DLL3 promotes progression of prostate cancer through MAPK pathway*
2024-01-17LIYuxuanYULinaZHANGWeiyangQIJingruZHAOZhuoyangHUAXing
LI Yuxuan,YU Lina,ZHANG Weiyang,QI Jingru,ZHAO Zhuoyang,HUA Xing
(1Department of Pathology,School of Basic Medicine and Public Health,Jinan University,Guangzhou 510632,China;2Departments of Pathology,Guangzhou Red Cross Hospital of Jinan University,Guangzhou 510220,China; 3Department of Pathology,School of Basic Medical Sciences,Southern Medical University,Guangzhou 510515,China; 4Department of Pathology,Nanfang Hospital,Guangzhou 510515,China; 5Guangdong Provincial Key Laboratory of Molecular Tumor Pathology,Guangzhou 510515,China. E-mail: Huaxingtime@ext.jnu.edu.cn)
[ABSTRACT] AIM: To explore the impact of Delta-like ligand 3(DLL3) on the progression of prostate cancer(PCa) and to elucidate its potential molecular mechanisms. METHODS: Overexpression and interference plasmids of DLL3 gene were constructed,and the effects of DLL3 on the proliferation,migration and invasion of PCa cells were assessed. Tumorigenesis assay was employed to determine whether DLL3 influenced the proliferation of PC-3 cells in vivo.The impact of DLL3 on epithelial-mesenchymal transition (EMT) and mitogen-activated protein kinase (MAPK) signaling was investigated through Western blotting. RESULTS: Overexpression of DLL3 significantly enhanced the proliferation,migration,invasion and EMT processes of PCa cells compared with empty vector group and blank control group (P<0.05). Conversely,knockdown of DLL3 expression yielded opposite effects (P<0.05). Moreover,up-regulation of DLL3 promoted tumor growth in the nude mouse xenograft model(P<0.05). Further investigation demonstrated that DLL3 upregulation led to increases in the levels of MAPK signaling pathway-related proteins. Interestingly,MAPK inhibitor effectively reduced the proliferation,migration and invasion of PCa cells caused by DLL3 overexpression (P<0.05). CONCLUSION: DLL3 promotes the proliferation,migration and invasion of PCa through the MAPK pathway.
[KEY WORDS] prostate cancer; Delta-like ligand 3; epithelial-mesenchymal transition; MAPK pathway
Over the past decade,several factors,such as population aging,urbanization,changes in the environment and lifestyle habits,and the increasing implementation of prostate-specific antigen screening,have contributed to rising prevalence of prostate cancer (PCa)[1-2]. Currently ranking as the fifth most common male malignant tumor in China,the recorded number of PCa cases reached 153 450 in 2019,and is projected to rise to 315 310 by 2030[3]. Accordingly,PCa has become a major public health concern. Despite progress in PCa management,including radiation therapy,surgical prostatectomy,androgen deprivation therapy and immunotherapy,the prognosis for patients with advanced prostate cancer remains poor[4]. Therefore,gaining a comprehensive understanding of the molecular mechanisms specific to the progression of PCa is crucial.
Delta-like ligand 3 (DLL3),initially discovered for its role in neural tube formation,notochord differentiation,spinal cord development,and other processes during embryonic development[5],reportedly exhibits limited expression or absent in normal tissues[6]. However,small-cell lung cancer (SCLS) and other neuroendocrine tumors was found to exhibit high levels of DLL3 on their membrane surface[6]. The potential function of DLL3 varies across tumor types,as it has been found to facilitate tumorigenesis and progression in SCLC[7],endometrial carcinoma[8],gastric carcinoma[9],breast cancer[10],melanoma[11],pituitary neoplasm[12]and small cell bladder cancer[13]. Conversely,DLL3 has been demonstrated to hinder the development of liver cancer[14]and malignant glioma[15]. The current understanding of DLL3's function and mechanism in PCa remains unclear. Therefore,additional research on the role and mechanism of DLL3 in PCa is essential for advancing treatment.
The epithelial-mesenchymal transition (EMT) process is crucial in both embryonic development and carcinogenesis. During EMT,epithelial cells lose their adhesive properties,including polarity and the presence of tight junctions[16]. Tumor cells undergoing EMT exhibit enhanced aggressiveness,including metastatic potential,invasive behavior,immunity evasion,and resistance to therapeutic agents[17]. The downregulation of E-cadherin expression is widely recognized as the most significant characteristic of EMT. Simultaneously,cells exhibit upregulation of mesenchymal markers,such as N-cadherin and vimentin.
Mitogen-activated protein kinase (MAPK) is a serine/threonine protein kinase ubiquitously present in mammalian cells[18]. The MAPK pathway facilitates the transmission of cytoplasmic signals to the nucleus,essential cellular processes,including proliferation,differentiation,apoptosis,and angiogenesis[19]. Under physiological conditions,the expression levels of MAPK family members maintain a dynamic balance between cell proliferation and apoptosis[18]. Growing evidence suggests that the initiation and progression of tumors are associated with the regulation of the MAPK signaling pathway[20],and the aberrant activation of specific proteins within this pathway is a prominent factor contributing to the pathogenesis of malignancies[21].
MATERIALS AND METHODS
1 Cell culture and transfection
The PCa cell lines (PC-3,DU145 and C4-2) were procured from the Cell Bank of the Chinese Academy of Science and authenticated by short tandem repeat(STR) genotyping. Cells were cultured in RPMI-1640 medium (KeyGen,Shanghai,China) supplemented with 10% fetal bovine serum (HyClone,USA) and 1% penicillin-streptomycin at 37 ℃,5% CO2,and 95% humidity. Cells were seeded in 6-well plates with an initial density to achieve a 70% confluence rate within 24 hours. Transfection was performed using Lipo-3000(GLPBIO,USA) with DLL3 plasmids (JiKai Gene,Shanghai,China). After 6 hours,the culture medium was replaced,and cells were collected after 48 hours for further experimentation.
2 RT-qPCR
The cells were collected into a sterile enzyme-free EP tube,and the following experimental steps were performed on ice. Trizol reagent(Invitrogen) was used to extract the total RNA,which was appropriately diluted with DEPC-treated water(Leagene,China). The density and purity (1.8-2.0) of the RNA were assessed by Nanodrop (Thermo Fisher). Subsequently,the total RNA was reverse transcribed into cDNA according to the guidelines of PrimeScript RT reagent Kit with gDNA Eraser (RR047,TaKaRa,Japan). Reverse transcription-quantitative polymerase chain reaction(RT-qPCR) was performed by TB Green Premix EX Taq (RR420,TaKaRa,Japan) and the Applied Biosystems 7500 system (ABI,USA) following the standard procedure for two-step PCR amplification provided in the kit instructions: The pre-denaturation stage was set at 95 ℃ for 30 sec; the subsequent stage involved 40 cycles of PCR reaction,with settings at 95 ℃ for 3 sec,60 ℃ for 30 sec,and finally,the melt curve stage. Glyceraldehyde 3-phosphate dehydrogenase(GAPDH) was used as a control to normalize the data to calculate gene relative expression. The primer(ShengGong,Shanghai,China) sequences included:DLL3,forward 5'-CGTCCGTAGATTGGAATCGCC-3',reverse 5'-TCCCGAGCGTAGATGGAAGG-3';GAPDH,forward 5'-GGAGCGAGATCCCTCCAAAAT-3',reverse 5'-TCCCGAGCGTAGATGGAAGG-3'.
3 CCK-8 assay
CCK-8 assay was performed at 24,48,72,and 96 h to measure cell proliferation. For this,cells were seeded in 96-well plates,and the original culture medium was aspirated after 6 h. Afterward,200 μl CCK-8 detection solution was introduced into each well according to the guidelines of CCK-8 Kit (GLPBIO,USA)and then incubated for 2 h. The microplate reader (Tecan) was used to measure the OD values at 450 nm.
4 Colony formation assay
Totally 1 000 cells per well were cultivated in 6-well plates,with three duplicate wells allocated for each experimental group. These cells were cultured in a 10% FBS medium for two weeks,and the medium was changed every three days. Next,the cells in every well were exposed to 4% paraformaldehyde for a duration of 15 minutes and then subjected to staining by crystal violet (Solarbio,G1046) for a period of 10 minutes. The number of colonies with over 50 cells was recorded.
5 Wound-healing assay
Wound-healing assays were conducted to evaluate the migration capability of cells. Prior to cell seeding,horizontal lines were pre-drawn on the bottom of a 6-well plate. Cells were allowed to grow in a complete medium until reaching 100% confluency,after which scratches were meticulously created using a 100 μL pipette. Images at both 0 h and 24 h time points were captured using an inverted microscope (Nikon). The qualitative assessment of cell migration was carried out using ImageJ software by measuring the cell-free area.
6 Transwell assay
The migration and invasion capacity of PCa cells were determined by transwell chambers (8 μm,Falcon) with and without Matrigel (356234,Corning). 5×104cells were resuspended in 200 μL serum-free 1640 medium and then introduced into the upper chamber.The lower chamber was filled with 500 μL medium containing 20% FBS,which served as a chemoattractant.Following 24 hours of culture,the non-migrating or non-invading cells were removed. The migrated and invasion cells attached to the underside were treated with 4% paraformaldehyde and then stained with crystal violet (Solarbio,G1046). Finally,the cells were captured and measured in five randomly chosen areas under a light microscope.
7 Tumor formation in nude mice
DLL3overexpression (OE) lentiviral particles and their control lentiviral counterparts (puromycin-resistant luciferase lentivirus,JiKai Gene Co.,Ltd.) were constructed and transfected into PC-3 cells. Stably transfected cells were selected using puromycin. Ten male nude mice (Guangdong Medical Laboratory Animal Center),approximately 5 weeks old and weighing around 16 g,were randomly divided into two groups:the empty vector group and the DLL3 overexpression group. Transfected cells in the logarithmic growth phase were suspended at a concentration of 4×106cells/mL. Following the experimental protocol,a needle was inserted into the upper 1/3 region of the groin of nude mice,and 0.5 mL of the cell suspension was injected to establish a tumor formation nude model. Tumor volume was measured every three days after tumor formation using the formulaV=L×W/2 (V,volume;L,length;W,width)[22]. The nude mice were anesthetized with 1.25% Tribromoethanol (0.2 mL/10 g,AIBI Bio-Technology Co.,Ltd.). XenoLight D-Luciferin Potassium Salt (Perkin Elmer) was injected through the tail vein,and mice were placed in the small animal live imaging system (Bruker In-Vivo MS FX Pro) to capture images for observing tumor size and growth. After the experiment,all nude mice were euthanized.
8 Western blotting
Proteins were extracted by RIPA Lysis buffer(Fdbio,China),which included protease and phosphatase inhibitors (Fdbio,China). The total protein concentration in each group was measured by the BCA protein analysis kit (Fdbio,China) and then standardized to an equal concentration. Loading buffer (Fdbio,FD002) was added to boil and denature. Afterwards,the protein samples were separated utilizing 10% SDSPAGE gels and subsequently transferred onto PVDF membranes (Merck Millipore,USA). Following one hour incubation at room temperature with 5% skim milk,the membranes were then incubated in primary antibody (1∶1 000,CST) at 4 ℃ overnight. After being washed three times with PBS-T,the membranes were treated with HRP-conjugated secondary antibodies(1∶10 000,CST) for 1 h at room temperature. The protein expression intensity was identified by the Enhanced Chemical Luminescence reagent (Fdbio,China),and images were captured by the Tanon 5200 chemical luminescence imaging system.
9 RNA sequencing (RNA-Seq)
PC-3 cells were seeded into 6-well plates and cultured for a duration of 24 h. Subsequently,DLL3 overexpression plasmids and empty control plasmids were transfected into cells based on the division of the group,with three duplicate wells allocated to each group. Following a continuous culture period of 48 h,the cells were collected into sterile enzyme-free EP tubes,appropriately labeled,and transported to Guangzhou IGE Biotechnology on dry ice for RNA-Seq. The raw data obtained from the sequencing process have been uploaded to the GEO database (GSE245415).
10 Statistical analysis
Statistical analyses were performed using Graph-Pad software. The experiments were conducted with three technical replicates,and the data were presented as means ± standard deviation (SD) unless otherwise stated. Two-group comparisons were assessed using Student'st-test,while multiple-group comparisons were conducted through one-way analysis of variance(ANOVA),followed by appropriate post-hoc tests. Statistical significance was established atP<0.05.
RESULTS
1 Overexpression of DLL3 promoted the proliferation,migration and invasion of PCa cells
TheDLL3overexpression plasmids (OE-DLL3) and empty vector control plasmids were transfected into PCa cell lines (PC-3,DU145 and C4-2). The successful construction of the plasmids was confirmed by increased DLL3 expression in OE-DLL3 group compared with blank control group (Figure 1A and B).Invitroexperiments revealed thatDLL3overexpression promoted cell proliferation,as observed in CCK-8 and colony formation assays(P<0.05 orP<0.01; Figure 1C,D and E). Moreover,DLL3overexpression enhanced cell migration,as demonstrated by wound-healing and Transwell migration assays(P<0.05 orP<0.01; Figure 1F and G). Transwell invasion assay further confirmed thatDLL3overexpression promoted the invasion of PCa cells (P<0.01; Figure 1H). Collectively,DLL3overexpression promoted the proliferation,migration and invasion of PCa cells.
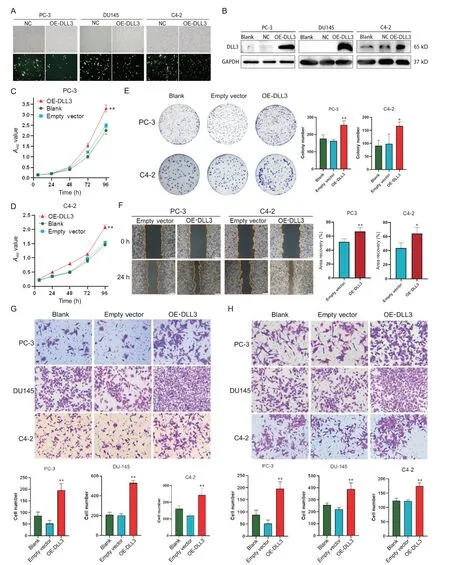
Figure 1. Overexpression of DLL3 (OE-DLL3) enhanced the proliferation,migration and invasion of prostate cancer cells.A and B: fluorescence microscopy and Western blotting were used to analyze the transfection efficiency; C and D: CCK-8 assay was used to assess the cell viability; E: colony formation assay was employed to examine the cell proliferation; F:the migration of the cells was evaluated by wound-healing assay (×40); G: the migration of the cells was observed by Transwell assay (×200); H: the invasion of the cells was detected by Transwell Matrigel assay (×200). Mean±SD. n=3. *P<0.05,**P<0.01 vs blank or empty vector group.
2 Overexpression of DLL3 promoted tumor formation of PCa cells in nude mice
StableDLL3-overexpressing PC-3 cells (PC-3-OE) were injected into the upper 1/3 groin of nude mice,resulting in a gradual increase in tumor volume over time. The tumor volume in PC-3-OE group significantly increased compared with control (PC-3-NC)group (P<0.05). See Figure 2. This demonstrated thatDLL3overexpression significantly enhanced the tumorigenicity of PC-3 cells.
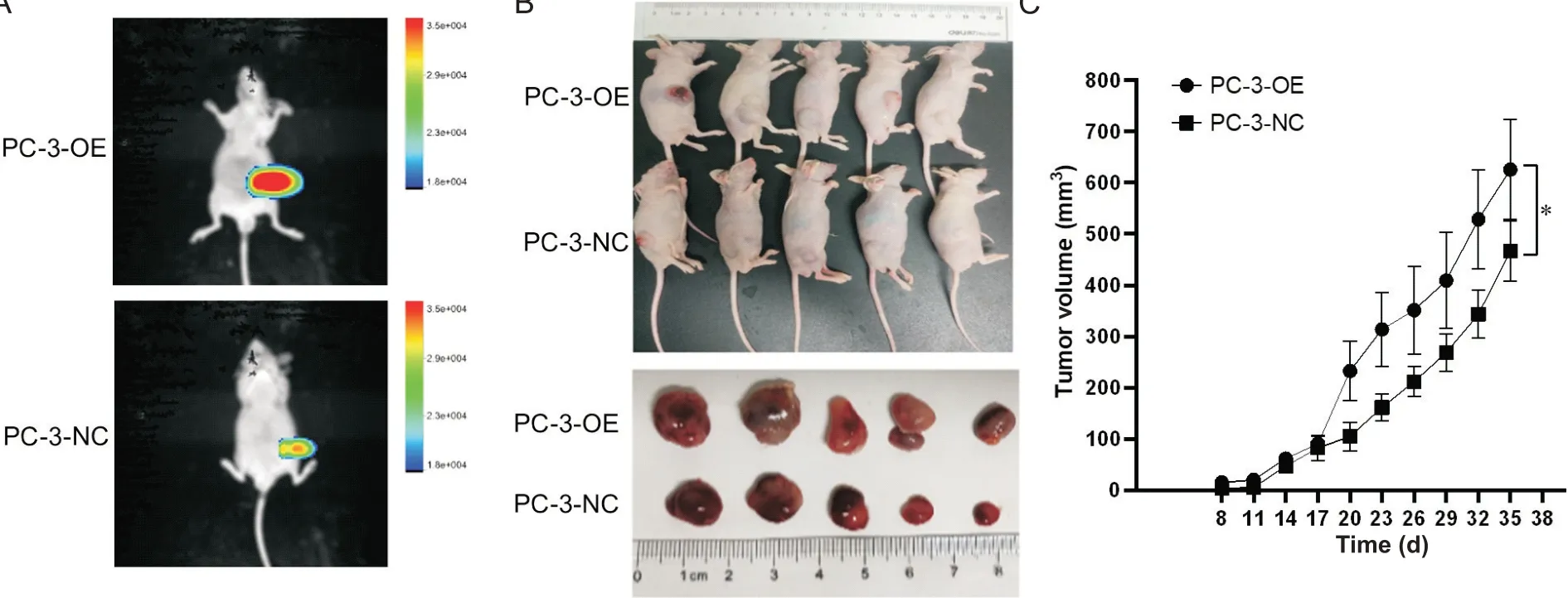
Figure 2. Overexpression of DLL3 in PC-3 cells (PC-3-OE) promoted the tumorigenicity of the cells in nude mice. A: representative images of GFP fluorescence in tumor-bearing nude mice; B: representative images of tumor-bearing nude mice in PC-3-negative control (NC) and PC-3-OE groups; C: the growth curves of the tumor volume in PC-3-NC and PC-3-OE groups. Mean±SD. n=5. *P<0.05.
3 Silencing of DLL3 suppressed the proliferation migration and invasion of PCa cells
Interference plasmids(sh-DLL3) were transfected into PCa cell lines(PC-3,DU145 and C4-2),resulting in a notable reduction of DLL3 expression at mRNA and protein levels(Figure 3A and B). The most effective interference plasmids,sh-DLL3#1 and sh-DLL3#2,were selected for subsequent experiments. Knockdown ofDLL3inhibited PCa cell proliferation,as evidenced by CCK-8 and colony formation assays (P<0.05 orP<0.01; Figure 3C,D and E). Transwell migration and wound-healing assays demonstrated reduced migratory capacity inDLL3-silencing PCa cells(P<0.01; Figure 3F and G). Additionally,Transwell invasion assays revealed suppressed invasive capacity of PCa cells afterDLL3silencing (P<0.01; Figure 3H).

Figure 3. Knockdown of DLL3 (sh-DLL3) suppressed the proliferation,migration and invasion of prostate cancer cells. A and B: RT-qPCR and Western blotting were used to analyze the transfection efficiency; C and D: the cell viability was evaluated by CCK-8 assay; E: the cell proliferation was evaluated by colony formation assay; F: the migration of the cells was assessed by wound-healing assay (×40); G: the migration of the cells was observed by Transwell assay (×200); H: the invasion of the cells was observed by Transwell Matrigel assay (×200). Mean±SD. n=3. *P<0.05,**P<0.01 vs sh-NC group.
4 DLL3 regulated the EMT of PCa cells
Overexpression ofDLL3decreased the epithelial marker E-cadherin but up-regulated the mesenchymal markers N-cadherin,vimentin,β-catenin and Slug (P<0.05 orP<0.01; Figure 4A). Conversely,DLL3silencing exhibited the opposite effect on the EMT phenotype (P<0.05 orP<0.01; Figure 4B),indicating that DLL3 promotes the EMT process and the progression of PCa.
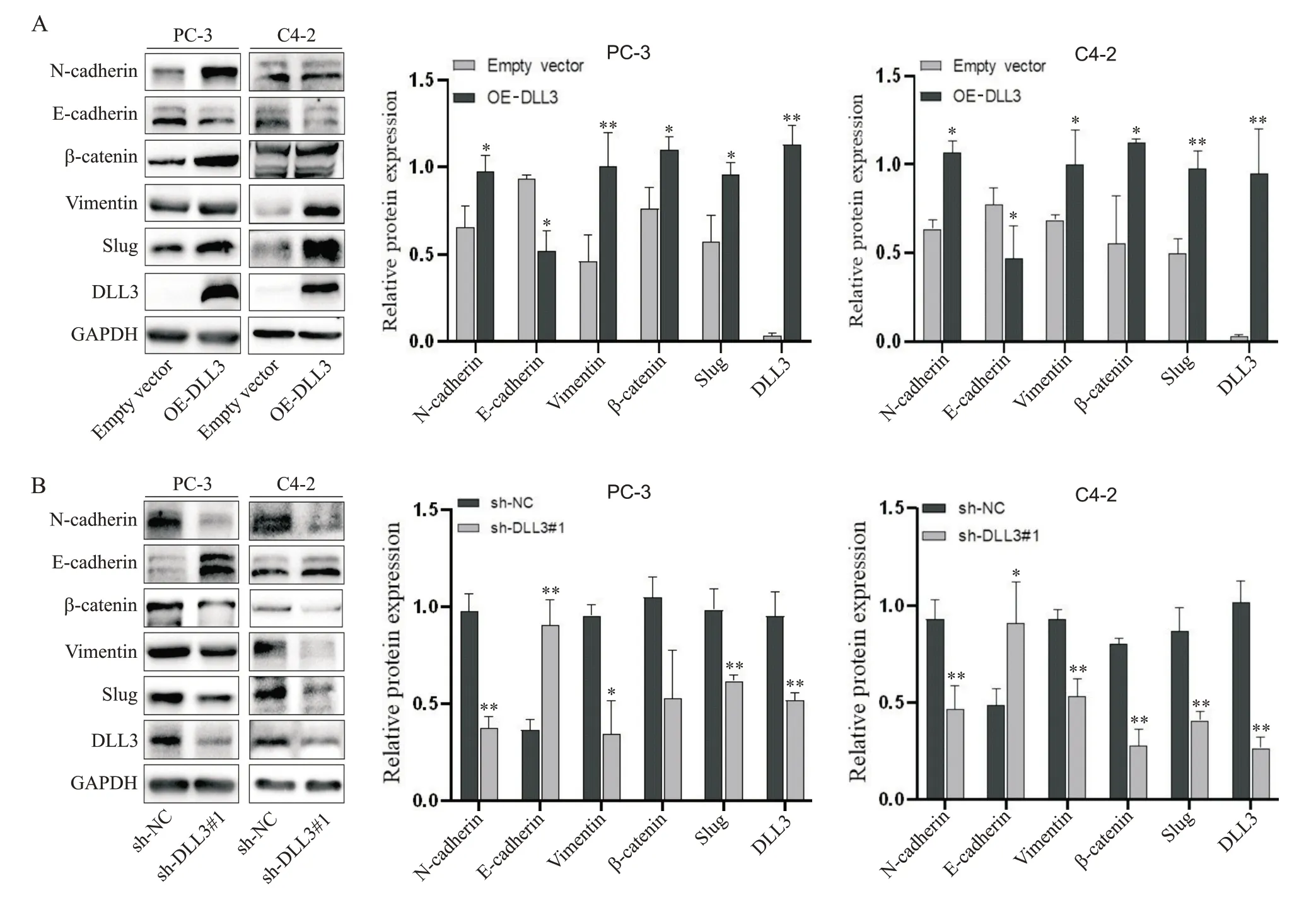
Figure 4. The protein expression of epithelial-mesenchymal transition markers in prostate cancer cells with overexpression(A) or knockdown(B) of DLL3 was detected by Western blotting. Mean±SD. n=3. *P<0.05,**P<0.01 vs empty vector or sh-NC group.
5 MAPK signaling pathway was involved in the,pr ogression of PCa mediated by DLL3
RNA-Seq results indicated differentially expressed genes in PC-3 cells betweenDLL3overexpression (PC-3-OE) and negative control (PC-3-NC) groups (Figure 5A). Gene Set Enrichment Analysis(GSEA) revealed thatDLL3triggered MAPK signaling pathway (Figure 5B). Western blotting demonstrated thatDLL3overexpression enhanced the phosphorylation levels of ERK1/2,MEK1/2,and P38 (P<0.05 orP<0.01; Figure 5C). Conversely,DLL3interference resulted in decreased phosphorylation of ERK1/2 and P38 in PCa cells (P<0.05 orP<0.01; Figure 5D). These findings suggested that DLL3 promotes PCa progression through the MAPK pathway.
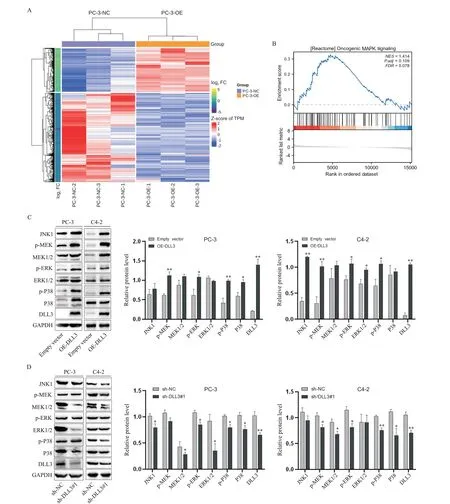
Figure 5. Effect of DLL3 on MAPK pathway. A: heatmap of the differentially expressed genes; B: the GSEA analysis demonstrated a positive correlation between DLL3 expression and MAPK signaling pathway; C and D: the protein levels of MAPK signaling pathway-related molecules in prostate cancer cells with overexpression (C) or knockdown (D) of DLL3 were detected by Western blotting. Mean±SD. n=3. *P<0.05,**P<0.01 vs empty vector or sh-NC group.
6 DLL3 promoted the progression of PCa through the MAPK pathway
PD0325901,an MEK/MAPK pathway inhibitor[23-24],was administered to PC-3 and DU145 cells,resulting in decreased p-ERK level (Figure 6A). Overexpression ofDLL3significantly promoted the viabilty of PCa cells,while PD0325901 partially mitigated this effect(P<0.01; Figure 6B and C). Additionally,PD0325901 effectively counteredDLL3-induced effects on the migration and invasion of PCa cells,as demonstrated by Transwell assays (P<0.01; Figure 6D). These findings indicated thatDLL3promotes the proliferation,invasion and migration of PCa cells through the MAPK pathway.
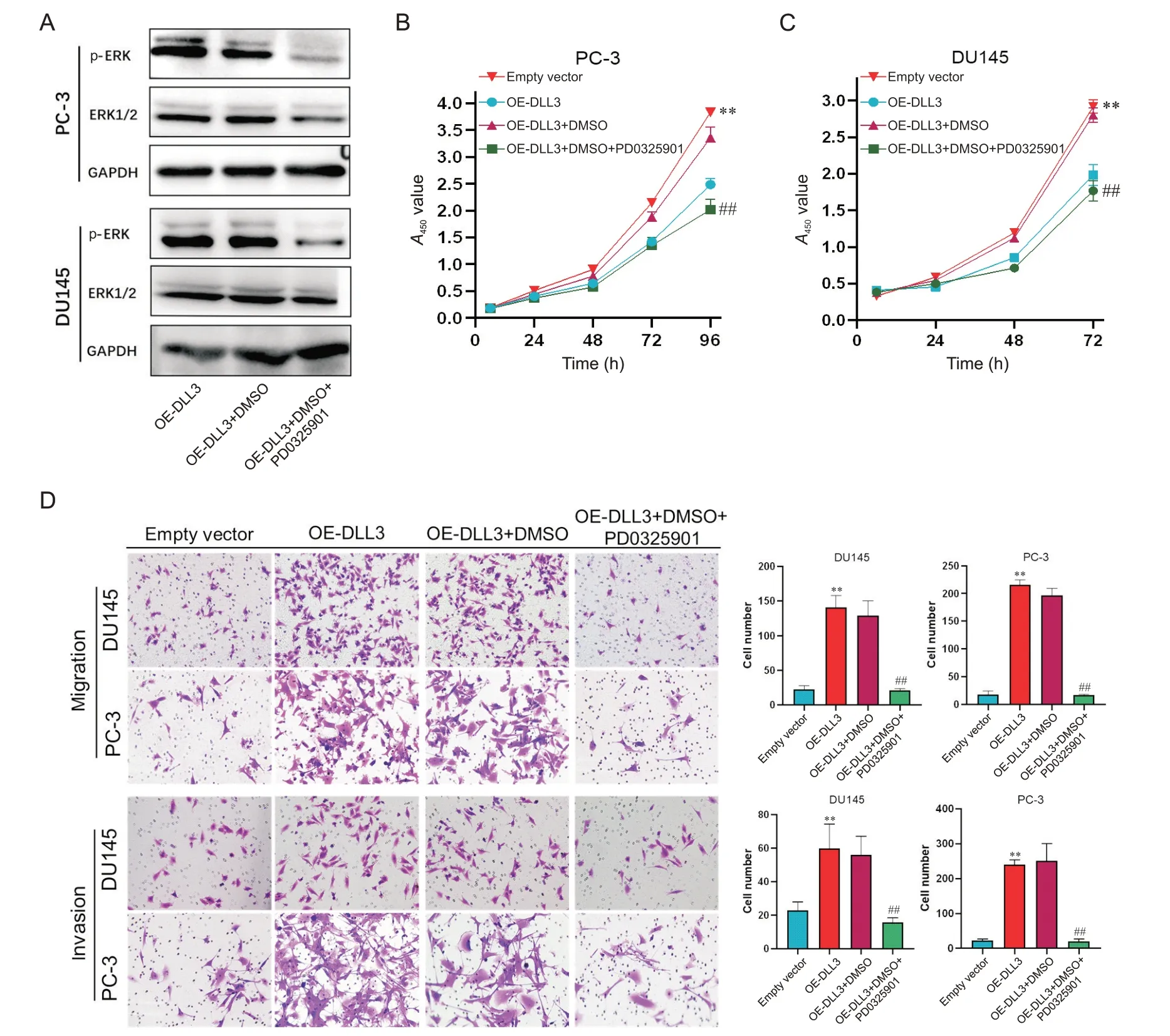
Figure 6. Overexpression of DLL3 (OE-DLL3) promoted the progression of prostate cancer cells through MAPK signaling pathway. A: Western blot was used to evaluate the protein levels of ERK and p-ERK in PC-3 and DU145 cells; B and C:the cell viability was evaluated by CCK-8 assay; D: the invasion and migration of the cells were assessed by Transwell assays (×200). Mean±SD. n=3. **P<0.01 vs empty vector group; ##P<0.01 vs OE-DLL3+DMSO group.
DISCUSSION
The rising prevalence of prostate cancer over recent decades has become a significant public health concern. Androgens,crucial in the progression of certain PCa types[25-26],have led to the development of androgen receptor pathway inhibitors,such as abiraterone acetate and enzalutamide,showing efficacy in the treatment of PCa[27-28]. While these therapies exhibit effectiveness in the initial stages,the majority of patients inevitably progress to castrate-resistant prostate cancer within 1 to 2 years,ultimately resulting in mortality for individuals with advanced PCa[29]. DLL3 is typically expressed exclusively in the Golgi apparatus of specific cells under physiological conditions[15]. Numerous studies have confirmed its significant expression in smallcell lung cancer and other neuroendocrine tumors,suggesting its involvement in complex tumor regulatory mechanisms[30]. However,the role of DLL3 in prostate cancer and its underlying mechanisms warrant clarification.
In this study,we manipulated the expression of DLL3 in PCa cell lines (PC-3,DU145 and C4-2) by both increasing and decreasing its levels. Through a comprehensive analysis using CCK-8,colony formation,wound healing,transwell assays,and Western blotting,we observed thatDLL3enhanced the proliferation,migration,invasion,and EMT of PCa cells. Notably,DLL3promoted the proliferation of PC-3 cells in a nude mouse model. Subsequent RNA-Seq analysis unveiled that the overexpression ofDLL3activated the MAPK pathway,a finding corroborated by Western blotting and subsequent reversal experiments.
The EMT process has emerged as a pivotal player in PCa progression[31]. A characteristic molecular alteration in EMT is the cadherin switch,involving the gradual downregulation of the epithelial marker E-cadherin and the gradual upregulation of the mesenchymal marker N-cadherin[32]. This downregulation of E-cadherin results in reduced cell adhesion,while cells undergoing EMT exhibit a mesenchymal phenotype,as evidenced by elevated expression of N-cadherin and vimentin[16].Besides,it has been reported that the Wnt signaling pathway mediator,β-Catenin,plays a crucial role in tumorigenesis[33]. Moreover,β -Catenin can activate slug,a transcription factor belonging to the snail family,which is instrumental in EMT. Slug inhibits the transcription of E-cadherin by binding to its promoter region,leading to a decrease in cell adhesion[34]. Additionally,various molecular mechanisms,including the involvement of growth factors,cytokines,and the regulation of signaling pathways,can directly or indirectly modulate EMT.
The MAPK signaling pathway plays a pivotal role in cellular systems,orchestrating various physiological and pathological processes. Notably,this pathway comprises multiple branches,including the P38/MAPK pathway,JNK/SAPK pathway,ERK1/2 pathway,and ERK5 pathway. The intricate cross-talk between these pathways can exert synergistic or inhibitory influences[35]. The ERK1/2-related intracellular signaling pathway is recognized as the classical MAPK signaling pathway[36]. Current evidence suggests that ERK1/2 assumes a central role in a series of kinase enzymatic cascades that facilitate the transmission of extracellular stimuli from the cellular membrane to the nucleus[21,37].The ERK1/2 signaling pathway is crucial within the MAPK signal transduction network and is closely associated with regulating cell proliferation,differentiation,apoptosis,migration,and other cellular processes[38].
In conclusion,DLL3expression is implicated in the progression of PCa,and this association is linked to the activation of the MAPK pathway byDLL3. Additionally,there are unexplored aspects in the RNA-Seq data. Preliminary experiments suggested potential associations ofDLL3with the PI3K-Akt signaling pathway,estrogen pathway,and heat shock proteins. However,due to space and experimental constraints,these associations have not been thoroughly investigated in this study. The regulation ofDLL3in PCa is highly complex,and to fully comprehend howDLL3promotes PCa progression,further extensive research is warranted.
