集(P<0.01)。见图3~4。
2.5 Western Blot:Western Blot检测三组各时期VEGF蛋白电泳测定结果,术后7 d达到峰值,Ad-HGF/VEGF165组的VEGF表达明显高于另两组,术后14 d蛋白表达有所回落。术后7 d时,Ad-HGF/VEGF165组显现出对VEGF和P-ERK1/2蛋白更高的活化潜能。见图5~6。
3 讨论
在整形与重建外科手术中,皮瓣移植术后早期促进新生血管网的形成与稳定性是提高皮瓣存活的重要因素[10-11]。经实验观察,高压氧治疗、血管扩张剂、磷酸二酯酶抑制剂等均可提高皮瓣移植后组织的氧含量并减轻炎症反应,使皮瓣的存活率一定程度上得到改善[12-14],但在扩大皮瓣运用面积效果方面并不理想。近年来,研究者们利用合适的载体,将外源性生长因子直接注射入皮瓣中远端,可有效提高小动脉供血及毛细血管网的形成,降低了皮瓣的坏死率[15-16]。其中,腺病毒作为常见的载体。在转染后的5~7 d,外源性基因蛋白的表达达到高峰,有效表达数周后消失。这种自限性能确保发挥细胞的生物学活性,并避免由于长期的基因表达而导致的病理性肿瘤[9,17-18]。在此基础上,本研究设想在术前7 d进行术区注射,避免外源性基因高表达前,皮瓣末端已发生缺血性坏死。通过免疫组织化学分析发现,术后初期,基因治疗组皮瓣远端成活面积和再血管化均有明显的改善。HE染色显可见大量的新生微血管及成纤维细胞,同时VEGF在内皮细胞胞质间的阳性表达及Western Blot的检测结果表明皮瓣组织间生长因子浓度明显增高,也证实了上述猜测。相关研究亦发现,于皮瓣缺血损伤前1周注射,使皮瓣远端有充足的时间构建支持性血管[8]。本研究结合现有理论基础,建立大鼠超比例随意皮瓣动物模型,术前7 d局部皮下注射Ad-HGF/VEGF165,论证基因联合治疗能有效提高并延长皮瓣移植术后局部生长因子生物学浓度,增强皮瓣远端血管新生和侧支循环的形成,为突破随意皮瓣長宽比例限制提供参考。
术后7 d观测皮瓣存活面积,发现Ad-HGF/VEGF165组可显著改善皮瓣远端的血运,仅有少数边缘区域组织缺血坏死。而Ad-VEGF165组皮瓣远端坏死相对严重。以往的研究中,Slobodkina E等[19]也发现联合应用后可明显提高毛细血管生成数目及侧支循环形成,微血管管腔长度增大,同时HGF与VEGF联合应用能更好的促进ERK1/2蛋白的磷酸化。这与本研究结果一致。其机制可能是:①除HGF和VEGF通过各自途径促进血管内皮细胞增殖,HGF还可通过磷脂酰肌醇3-激酶(PI3K)诱导VEGF的分泌和表达,并在细胞外基质促进VEGF的活性成倍增加[20-23]。②VEGF与HGF联合应用后,ERK1/2蛋白磷酸化水平明显高于单独应用一种因子时[19]。ERK1/2通过诱导特定蛋白的表达或活化,进而调控细胞的增殖和代谢[24-25]。在促进糖尿病大鼠创面愈合的实验中抑制ERK1/2通路,发现血管生成数目、创面愈合速率明显降低[26]。③基因联合应用能增强Rho的活性及Rac的调控,使皮瓣远端的新生血管与和创缘预先存在的血管网进行功能性重塑,形成了新的小动脉分支。同时HGF与VEGF联合应用可提高抗凋亡基因Bcl2和A1的mRNA水平,增强血管内皮细胞在组织严重缺血和炎性反应环境中的存活能力[27-29]。
综上所述,本实验证实了HGF和VEGF165联合应用能显著增强ERK1/2蛋白的磷酸化和VEGF-A的分泌与表达,并且术前7 d注射使受损组织有充足的时间构建支持性血管,从而有效改善术后早期皮瓣中远端成活能力。但是,其协同效应的机制仍需更深入研究。虽然目前尚无关于两种基因联合应用在体内的适当药物剂量和注射时间的可复制性数据,以确保患者治疗后的长期安全性,但在促进血管新生和改善皮瓣存活方面,基因治疗仍是值得期待的研究方向。
[参考文献]
[1]Wu S,Hu X,Wang Z H,et al.Extracellular vesicles isolated from hypoxia-preconditioned adipose-derived stem cells promote hypoxia-inducible factor 1alpha-mediated neovascularization of random skin flap in rats[J].Ann Plast Surg,2022,89(2):225-229.
[2]Sanada F,Fujikawa T,Shibata K,et al.Therapeutic angiogenesis using HGF plasmid[J].Ann Vasc Dis,2020,13(2):109-115.
[3]Seyed J S,Blank F,Ramser H E,et al.Efficacy of combined in-vivo electroporation-mediated gene transfer of vegf, hgf, and IL-10 on skin flap survival, monitored by label-free optical imaging: a feasibility study[J].Front Surg,2021,8:639661.
[4]Jin Z,Yao C,Poonit K,et al.Allogenic endothelial progenitor cell transplantation increases flap survival through an upregulation of eNOs and VEGF on venous flap survival in rabbits[J].J Plast Reconstr Aesthet Surg,2019,72(4):581-589.
[5]Wang L S,Wang H,Zhang Q L,et al.Hepatocyte growth factor gene therapy for ischemic diseases[J].Hum Gene Ther,2018,29(4):413-423.
[6]Chang H K,Kim P H,Kim D W,et al.Coronary stents with inducible VEGF/HGF-secreting UCB-MSCs reduced restenosis and increased re-endothelialization in a swine model[J]. Exp Mol Med,2018,50(9):1-14.
[7]Makarevich P I,Dergilev K V,Tsokolaeva Z I,et al.Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene therapy in a rat model of myocardial infarction[J].PLoS One,2018,13(5):e197566.
[8]Fang T,Lineaweaver W C,Chen M B,et al.Effects of vascular endothelial growth factor on survival of surgical flaps: a review of experimental studies[J].J Reconstr Microsurg,2014,30(1):1-13.
[9]Afrough S,Rhodes S,Evans T,et al.Immunologic dose-response to adenovirus-vectored vaccines in animals and humans: a systematic review of dose-response studies of replication incompetent adenoviral vaccine vectors when given via an intramuscular or subcutaneous route[J].Vaccines (Basel),2020,8(1):131.
[10]趙黎君,周琴,邹小梅.鼠神经生长因子联合腹部超薄皮瓣修复手部深度电烧伤创面[J].中国美容医学,2022,31(10):31-34.
[11]Altinel D,Serin M,Erdem H,et al.Comparison of incisional delay patterns on a rat random flap model[J].J Plast Surg Hand Surg,2019,53(4):247-253.
[12]Seth R,Badran K W,Cedars E,et al.Vasodilation by verapamil-nitroglycerin solution in microvascular surgery[J].Otolaryngol Head Neck Surg,2021,164(1):104-109.
[13]吕春风,刘胜达,种红,等.皮瓣移植术后应用高压氧治疗对皮瓣存活的影响研究[J].中国美容医学,2019,28(1):31-33.
[14]Pedretti S,Rena C L,Orellano L,et al.Benefits of pentoxifylline for skin flap tissue repair in rats[J].Acta Cir Bras,2020,35(11):e301105.
[15]Chehelcheraghi F,Chien S,Bayat M.Mesenchymal stem cells improve survival in ischemic diabetic random skin flap via increased angiogenesis and VEGF expression[J].J Cell Biochem,2019,120(10):17491-17499.
[16]Kushibiki T,Mayumi Y,Nakayama E,et al.Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor[J].Sci Rep,2021,11(1):23094.
[17]Bertzbach L D,Ip W H,Dobner T.Animal models in human adenovirus research[J].Biology (Basel),2021,10(12):1253.
[18]陈金逸,陈宗存,饶朗毓,等.hPlGF-2基因修饰的骨髓间充质干细胞对皮肤创伤修复及血管形成的作用研究[J].中国美容医学,2020,29(10):106-111.
[19]Slobodkina E,Boldyreva M,Karagyaur M,et al.Therapeutic angiogenesis by a "dynamic duo": simultaneous expression of HGF and VEGF165 by novel bicistronic plasmid restores blood flow in ischemic skeletal muscle[J].Pharmaceutics,2020,12(12):1231.
[20]Ntellas P,Mavroeidis L,Gkoura S,et al.Old player-new tricks: non angiogenic effects of the VEGF/VEGFR pathway in cancer[J].Cancers (Basel),2020,12(11):3145.
[21]Moosavi F,Giovannetti E,Saso L,et al.HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers[J].Crit Rev Clin Lab Sci,2019,56(8):533-566.
[22]Melincovici C S,Bosca A B,Susman S,et al.Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis[J].Rom J Morphol Embryol,2018,59(2):455-467.
[23]Barc P,Antkiewicz M,Sliwa B,et al.Treatment of critical limb ischemia by pires/vegf165/hgf administration[J].Ann Vasc Surg,2019,60:346-354.
[24]Wu J,Kong M,Lou Y,et al.Simultaneous activation of Erk1/2 and Akt signaling is critical for formononetin-induced promotion of endothelial function[J].Front Pharmacol,2020,11:608518.
[25]Medfai H,Khalil A,Rousseau A,et alHuman peroxidasin 1 promotes angiogenesis through ERK1/2, Akt, and FAK pathways[J].Cardiovasc Res,2019,115(2):463-475.
[26]Zhang J,Chen C,Hu B,et al.Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling[J].Int J Biol Sci,2016,12(12):1472-1487.
[27]Barc P,Antkiewicz M,Sliwa B,et al.Double VEGF/HGF gene therapy in critical limb ischemia complicated by diabetes mellitus[J].J Cardiovasc Transl Res,2021,14(3):409-415.
[28]Zhong W,Zhao Y,Tian Y,et al.The protective effects of HGF against apoptosis in vascular endothelial cells caused by peripheral vascular injury[J].Acta Biochim Biophys Sin (Shanghai),2018,50(7):701-708.
[29]Makarevich P,Tsokolaeva Z,Shevelev A,et al.Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle[J].PLoS One,2012,7(6):e38776.
[收稿日期]2022-10-28
本文引用格式:趙毅,闫洪伟,王玉琦,等.腺病毒介导的肝细胞生长因子/血管内皮生长因子165对大鼠超比例随意皮瓣成活影响及机制研究[J].中国美容医学,2024,33(2):60-63,97.
