艰难梭菌感染的临床治疗策略及新药物研究进展
2023-12-26郭银莉洪守强陈渺渺苏少硼崔润博赵西林牛建军
郭银莉 洪守强 陈渺渺 苏少硼 崔润博 赵西林 牛建军

摘要:艱难梭菌(Clostridium difficile)是一种革兰阳性厌氧芽孢杆菌,因导致医院内腹泻备受关注。由于C. difficile的分离培养需要在厌氧状态下实现,这给国内外研究者带来非常大的限制和挑战。目前临床治疗手段主要采取抗生素和粪便微生物群移植(fecal microbiota transplantation, FMT)。据统计艰难梭菌感染(C. difficile infection, CDI)经万古霉素治疗后复发率高达30%,死亡率达15%,是临床上亟待解决的难题。目前,许多国家在大力开发有效的新型药物来治疗CDI。本文根据目前的临床治疗现状和正在开发中的新药物进行了简要总结,更新了目前5种已有临床实验的新型抗菌药物和11种新开发的不同抗菌剂,前者包括ridinilazole、surotomycin、cadazolid、CRS3123和DS-2969b;后者包括黄芩苷、rhodomyrtone、OPS-2071、rakicidin B、依拉环素、双环胍、CM-A、月桂酸、NCK-10、Raja 42和AJ-024。针对现有临床前和临床抗菌数据,将这些潜在药物与传统抗生素进行比较,分析其所具有的药代动力学和药效特征优势,以期为CDI的新药研发提供新思路。
关键词:艰难梭菌;艰难梭菌感染;临床治疗;新药
中图分类号:R978.1文献标志码:A
Research progress of clinical treatment strategies and new drugs for
Clostridium difficile infection
Guo Yinli1,2, Hong Shouqiang1, Chen Miaomiao1, Su Shaopeng1, Cui Runbo1, Zhao Xilin1, and Niu Jianjun1,2
(1 State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen 361102; 2 Zhongshan Hospital Affiliated to Xiamen University, Xiamen 361004)
Abstract Clostridium difficile, a Gram-positive anaerobic bacillus, has attracted much attention for causing nosocomial diarrhea. The isolation and cultivation of C. difficile need to be achieved under anaerobic conditions, which brings great limitations and challenges to researchers. At present, the main clinical treatment methods are antibiotics and fecal microbiota transplantation (FMT). According to the survey analysis, the recurrence rate of
C. difficile infection (CDI) after vancomycin treatment is as high as 30%, and the mortality rate is 15%, which is an urgent clinical problem needs to be solved. At present, many countries are vigorously developing effective new drugs to treat CDI. This article briefly summarizes the current status of clinical treatment and new drugs in development, and updates the current five new antibacterial agents that have been tested in clinical trials and 11 different newly developed antimicrobials. The former category includes ridinilazole, surotomycin, cadazolid, CRS3123, and DS-2969b. The latter includes baicalin, rhodomyrtone, OPS-2071, rakicidin B, eravacycline, bicycloguanidine, CM-A, lauric acid, NCK-10, Raja 42, and AJ-024. Based on the existing pre-clinical and clinical antibacterial data, these potential drugs were compared with traditional antibiotics for their pharmacokinetic and pharmacodynamic characteristics in order to provide new ideas for the development of new drugs for CDI.
Key words Clostridium difficile; Clostridium difficile infection; Clinical treatment; New drugs
多年来,抗菌药物在治疗细菌性感染疾病方面做出了巨大贡献,但其高频率使用为C. difficile感染、繁殖和传播创造了有利条件。经研究发现,在抗菌药物高使用率的急诊医院中C. difficile发病率与抗菌药物使用量之间呈正相关关系[1]。C. difficile是一种厌氧、产芽孢的革兰阳性杆菌,于1935年由美国学者在健康新生儿粪便中首次分离发现,被认为是新生儿肠道菌群的一部分,具有生长缓慢、分离困难和条件致病的特性[2]。C. difficile主要通过粪口传播,因其产生的孢子可在有氧的环境中休眠数月,条件合适时即萌发为营养体从而导致CDI感染或复发。经统计,C. difficile的易感人群为65岁以上且存在抗菌药物暴露的住院或门诊医疗患者,主要危险因素是年长体弱者的肠道稳态容易被抗菌药物破坏和免疫力降低。其中,女性的发病率高于男性[3],随着高毒菌株PCR-核糖体分型027 R20291(NAP1/027)在北美和欧洲出现并快速传播[4],C. difficile的感染人群逐渐扩大到近期未接触抗菌药物的儿童或成年人,目前该毒株已被证实与CDI复发、治愈率降低和死亡率上升有关[5]。临床上,约有25%~33%的抗生素相关性腹泻(antibiotic associated diarrhea, AAD)和90%的伪膜性结肠炎是由CDI引起[6],疾病可由轻度腹泻发展为中毒性巨结肠,严重时造成死亡。
CDI的治愈有两大难点:第一,有效治疗药物有限,在细菌耐药问题非常严峻的背景下,一旦发生耐药菌的传播不仅会提高治疗难度,还会带来用药成本增加、延长住院时间和影响患者愈后等一系列问题;第二,约30%的复发率给医疗系统造成很大的经济负担。因此,研究人员需要不断尝试研发新的抗菌药物和治疗方法来缓解C. difficile难治愈的问题。除发现新的抗菌药物之外,一些替代疗法也在不断开发,如噬菌体介导的CRISPR-Cas系统靶向细菌基因组疗法[7]、口服微生物组治疗剂SER-109[8]和单克隆抗体bezlotoxumab[9]等。虽然这些手段在一定程度上具有潜在预防和治疗CDI的能力,但是新疗法的作用范围、是否安全有效和不良反应程度还需要进一步证实。近年来,抗生素治疗失败和高复发率使得抗CDI的潜在药物备受关注,已进入临床评估阶段的新型抗菌药物和正处于研发中的潜在药物都将为辅助临床用药、治疗CDI和复发防治提供新的思路和策略。
1 C. difficile的致病与治疗现状
1.1 致病及复发机制
C. difficile属于人类正常肠道菌群的一部分,约5%的健康成年人无症状携带C. difficile孢子。在没有抗菌药物暴露和免疫力正常的情况下,健康者的肠道菌群可以抵抗C. difficile孢子的萌发与定殖。一旦患者接受抗菌药物治疗引起正常肠道菌群紊乱,将会降低肠道微生物的多样性和产生抑制性代谢物,导致肠道对C. difficile孢子萌发的抵抗力降低,給孢子萌发与C. difficile菌株定殖提供有利条件。C. difficile有很多种致病因子:产生毒素、黏膜粘附、生成荚膜和分泌组织降解酶等,其中与致病相关且最重要的是C. difficile毒素[10]。C. difficile产生3种蛋白毒素,分别是C. difficile毒素A (C. difficile toxin A, TcdA)、C. difficile毒素B (C. difficile toxin B, TcdB)和C.difficile转移酶毒素(Clostridium difficile transferase toxin, CDT)。其中外毒素TcdA和TcdB通过灭活Rho家族的Rho GTP酶引起结肠细胞死亡、肠屏障功能缺失以及嗜中性细胞浸润进而导致结肠炎,是介导C. difficile感染性腹泻发展过程中最重要的两大毒素。CDT是一种二元肌动蛋白-ADP-核糖基化毒素,可导致肌动蛋白解聚,从而诱导形成基于微管的突起[11-12]。除此之外,C. difficile能够产生孢子抵抗极端环境从而造成疾病复发和二次传播。孢子在人体分泌的胆汁酸中的牛磺胆酸盐(taurocholate, TCA)促进下启动萌发为营养体菌株,同时产生TcdA和TcdB[11]并且可以在24 h内诱导胆汁酸快速流入肠道,以便继续利于营养体的生长和繁殖[13]。当C. difficile在结肠定植后,其代谢物和毒素不断破坏肠上皮细胞、损伤细胞骨架、释放黏液和炎症产物,随后产生炎症反应伴随腹泻、腹痛症状,严重时发展为中毒性巨结肠、肠穿孔、休克甚至危及患者生命[11]。在感染过程中,致病菌株的毒素和表达程度与CDI的疾病严重程度相关,研究发现C. difficile可以利用毒素介导的炎症反应来抑制肠道菌群中抵抗C. difficile定植的微生物生存以获得利己生存的条件[14],这些特点对CDI的治疗造成了挑战。
对初次CDI的患者进行抗生素治疗后常常出现复发型CDI (recurrent C. difficile infection, rCDI),复发率高达30%[15],并且复发风险会随着药物治疗的频率增加而提高[16]。引起rCDI的因素有很多种,主要包括致病菌株的产毒能力、耐药菌株出现、感染过程中肠道菌群的持续改变和未能对C. difficile毒素产生有效的抗体反应。耐药菌株的出现通常是复发的主要原因,除此之外有研究表明抗菌剂的使用可以诱导生物膜的产生导致C. difficile躲避抗菌药物的杀伤[17],这提示我们C. difficile形成生物膜并留存在宿主细胞中可能是导致治疗失败与复发的重要机制。
1.2 临床治疗现状
甲硝唑(metronidazole, Mtr)和万古霉素(vancomycin, Van)是几十年来治疗CDI的一线药物,由于耐Mtr菌株的出现导致治疗失败,并且Mtr对肠道菌群的广谱杀灭也不利于CDI治疗,Mtr现已逐渐退出C. difficile抗生素药物的第一选择行列。Mtr是一种硝基咪唑类药物,其作用方式是通过被动扩散作为前药进入细菌,随后在胞质内被还原成为亚硝基自由基与DNA分子非特异性结合(包括氧化抑制DNA合成和DNA损伤,导致单链和双链断裂),破坏DNA双链使细菌死亡但不裂解。对于需氧细菌来说,由于自身缺乏负氧化还原电位的电子转运蛋白,Mtr的活性远不如对厌氧细菌有效。Mtr的耐药机制比较复杂且因菌类不同而异,厌氧菌对Mtr产生耐药主要是硝基还原酶活性降低导致前药还原活化速率变低以及外排泵系统减少胞内对药物的摄取使得菌体内药物浓度降低两种机制共同作用,其他机制包括药物失活和DNA损伤修复增加等[18]。Van是一种通过抑制细菌细胞壁合成发挥作用的糖肽类抗菌药物,是临床治疗革兰阳性菌引起重度感染的重要药物。该药主要通过与未交联脂质II(十一碳烯基-二磷酸-N-乙酰胞壁酰[N-乙酰葡糖胺]-L-丙氨酰-γ-D-谷氨酰-L-赖氨酰-D-丙氨酰-D-丙氨酸)的末端D-丙氨酰-D-丙氨酸(D-alanyl-D-alanine, D-Ala-D-Ala)结合靶向革兰阳性菌细胞壁合成,阻碍青霉素结合蛋白(penicillin binding protein, PBP)将脂质II交联到肽聚糖上,从而抑制细胞壁合成导致在不利的渗透压环境下细胞破裂而死亡[19]。经过研究发现,C. difficile中Van耐药性的相关机制主要包括由van基因介导的靶点改变、外排泵、RNA聚合酶突变和生物膜形成[20]。在2021年,研究人员分离到对Van敏感性降低的C. difficile临床菌株[21],这使得CDI治疗面临新的挑战。
在近几年的研究中新发现了非达霉素(fidaxomicin, Fid),是一种窄谱大环内酯类抗生素,比Mtr和Van抗菌效果更特异、持久,不仅能够提高整体治愈率(Fid 73%, Van 62.9%),还能降低复发风险(Fid 3.3%, Van 4%)[22]。研究表明Fid对革兰阴性菌没有影响,主要作用机制是通过抑制RNA聚合酶阻止转录[23]。Fid于2011年首次获得美国食品药品监督管理局(Food and Drug Administration, FDA)批准用于治疗成人CDI相关性腹泻,但由于该药成本昂贵暂未被我国引入临床使用。目前有实验室通过诱导耐药研究发现RNA聚合酶β-亚基rpoB的单核苷酸变异(single-nucleotide polymorphisms, SNP)将导致该药的敏感性降低[24]。Ⅲ期临床试验表明,该药口服给药的疗效不劣于Van。2021年,欧洲和美国传染病学会发布了有关C. difficile结肠炎治疗的新指南,建议将Fid作为一线治疗,Van作为第二选择,Mtr仅在没有其他治疗方法的情况下才被推荐使用,因其在轻型CDI且复发概率低的患者中仍然具有很好的疗效[25]。
以上3种抗生素都具有较强的杀菌效果,是控制C. difficile感染和传播公认的有效方法,但是在高频率使用下可能会诱导C. difficile生物膜形成、降低具有定殖抗性的微生物水平从而加重感染甚至引起复发。
近些年各国研究者都非常关注粪菌移植(fecal microbiota transplantation, FMT)疗法,该疗法通过将健康供体粪便的微生物组转移到患者体内,理论上可以达到恢复肠道菌群的平衡来抵抗C. difficile的生长繁殖[26]。有研究证实,FMT诱导肠道菌群重建和代谢物恢复能促进小鼠定植抵抗的恢复,从而为预防CDI复发提供新的方法[27]。最近發表的一项II期研究表明,使用一次性活体微生物治疗药物,可治愈近80%的rCDI患者[28]。FMT的发现给临床治疗提供了一种新思路,但其实际治疗的副作用及对患者未来生存质量的影响处于未知阶段,并且存在传播新病原体的潜在风险。
2 新型CDI治疗药物研究进展
2.1 进入临床评估阶段的CDI治疗药物
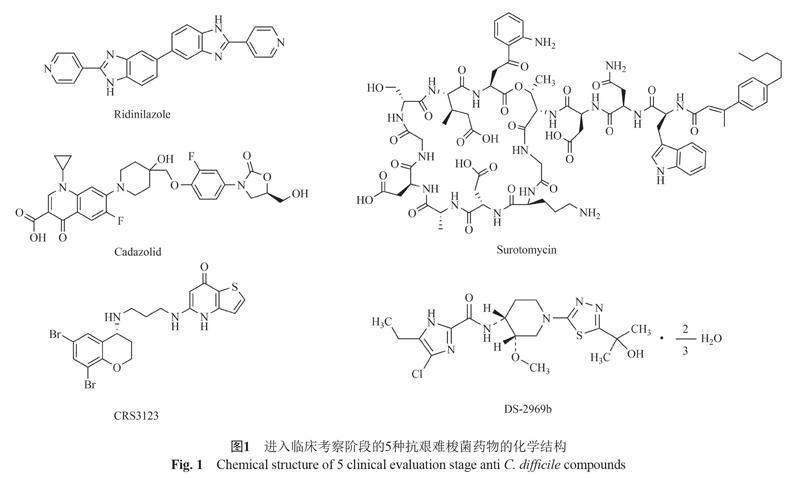
在过去的几十年里,真正应用到临床治疗CDI的新型抗菌药物很少,这不仅与C. difficile自身的抵抗特性及产孢子易复发有关,还与实现对该菌的稳定研究比较困难有关。相对于大肠埃希菌来说,C. difficile的研究进程是困难缓慢的。下面对近些年来进入临床评估阶段的5种抗C. difficile药物的最新结果进行总结。药物的结构与临床评估结果分别见图1及表1。
2.1.1 Ridinilazole
Ridinilazole (Rdz, 原称为SMT19969),是一种窄谱、不可吸收的新型抗生素,对C. difficile具有高选择抗菌活性和低耐药率[47]。由于其表现出降低CDI复发的优势,因此在各类新型药物中脱颖而出。目前Rdz的抗菌机制尚不清楚,但是作为双苯并咪唑(bis-benzimidazoles, BBZ)的衍生物,其作用机制可能与BBZ类似,而研究表明BBZ抗肿瘤和抗菌活性的作用机制是抑制DNA合成[48-49]。研究发现暴露于Rdz后细菌细胞分裂停止,这表明其可能通过改变C. difficile细胞分裂增殖影响细菌生长。该药物对英国和亚洲北部的一系列临床分离株、超百种有耐药表型的分离株均有抑制活性[50-52]。在仓鼠肠道模型中,对其他正常肠道菌群(不包括梭菌属)没有抗菌活性[53]。Rdz表现出的窄谱活性对肠道中维持C. difficile定植抗性的微生物影响较小,尤其是对脆弱拟杆菌[54]。动物实验表明,给药期间Rdz与Van均有100%存活率,停止给药后Van处理组第8天死亡率高达100%,Rdz处理组在第13天开始出现死亡,并且维持80%的生存率直到研究结束[55]。这表明,Rdz不仅可以应用在急性和重症CDI的治疗,同时能够预防复发。
目前Rdz的最新临床阶段是Ⅱ期研究,Ⅲ期临床试验正在进行中。Ⅰ期结果显示,健康志愿者单次和多次口服后对Rdz具有良好的耐受性。给药后全身暴露量低,且出现的所有不良反应都是轻度的[29]。Ⅱ期实验发现该药物导致的不良反应症状及发生率与Van几乎一致,进一步证实了其安全性和有效性[30]。目前口服Rdz疗法已有的Ⅰ期、Ⅱ期临床评估结果良好,也让我们期待更大规模的Ⅲ期临床试验结果来支持Rdz应用于临床。
2.1.2 Surotomycin
Surotomycin (Sur, 原称为CB-183, 315)是一种口服给药、吸收少、具选择性的新型环状脂肽类膜活性抗生素,可以引起膜去极化而不增加膜的通透性从而导致细胞死亡[56]。Sur对革兰阴性厌氧菌和兼性厌氧菌没有活性[57]。与Van相比,Sur对指数生长期的细菌杀伤速率远高于Van,高浓度时能够快速杀死平台期细菌,这表明Sur对生长和非生长状态的C. difficile均表现出杀菌活性[58-59]。由于平台期细菌可以形成孢子并产生毒素,也就是说,Sur通过直接杀伤作用能够降低结肠和粪便中的孢子滴度和毒素水平[60]。在体外肠道模型中,滴注Sur后对脆弱拟杆菌没有影响[61]。另外,采用Sur治疗CDI可以降低万古霉素耐药肠球菌(vancomycin-resistant Enterococcus, VRE)水平[62]。
在临床高剂量Ⅰ期研究中,Sur对革兰阴性厌氧菌没有影响[33]。所有不良反应为轻度至中度,证明了其安全性与耐受性。在多中心的Ⅱ期临床试验中,Sur受试组与Van对照组治愈率接近,Sur的复发率较低且4周内的持续临床缓解率更高[34]。另一项Ⅱ期临床试验研究中也证实了Sur对微生物群的影响更小[35]。在2项更大规模、随访30~50 d内的国际多中心Ⅲ期临床实验中,Sur的持续临床反应率(治愈且随访未复发)低于Van[36-37]。这表明,Sur的持续治疗效果劣于Van。因此,Sur的Ⅲ期临床实验没有达到预期目标疗效。
2.1.3 Cadazolid
Cadazolid (Cdz, 原称为ACT-179811)是一种新型恶唑烷酮抗生素,对多种C. difficile临床菌株抑制细菌蛋白和毒素合成的能力优于Van和Mtr,24 h杀菌率高达99.9%[63-64]。通过大分子标记实验证实了Cdz的杀菌机制主要是抑制蛋白质合成(仅在药物浓度高时抑制DNA合成)[65]。在肠道模型中,Cdz与Van的治疗效果相当[66]。在小鼠CDI模型中,Cdz不会促进VRE的定植[67]。作为医院中重要的医疗保健目标,Cdz在降低VRE定植方面具有突出优势。
在2项Ⅰ期临床研究中,单次和多次递增口服剂量后发现健康受试者对Cdz的耐受性良好,其常见不良反应是头痛[38]。重症CDI患者口服给药后粪便中的药物浓度维持高水平[39]。一项在4个国家(加拿大、德国、英国和美国)进行的多中心Ⅱ期临床实验确认了Cdz对不同菌株感染的CDI患者具有良好疗效,患者口服最佳剂量为250 mg[40-41]。因此,在25个国家同时进行的2项Ⅲ期临床试验中,重点评估了Cdz每日2次250 mg剂量治疗的有效性。结果显示,项目一中Cdz的治愈率与Van接近,但项目二中Cdz的治愈率低于Van[42]。因此,与Van相比,不能认为Cdz具有优越的临床治愈能力。
2.1.4 MetRS抑制剂:CRS3123
CRS3123 (原称REP3123)是一种新型二芳基二胺,其抗菌机制是特异性抑制甲硫酰-tRNA合成酶(methionyl-tRNA synthetase, MetRS)。通过降低带电与不带电tRNA的比例来模拟氨基酸饥饿,并诱导严谨反应,進而导致RNA合成减少。该药主要对革兰阳性细菌的MetRS具有抑制作用,对大多数革兰阴性细菌和哺乳动物(包括人类)的MetRS没有影响[68]。在低至1 ?g/mL的浓度下,CRS3123能够抑制C. difficile外毒素产生,并减少10倍以上的孢子形成率[69]。在仓鼠CDI模型中,给药后33 d内的总存活率CRS3123高于Van和Mtr[68-69]。因此,该药物具有抑制C. difficile孢子形成及萌发的能力,并且在降低复发方面有一定潜力。
目前CRS3123正处于临床Ⅰ期研究阶段,单次给药剂量递增至1200 mg时不良反应为轻度至中度[43]。因此,CRS3123在较宽的剂量范围内具有良好的安全性和耐受性。在另一项Ⅰ期临床研究中,每日2次持续给药10 d没有报告严重不良事件(serious adverse events, SAEs)或严重治疗紧急不良事件(serious treatment-emergent adverse events, STEAEs),进一步证实了CRS3123的安全性和耐受性。更重要的是,CRS3123对共生厌氧菌没有活性(包括拟杆菌、双歧杆菌和共生梭菌),这些结果支持CRS3123作为窄谱药物进行下一步的临床研究[44]。由于CDI患者的肠上皮受损可能会影响药物的吸收程度,因此在未来的Ⅱ期试验中需要重点评估该药的全身暴露水平和持续临床治愈率。
2.1.5 GyrB抑制剂:DS-2969b
DS-2969b是一种新型GyrB抑制剂,其作用方式是与DNA旋转酶的ATP结合位点结合从而抑制DNA旋转酶的活性。DS-2696b对革兰阳性菌具有活性,特别是C. difficile和金黄色葡萄球菌(MRSA)[70]。在CDI模型中,皮下和口服给药同样有效,静脉和口服给药后粪便中排泄水平接近[71]。对大鼠连续灌胃7 d后,部分肠道微生物(主要包括球状梭菌和乳酸杆菌)的数量受到显著抑制,但是主要肠道菌群能够快速恢复到用药前水平,因此该药对肠道微生物的影响是短暂且可逆的[72]。这些数据支持DS-2969b进一步开发成为CDI口服或静脉治疗药物。
DS-2969b已进行Ⅰ期临床研究,结果支持下一步临床试验。在Ⅰ期评估中,不良反应主要与胃肠道有关且症状为轻度,因此该药安全和人体耐受性良好。首次给药后粪便中DS-2969a水平能够保持17 d以上[45]。在另一项Ⅰ期临床实验中证实了人体对DS-2969a耐受性良好,在剂量≥60 mg给药24 h内达到杀灭C. difficile的目标水平[46]。这些数据支持DS-2969b作为新药物继续开发,接下来需要充分了解该药对肠道微生物群的影响及临床治疗效果。
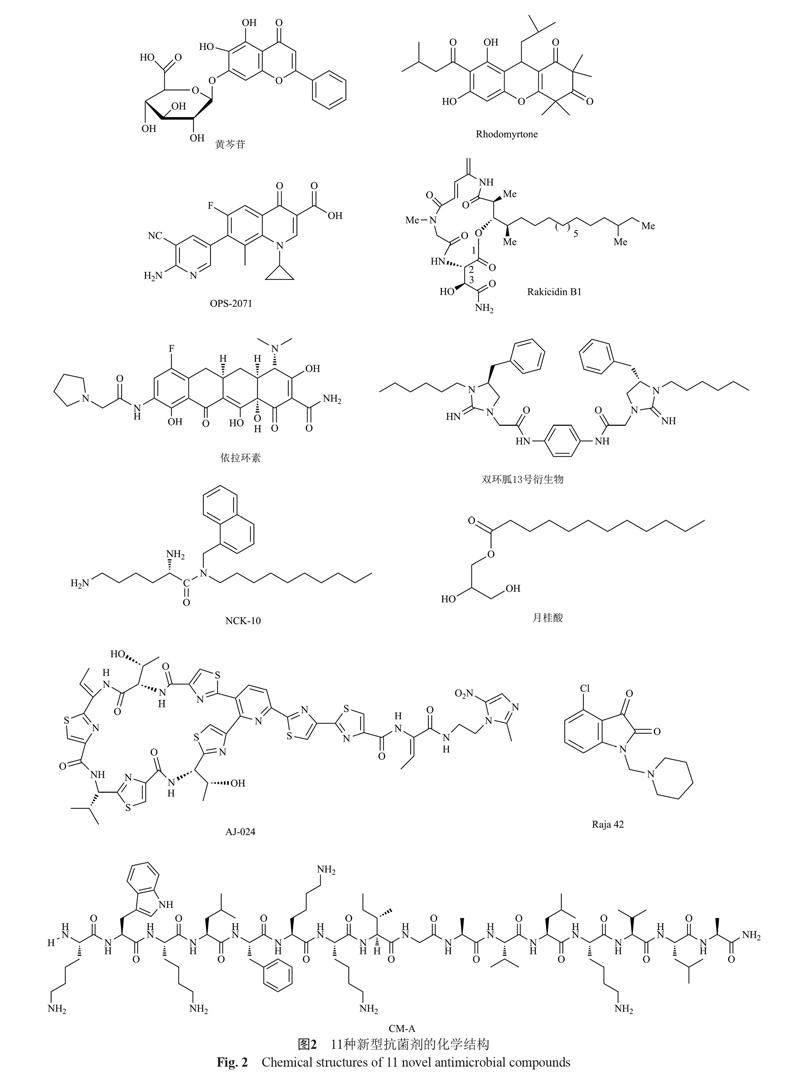
2.2 处于临床前开发阶段的抗菌剂
在治疗CDI的抗生素数量有限且复发率高的背景下,近5年研究人员新开发出以下11种有潜力的抗菌剂,下面对其化学结构、抗菌活性及主要作用机制进行汇总(见图2和表2)。
2.2.1 天然抗菌药物
据报道,天然存在的植物营养素类黄酮对多种病原体具有抗菌活性。研究发现黄酮类化合物在炎症性肠病(inflammatory bowel disease, IBD)中具有抗炎、抗腹泻的作用[86],其中黄芩苷通过抑制PI3K/AKT信号通路而影响IBD的发展[87]。近期研究发现,亚抑制浓度(subinhibitory concentration, SIC)的黄芩苷可以使C. difficile高毒株(ATCCBAA 1870和1803菌株)毒素合成降低70%~85%、孢子形成减少1.1~1.3 lgCFU/mL,其主要作用机制是抑制毒素TcdA、TcdB和孢子形成相关基因的转录[73-74]。月桂酸(lauric acid, LA)是一种脂肪酸(fatty acids, FA),在椰子油中占比高达45%~53%。經体外试验发现LA可以显著抑制C. difficile ATCC9689菌株的生长[88]。LA对于多种临床分离株的最低杀菌浓度(minimum bactericidal concentration, MBC)范围为0.312~0.625 mg/mL,0.25×MBC LA与20 μg/mL Van抑制生物膜形成的效果一致,且能以剂量依赖性降低孢子的萌发速率[82]。因此,黄芩苷和LA作为抑制C. difficile生长和毒力的天然成分,均有望开发为治疗CDI的辅助药物。
2.2.2 人工合成或衍生类抗菌药物
抗菌肽(antimicrobial peptides, AMPs)因拥有广谱抗菌活性、热稳定性和降低细菌耐药性等优点[89],近年来逐渐成为抗生素替代药物的热门研究对象。CM-A是由CM肽(天蚕素A和蜂毒肽)在第16位添加丙氨酸后获得的衍生肽,CM-A对C. difficile的最低抑菌浓度(minimum inhibitory concentration, MIC)低于CM近2倍,且在MIC浓度下对人结肠腺癌细胞(the human colon adenocarcinomacell lines, Caco-2 细胞)没有毒性[81]。CM-A主要以细胞膜作为抗菌作用靶点,然而是否可以更彻底地杀灭处于缓慢生长期的细菌和减少细菌耐药性产生还需进一步验证。同样靶向细胞膜的芳基-烷基-赖氨酸是一类小分子拟肽抗菌剂[90]。其中含有癸基链的NCK-10化合物对C. difficile的MIC值与Van接近,并且多种Van耐药型菌株对其敏感,该化合物主要集中于胃肠道不会进入血液,且对人结肠上皮细胞无毒[83]。
国内研究者筛选发现rakicidin B是一种从海洋小单孢菌的发酵液中分离得到的缩肽类化合物,其对CDI小鼠有保护作用,被感染小鼠干预后存活率高达80%且复发率低于Van,其衍生物rakicidin B1具有更强的抗C. difficile活性[77,91]。本实验室前期研究发现rakicidin B1在培养基中稳定性下降、艰难梭菌诱导耐药等原因,测定MIC时间较长,药物降解导致MIC值偏高[92]。为避免复杂性问题及方便后续研究,我们在极低的药物浓度下(0.4 ?g/mL)进行杀菌试验,结果发现rakicidin B1作用5 min时即可快速杀菌100倍,与临床上的使用剂量相比该浓度相对较低杀菌速度较快,具有非常大的治疗潜力。Rhodomyrtone是1种三酮-酰基间苯三酚,比Van杀菌所需浓度更低且速度更快,0.5×MIC时能够抑制孢子萌发,还可以减少生物膜形成从而降低CDI复发率[75]。具有两亲结构的化合物双环胍不仅MIC值与Van接近,对CDI小鼠有一定保护作用且粪便中C. difficile水平降低80%,其中化合物13最具选择性[80]。因此,rakicidin B1、rhodomyrtone和双环胍13号化合物均具有较强的抗菌活性,可以作为治疗CDI的候选开发药物,但是其毒力影响及细胞毒性需进一步探究。
Raja 42是一种靛红-苯并噻唑类似物(含有γ-内酰胺结构),不仅对多种临床分离菌株有抗菌活性,而且Mtr耐药菌株也对其敏感。Raja 42的潜在治疗指数(therapeutic index, TI:12.15)优于Van(TI:7.45),表明其毒性低于Van,可考虑开发为临床二线药物,在Mtr无效时辅以治疗[84]。AJ-024是一种含有硝基咪唑结构的新型硫肽抗生素(26元硫肽的硝基咪唑衍生物),体外试验发现在4×MIC时,AJ-024的杀菌活性明显优于Van并且维持长达24 h,0.25 ?g/mL时该衍生物的杀菌程度与2 ?g/mL Van相当,其作用机制是26元硫肽与细菌23S核糖体RNA亚基和蛋白质L11之间紧密结合而干扰蛋白质合成。在小鼠IBD合并症模型中AJ-024与Van均有80%的存活率[85]。OPS-2071是一种新型喹诺酮类抗菌剂,主要作用机制是抑制细菌DNA复制,1×MIC OPS-2071与2×MIC Mtr和Van杀菌程度相当,且48 h后未恢复。在仓鼠CDI模型中,OPS-2071的50%有效剂量(effective dose 50, ED50)比Van低39.0倍,自发突变率低且抗菌效力持续时间长于Van,不易产生耐药性[76]。不难发现,在体内和体外抗菌效果上,AJ-024与OPS-2071均拥有比Van更优秀的活性,因此非常适于单药治疗CDI。
2.2.3 老藥新用
随着全球对临床抗生素使用管理的加强推广,“老药新用”在抗感染行业逐渐展现优势。依拉环素(eravacyclin, 原称为TP-434)作为一种新型合成四环素抗菌剂,由于对需氧和厌氧的革兰阳性及革兰阴性病原体具有广谱活性,已于2018年获得FDA批准用于成人复杂腹腔感染的治疗[79]。研究发现它能与30S核糖体亚基结合抑制蛋白质合成来发挥抗C. difficile活性,MBC值低于Van的情况下拥有相似的时间杀伤动力学特征[78]。因此,可以尝试作为老药新用药物来治疗CDI。
3 展望
C. difficile复杂的发病机制和高复发率使得新药物研发和临床试验道路上遇到多重障碍。此外,其耐药机制也因遗传操作等技术瓶颈而难以阐明,造成细菌耐药问题日趋严重。FMT疗法的出现将很好地降低细菌耐药影响,其肠道微生物重建作用在治疗rCDI方面非常有潜力。虽然FMT疗法在II期临床实验中显示出比较好的安全性和有效性,但由于存在潜在的新病原体传播以及对受试者长远健康的风险未知,在未来的临床研究中需要更仔细且长期的随访。Ridinilazole作为脱颖而出的新型抗生素,在临床Ⅱ期实验中表现出良好的疗效,其Ⅲ期临床实验非常值得期待。在对C. difficile的新药物研究中,化合物的筛选、临床前研究、临床评价结果、试验失败的经验都是值得积累的。虽然有失败的结果,但是,所发现的更多有潜力的新药物和老药新用药物将进入下一步研究。通过对16种抗菌化合物的总结,发现抗C. difficile的新药研发趋势是:窄谱、特异,对肠道菌群影响小、肠道浓度高、作用时间长但系统吸收差;抑制或杀伤孢子、生物膜,停药后复发率低。在今后的药物探索过程中,除了对抗菌机制和耐药机制的进一步探究,找到针对rCDI的最佳治疗方法也是研究重点之一,结合适当的预防和抗生素管控对控制CDI感染和传播具有重要意义。希望通过对C. difficile的感染与治疗现状进行综述为后续治疗CDI的药物研发提供建议与方向。
参 考 文 献
Kazakova S V, Baggs J, Yi S H, et al. Associations of facility-level antibiotic use and hospital-onset Clostridioides difficile infection in US acute-care hospitals, 2012—2018[J]. Infect Control Hosp Epidemiol, 2022, 43(8): 1067-1069.
Hall I C. Intestinal flora in new-born infants[J]. Amer J Dis Childr, 1935, 49(2): 390-402.
Lessa F C, Mu Y, Bamberg W M, et al. Burden of Clostridium difficile infection in the United States[J]. N Engl J Med, 2015, 372(9): 825-834.
Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe[J]. Lancet, 2005, 366(9491): 1079-1084.
Petrella L A, Sambol S P, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain[J]. Clin Infect Dis, 2012, 55(3): 351-357.
Banawas S S. Clostridium difficile infections: A global overview of drug sensitivity and resistance mechanisms[J]. Biomed Res Int, 2018. doi: 10.1155/2018/8414257.
Selle K, Fletcher J R, Tuson H, et al. In vivo targeting of Clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials[J]. mBio, 2020, 11(2): e00019-20.
Feuerstadt P, Louie T J, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection[J]. N Engl J Med, 2022, 386(3): 220-229.
Hyte M L, Arphai L J, Vaughn C J, et al. The role of bezlotoxumab for the prevention of recurrent Clostridioides difficile infections: A review of the current literature and paradigm shift after 2021[J]. Antibiotics (Basel), 2022, 11(9): 1211.
Borriello S P. 12th C. L. Oakley lecture. Pathogenesis of Clostridium difficile infection of the gut[J]. J Med Microbiol, 1990, 33(4): 207-215.
Aktories K, Schwan C, Jank T. Clostridium difficile toxin biology[J]. Annu Rev Microbiol, 2017, 71: 281-307.
Jank T, Giesemann T, Aktories K. Rho-glucosylating Clostridium difficile toxins A and B: New insights into structure and function[J]. Glycobiology (Oxford), 2007, 17(4): 15R-22R.
Wexler A G, Guiberson E R, Beavers W N, et al. Clostridioides difficile infection induces a rapid influx of bile acids into the gut during colonization of the host[J]. Cell Rep, 2021, 36(10): 109683.
Fletcher J R, Pike C M, Parsons R J, et al. Clostridioides difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota[J]. Nat Commun, 2021, 12(1): 462.
Cornely O A, Crook D W, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial[J]. Lancet Infect Dis, 2012, 12(4): 281-289.
Pepin J, Alary M E, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada[J]. Clin Infect Dis, 2005, 40(11): 1591-1597.
Ethapa T, Leuzzi R, Ng Y K, et al. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile[J]. J Bacteriol, 2013, 195(3): 545-555.
L?fmark S, Edlund C, Nord C E. Metronidazole is still the drug of choice for treatment of anaerobic infections[J]. Clin Infect Dis, 2010, 50(Supplement_1): S16-S23.
Stogios P J, Savchenko A. Molecular mechanisms of vancomycin resistance[J]. Protein Sci, 2020, 29(3): 654-669.
Eubank T A, Gonzales-Luna A J, Hurdle J G, et al. Genetic mechanisms of vancomycin resistance in Clostridioides difficile: A systematic review[J]. Antibiotics (Basel), 2022, 11(2): 258.
Darkoh C, Keita K, Odo C, et al. Emergence of clinical Clostridioides difficile isolates with decreased susceptibility to vancomycin[J]. Clin Infect Dis, 2022, 74(1): 120-126.
Giacobbe D R, Vena A, Falcone M, et al. Fidaxomicin for the treatment of Clostridioides difficile infection in adult patients: An update on results from randomized controlled trials[J]. Antibiotics (Basel), 2022, 11(10): 1365.
Zhanel G G, Walkty A J, Karlowsky J A. Fidaxomicin: A novel agent for the treatment of Clostridium difficile infection[J]. Can J Infect Dis Med Microbiol, 2015, 26(6): 305-312.
Kuehne S A, Dempster A W, Collery M M, et al. Characterization of the impact of rpoB mutations on the in vitro and in vivo competitive fitness of Clostridium difficile and susceptibility to fidaxomicin[J]. J Antimicrob Chemother, 2018, 73(4): 973-980.
Meuwly M, Chuard C. Clostridioides difficile infection: Various therapeutic approaches[J]. Rev Med Suisse, 2022, 18(799): 1896-1899.
Stallmach A, Steube A, Stallhofer J, et al. Fecal microbiota transplantation: Indications, risks and opportunities[J]. Inn Med (Heidelb), 2022, 63(10): 1036-1042.
Xu Q, Zhang S, Quan J, et al. The evaluation of fecal microbiota transplantation vs vancomycin in a Clostridioides difficile infection model[J]. Appl Microbiol Biotechnol, 2022, 106(19): 6689-6700.
Orenstein R, Dubberke E R, Khanna S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: Results from an open-label phase 2 clinical trial[J]. BMC Infect Dis, 2022, 22(1): 245.
Vickers R, Robinson N, Best E, et al. A randomised phase 1 study to investigate safety, pharmacokinetics and impact on gut microbiota following single and multiple oral doses in healthy male subjects of SMT19969, a novel agent for Clostridium difficile infections[J]. BMC Infect Dis, 2015, 15: 91.
Vickers R J, Tillotson G S, Nathan R, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: A phase 2, randomised, double-blind, active-controlled, non-inferiority study[J]. Lancet Infect Dis, 2017, 17(7): 735-744.
Snydman D R, McDermott L A, Thorpe C M, et al. Antimicrobial susceptibility and ribotypes of Clostridium difficile isolates from a phase 2 clinical trial of ridinilazole (SMT19969) and vancomycin[J]. J Antimicrob Chemother, 2018, 73(8): 2078-2084.
Chandorkar G, Zhan Q, Donovan J, et al. Pharmacokinetics of surotomycin from phase 1 single and multiple ascending dose studies in healthy volunteers[J]. BMC Pharmacol Toxicol, 2017, 18(1): 24.
Citron D M, Tyrrell K L, Dale S E, et al. Impact of surotomycin on the gut microbiota of healthy volunteers in a phase 1 clinical trial[J]. Antimicrob Agents Chemother, 2016, 60(4): 2069-2074.
Lee C H, Patino H, Stevens C, et al. Surotomycin versus vancomycin for Clostridium difficile infection: Phase 2, randomized, controlled, double-blind, non-inferiority, multicentre trial[J]. J Antimicrob Chemother, 2016, 71(10): 2964-2971.
Cannon K, Byrne B, Happe J, et al. Enteric microbiome profiles during a randomized phase 2 clinical trial of surotomycin versus vancomycin for the treatment of Clostridium difficile infection[J]. J Antimicrob Chemother, 2017, 72(12): 3453-3461.
Daley P, Louie T, Lutz J E, et al. Surotomycin versus vancomycin in adults with Clostridium difficile infection: Primary clinical outcomes from the second pivotal, randomized, double-blind, phase 3 trial[J]. J Antimicrob Chemother, 2017, 72(12): 3462-3470.
Boix V, Fedorak R N, Mullane K M, et al. Primary outcomes from a phase 3, randomized, double-blind, active-controlled trial of surotomycin in subjects with Clostridium difficile infection[J]. Open Forum Infect Dis, 2017, 4(1): ofw275.
Baldoni D, Gutierrez M, Timmer W, et al. Cadazolid, a novel antibiotic with potent activity against Clostridium difficile: Safety, tolerability and pharmacokinetics in healthy subjects following single and multiple oral doses[J]. J Antimicrob Chemother, 2014, 69(3): 706-714.
Gehin M, Desnica B, Dingemanse J. Minimal systemic and high faecal exposure to cadazolid in patients with severe Clostridium difficile infection[J]. Int J Antimicrob Agents, 2015, 46(5): 576-581.
Louie T, Nord C E, Talbot G H, et al. Multicenter, double-blind, randomized, phase 2 study evaluating the novel antibiotic cadazolid in patients with Clostridium difficile infection[J]. Antimicrob Agents Chemother, 2015, 59(10): 6266-6273.
Gerding D N, Hecht D W, Louie T, et al. Susceptibility of Clostridium difficile isolates from a phase 2 clinical trial of cadazolid and vancomycin in C. difficile infection[J]. J Antimicrob Chemother, 2016, 71(1): 213-219.
Gerding D N, Cornely O A, Grill S, et al. Cadazolid for the treatment of Clostridium difficile infection: Results of two double-blind, placebo-controlled, non-inferiority, randomised phase 3 trials[J]. Lancet Infect Dis, 2019, 19(3): 265-274.
Nayak S U, Griffiss J M, Blumer J, et al. Safety, tolerability, systemic exposure, and metabolism of CRS3123, a Methionyl-tRNA synthetase inhibitor developed for treatment of Clostridium difficile, in a phase 1 study[J]. Antimicrob Agents Chemother, 2017, 61(8). doi: 10.1128/AAC.02760-16.
Lomeli B K, Galbraith H, Schettler J, et al. Multiple-ascending-dose phase 1 clinical study of the safety, tolerability, and pharmacokinetics of CRS3123, a narrow-spectrum agent with minimal disruption of normal gut microbiota[J]. Antimicrob Agents Chemother, 2019, 64(1): e01395-19.
Vandell A G, Inoue S, Dennie J, et al. Phase 1 study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple oral doses of DS-2969b, a novel GyrB inhibitor, in healthy subjects[J]. Antimicrob Agents Chemother, 2018, 62(5): e02537-17.
Dennie J, Vandell A G, Inoue S, et al. A phase I, single-ascending-dose study in healthy subjects to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of DS-2969b, a novel GyrB inhibitor[J]. J Clin Pharmacol, 2018, 58(12): 1557-1565.
Vickers R, Tinsley J, Storer R, et al. SMT19969–a novel antibiotic for C. difficile: Clostridium difficile growth inhibition, spectrum of activity and resistance development[C]. 51st Interscience Conf on Antimicrobial Agents and Chemotherapy (ICAAC), 2011.
Baron A, Sann C L, Mann J. Symmetric bis-benzimidazoles as DNA minor groove-binding agents with anti-tumour and antibacterial activity, and the evolution of the drug ridinilazole for the treatment of Clostridium difficile infections[J]. Bioorg Med Chem, 2022, 58: 116656.
Vickers R J, Tillotson G, Goldstein E J, et al. Ridinilazole: A novel therapy for Clostridium difficile infection[J]. Int J Antimicrob Agents, 2016, 48(2): 137-143.
Corbett D, Wise A, Birchall S, et al. In vitro susceptibility of Clostridium difficile to SMT19969 and comparators, as well as the killing kinetics and post-antibiotic effects of SMT19969 and comparators against C. difficile[J]. J Antimicrob Chemother, 2015, 70(6): 1751-1756.
Collins D A, Wu Y, Tateda K, et al. Evaluation of the antimicrobial activity of ridinilazole and six comparators against Chinese, Japanese and South Korean strains of Clostridioides difficile[J]. J Antimicrob Chemother, 2021, 76(4): 967-972.
Freeman J, Vernon J, Vickers R, et al. Susceptibility of Clostridium difficile isolates of varying antimicrobial resistance phenotypes to SMT19969 and 11 comparators[J]. Antimicrob Agents Chemother, 2016, 60(1): 689-692.
Baines S D, Crowther G S, Freeman J, et al. SMT19969 as a treatment for Clostridium difficile infection: An assessment of antimicrobial activity using conventional susceptibility testing and an in vitro gut model[J]. J Antimicrob Chemother, 2015, 70(1): 182-189.
Goldstein E J, Citron D M, Tyrrell K L, et al. Comparative in vitro activities of SMT19969, a new antimicrobial agent, against Clostridium difficile and 350 Gram-positive and Gram-negative aerobic and anaerobic intestinal flora isolates[J]. Antimicrob Agents Chemother, 2013, 57(10): 4872-4876.
Sattar A, Thommes P, Payne L, et al. SMT19969 for Clostridium difficile infection (CDI): In vivo efficacy compared with fidaxomicin and vancomycin in the hamster model of CDI[J]. J Antimicrob Chemother, 2015, 70(6): 1757-1762.
Mascio C T, Mortin L I, Howland K T, et al. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile[J]. Antimicrob Agents Chemother, 2012, 56(10): 5023-5030.
Knight-Connoni V, Mascio C, Chesnel L, et al. Discovery and development of surotomycin for the treatment of Clostridium difficile[J]. J Ind Microbiol Biotechnol, 2016, 43(2-3): 195-204.
Bouillaut L, McBride S, Sorg J A, et al. Effects of surotomycin on Clostridium difficile viability and toxin production in vitro[J]. Antimicrob Agents Chemother, 2015, 59(7): 4199-4205.
Alam M Z, Wu X, Mascio C, et al. Mode of action and bactericidal properties of surotomycin against growing and nongrowing Clostridium difficile[J]. Antimicrob Agents Chemother, 2015, 59(9): 5165-5170.
Endres B T, Bassères E, Khaleduzzaman M, et al. Evaluating the effects of surotomycin treatment on Clostridium difficile toxin A and B production, immune response, and morphological changes[J]. Antimicrobial agents and chemotherapy, 2016, 60(6): 3519-3523.
Chilton C H, Crowther G S, Todhunter S L, et al. Efficacy of surotomycin in an in vitro gut model of Clostridium difficile infection[J]. J Antimicrob Chemother, 2014, 69(9): 2426-2433.
Deshpande A, Hurless K, Cadnum J L, et al. Effect of surotomycin, a novel cyclic lipopeptide antibiotic, on intestinal colonization with vancomycin-resistant Enterococci and Klebsiella pneumoniae in mice[J]. Antimicrob Agents Chemother, 2016, 60(6): 3333-3339.
Rashid M U, Lozano H M, Weintraub A, et al. In vitro activity of cadazolid against Clostridium difficile strains isolated from primary and recurrent infections in Stockholm, Sweden[J]. Anaerobe, 2013, 20: 32-35.
Locher H H, Seiler P, Chen X, et al. In vitro and in vivo antibacterial evaluation of cadazolid, a new antibiotic for treatment of Clostridium difficile infections[J]. Antimicrob Agents Chemother, 2014, 58(2): 892-900.
Locher H H, Caspers P, Bruyere T, et al. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections[J]. Antimicrob Agents Chemother, 2014, 58(2): 901-908.
Chilton C H, Crowther G S, Baines S D, et al. In vitro activity of cadazolid against clinically relevant Clostridium difficile isolates and in an in vitro gut model of C. difficile infection[J]. J Antimicrob Chemother, 2013, 69(3): 697-705.
Seiler P, Enderlin-Paput M, Pfaff P, et al. Cadazolid does not promote intestinal colonization of vancomycin-resistant Enterococci in mice[J]. Antimicrob Agents Chemother, 2016, 60(1): 628-631.
Critchley I A, Green L S, Young C L, et al. Spectrum of activity and mode of action of REP3123, a new antibiotic to treat Clostridium difficile infections[J]. J Antimicrob Chemother, 2009, 63(5): 954-963.
Ochsner U A, Bell S J, O'Leary A L, et al. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model[J]. J Antimicrob Chemother, 2009, 63(5): 964-971.
Tyrrell K L, Citron D M, Merriam C V, et al. In vitro activity of DS-2969b and comparator antimicrobial agents against Clostridioides (Clostridium) difficile, methicillin-resistant Staphylococcus aureus, and other anaerobic bacteria[J]. Anaerobe, 2018, 54: 39-41.
Mathur T, Barman T K, Kumar M, et al. In vitro and in vivo activities of DS-2969b, a novel GyrB inhibitor, against Clostridium difficile[J]. Antimicrob Agents Chemother, 2018, 62(4): e02157-17.
Kumar M, Mathur T, Joshi V, et al. Effect of DS-2969b, a novel GyrB inhibitor, on rat and monkey intestinal microbiota[J]. Anaerobe, 2018, 51: 120-123.
Pellissery A J, Vinayamohan P G, Venkitanarayanan K. In vitro antivirulence activity of baicalin against Clostridioides difficile[J]. J Med Microbiol, 2020, 69(4): 631-639.
Pellissery A J, Vinayamohan P G, Kuttappan D A, et al. Protective effect of baicalin against Clostridioides difficile infection in mice[J]. Antibiotics (Basel), 2021, 10(8): 926.
Srisuwan S, Mackin K E, Hocking D, et al. Antibacterial activity of rhodomyrtone on Clostridium difficile vegetative cells and spores in vitro[J]. Int J Antimicrob Agents, 2018, 52(5): 724-729.
Oka D, Yamaya N, Kuno T, et al. In vitro and in vivo antibacterial activities of a novel quinolone compound, OPS-2071, against Clostridioides difficile[J]. Antimicrob Agents Chemother, 2022, 66(3): e0233821.
林風, 陈丽, 赵薇, 等. 缩肽类化合物rakicidins体内外抗艰难梭菌活性研究[J]. 中国抗生素杂志, 2017, 42(5): 343-347.
Basseres E, Begum K, Lancaster C, et al. In vitro activity of eravacycline against common ribotypes of Clostridioides difficile[J]. J Antimicrob Chemother, 2020, 75(10): 2879.
Alosaimy S, Abdul-Mutakabbir J C, Kebriaei R, et al. Evaluation of eravacycline: A novel fluorocycline[J]. Pharmacotherapy, 2020, 40(3): 221-238.
Li C, Teng P, Peng Z, et al. Bis-cyclic guanidines as a novel class of compounds potent against Clostridium difficile[J]. ChemMedChem, 2018, 13(14): 1414-1420.
Arthithanyaroj S, Chankhamhaengdecha S, Chaisri U, et al. Effective inhibition of Clostridioides difficile by the novel peptide CM-A[J]. PLoS One, 2021, 16(9): e257431.
Yang H, Chen J, Rathod J, et al. Lauric acid is an inhibitor of Clostridium difficile growth in vitro and reduces inflammation in a mouse infection model[J]. Front Microbiol, 2017, 8: 2635.
Ghosh C, AbdelKhalek A, Mohammad H, et al. Aryl-alkyl-lysines: Novel agents for treatment of C. difficile infection[J]. Sci Rep, 2020, 10(1): 5624.
Fong A, Ross M, Boudreau J, et al. Raja 42, a novel gamma lactam compound, is effective against Clostridioides difficile[J]. PLoS One, 2021, 16(9): e257143.
Kim D, Kim Y R, Hwang H J, et al. Nitro-group-containing thiopeptide derivatives as promising agents to target Clostridioides difficile[J]. Pharmaceuticals (Basel), 2022, 15(5): 623.
Wang L, Gao M, Kang G, et al. The potential role of phytonutrients flavonoids influencing gut microbiota in the prophylaxis and treatment of inflammatory bowel disease[J]. Front Nutr, 2021, 8: 798038.
Zhu L, Shen H, Gu P Q, et al. Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation[J]. Exp Ther Med, 2020, 20(1): 581-590.
Shilling M, Matt L, Rubin E, et al. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile[J]. J Med Food, 2013, 16(12): 1079-1085.
肖懷秋, 李玉珍, 林亲录, 等. 抗菌肽多靶点作用抑菌机理研究进展[J]. 食品与生物技术学报, 2022, 41(5): 11-19.
Ghosh C, Manjunath G B, Akkapeddi P, et al. Small molecular antibacterial peptoid mimics: The simpler the better![J]. J Med Chem, 2014, 57(4): 1428-1436.
谢立君, 陈丽, 赵薇, 等. 抗艰难梭菌rakicidin B1新衍生物的设计、合成及生物学活性评价(英文)[J]. 中国抗生素杂志, 2019, 44(12): 1334-1340.
郭超勤.天然产物抗厌氧菌活性评价及抗艰难梭菌联合用药研究[D]. 厦门: 厦门大学, 2019.
收稿日期:2022-12-26
基金项目:国家自然科学基金(No. 82172316)
作者简介:郭银莉,女,生于1998年,在读硕士研究生,主要研究方向为病原微生物抗感染及耐药机制,E-mail: gyl_guo@163.com
*通信作者,赵西林,E-mail: zhaox5@xmu.edu.cn;牛建军, E-mail: Niujianjun211@xmu.edu.cn
