Ultimate fate of halosulfuron methyl and its effects on enzymatic and microbial activities in three differently textured soils
2023-12-21PervinderKAURJasleenKAURandHarshdeepKAUR
Pervinder KAUR,Jasleen KAUR and Harshdeep KAUR
1Department of Agronomy,Punjab Agricultural University,Ludhiana,Punjab 141001(India)
2Department of Chemistry,Punjab Agricultural University,Ludhiana,Punjab 141001(India)
ABSTRACT Halosulfuron methyl is a sulfonylurea herbicide used worldwide for weed control in sugarcane,maize,wheat,and rice production.Considering its environmental impact,this study evaluated the effects of soil type,application rate,and temperature on the dynamics of halosulfuron methyl degradation.Additionally, as soil microbes and enzymes are reliable indicators of the impacts of anthropogenic activities on soil health, the effects of halosulfuron methyl on soil enzymatic and microbial activities were also assessed.The half-life(DT50)of halosulfuron methyl varied from 9.38 to 33.77 d.Increase in temperature accelerated the degradation and DT50 varied from 14.39 to 33.77,11.05 to 28.94,and 9.38 to 25.41 d at 5,15,and 25 ◦C,respectively.The metabolites of halosulfuron methyl,including halosulfuron,methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate,4,6-dimethoxy-2-pyrimidinamine, and methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate, were detected in the studied soils, and their appearance and disappearance varied with application rate,soil type,and incubation temperature.Halosulfuron methyl had transitory harmful effects on soil enzymatic and microbial activities depending on its application rate.The results suggest that the application rate of halosulfuron methyl,soil physicochemical parameters,and temperature should be considered together to ensure satisfactory weed control with reduced environmental risk.
KeyWords: degradation,environmental risks,metabolites,principal component analysis,soil type,sulfonylurea herbicides,weed control
INTRODUCTION
Herbicide application is important for effective weed control,as weeds compete with crops for available nutrients and moisture and reduce crop yield by 30%—80%(Scavo and Mauromicale,2020).However,extensive use of herbicides may have adverse environmental effects (Baćmagaet al.,2015).Once herbicides are released into the environment,they may impair soil, water, and air quality.Furthermore,sometimes they show toxicity to crops and residual effects on succeeding crops and non-target organisms,depending upon their persistence(Sondhia,2014).Herbicides penetrate soils and affect soil enzymatic and microbial activities(García-Delgadoet al.,2019;Carpioet al.,2020).
Halosulfuron methyl is a pyrazol sulfonylurea herbicide used to control broadleaf and cyperaceous weeds in graminaceous crops, such as wheat, maize, sugarcane, and rice(Fenget al., 2014).It is a weakly acidic herbicide, with an acid dissociation constant of 3.44 and low to moderate persistence.Its half-life (DT50) varies from 9 to 55 d in aerobic and anaerobic soils.Its degradation decreases with increasing soil pH(EFSA,2012;Rajasekharam and Ramesh,2014), and under unfavorable conditions, it causes slight toxicity in succeeding susceptible crops such as watermelon,alfalfa, spinach, turnip green, and cabbage (Chandet al.,2014).It is moderately adsorbed to soil particles,with an adsorption coefficient of 1.12—1.48 mL g-1,and it has high potential to leach and contaminate ground and surface waters(EFSA,2012;Deviet al.,2019).Residues of halosulfuron methyl and its metabolite chlorosulfonamide acid have been detected in groundwater at levels that potentially exceed the permissible maximum residue limit of 0.1µg L-1(EFSA,2012).It is toxic to aquatic and terrestrial plants and shows high bioaccumulation potential(Lewiset al.,2016).
Deviet al.(2019),Rajasekharam and Ramesh(2014),and Wanget al.(2020) reported contrary results on the degradation of halosulfuron methyl in soil depending on soil physicochemical properties,agronomic practices,and environmental conditions.As these factors and their subtle interplay vary significantly with geographical conditions,extrapolation of the results will lead to erroneous estimation of dissipation behavior and consequently inaccurate risk assessment based on these values.Additionally,soil pH in the cereal and sugarcane-growing regions of Indian Punjab ranges from 7.7 to 9.0,and halosulfuron methyl is expected to predominantly be degraded by soil microbes in these regions, as chemical hydrolysis is very slow at this pH.Hence,halosulfuron methyl and its metabolites may persist for longer periods and hinder the growth and development of certain rotational crops.
Soil microbes and enzymes are reliable indicators for evaluating the effects of anthropogenic activities on soil health.Herbicide application can inhibit some of the natural processes and decrease the performance of non-target organisms,indicating that herbicides applied with the goal of maximizing productivity and economic returns potentially act at the expense of ecosystem functions(MacLarenet al.,2020).Limited information is available on the effect of halosulfuron methyl on microbial activity in acidic and neutral soils (Wanget al.2020), and information on its effect on enzymatic and microbial activity in alkaline soils is scanty.Some herbicides stimulate the growth of microorganisms, as they act as a carbon source for soil microbiota(Minet al.,2001;Singh and Ghoshal,2013;Tomkielet al.,2019).However, Wyszkowskaet al.(2008)and Liuet al.(2020) reported the inhibition of soil enzymatic activity due to the toxic effect of applied herbicides.This variability in results is probably due to the effect of herbicides on soil biota, depending on the physicochemical characteristics of soils and herbicides,environmental conditions,and agronomical practices(García-Delgadoet al.,2019).In addition,soil microorganisms are influenced not only by the parent herbicide, but also by its transformation products,which are sometimes more injurious than the parent compound.Considering the environmental impact and variability reported in the literature,a systematic detailed study on the degradation kinetics of halosulfuron methyl under different agroclimatic conditions and its effects on soil enzymatic and microbial activities is very important.
The present study investigated the impact of soil physicochemical properties,application rate,and temperature on the degradation of halosulfuron methyl and its effects on soil enzymatic and microbial activities during degradation.The findings will help in the development of weed management strategies that make a more effective and efficient use of halosulfuron methyl.
MATERIALS AND METHODS
Herbicide and chemicals
A certified standard of halosulfuron methyl(99.9%purity) was supplied by Bayer Crop Science, India.Stock solution(1 000µg mL-1)and serial dilutions(0.008—100µg mL-1)of halosulfuron methyl were prepared with acetonitrile.Water,acetonitrile,and other chemicals used in this study were of high-pressure liquid chromatography(HPLC)-grade and procured from Qualikems Chemicals Pvt.Ltd.,Mumbai,India.The chemicals required for the assay of soil enzymatic activities were procured from Hi Media Laboratories Pvt.Ltd., Mumbai, India.Commercial formulation of halosulfuron methyl(Sencor 75%wettable granules)was purchased in a local market.
Experimental design
Degradation studies were carried out in three soils under laboratory conditions.The sandy loam(SL)was collected from Shaheed Bhagat Singh Nagar,Punjab,India(31◦10′11′′N,65◦58′91′′E),and the loamy sand(LS)and clay loam(CL)were collected from Punjab Agricultural University,Ludhiana, Punjab, India (30◦52′97′′N, 75◦47′32′′E and 30◦52′75′′N,75◦47′24′′E,respectively).The physicochemical properties of the soils were assessed using the methods reported by Piper(1966),Richard(1954),Jackson(1958),and Walkley and Black(1934)(Table I).

TABLE I Properties of the three differently textured soils used in this study
The experiment was conducted in a randomized block design with three replications.For each soil type,45 pots(10 cm diameter×8.5 cm height)were filled with 500 g soil and divided into three batches equally for incubation at 5,15,and 25◦C,respectively.In each batch,five application rates(R0—R4)of halosulfuron methyl were set up:0,67.5,135,202.5,and 270 g ha-1(or 0,0.033 7,0.067 4,0.101 1,and 0.134 8 mg kg-1,respectively).Distilled water was added to the pots daily to maintain soil moisture at 75%of field capacity.To determine the residue of halosulfuron methyl,soil samples were collected from each pot at 0(3 h),7,10,15,21,30,45,60,90,and 120 d after application(DAA)of the herbicide.Soil samples collected at 0, 15, 30, 45,60,90,and 120 DAA were also analyzed for enzymatic and microbial activities.Dehydrogenase(DH)activity was analyzed by determining the triphenyl formazan(TPF)released from 2,3,5-triphenyl tetrazolium chloride photometrically at 485 nm(Tabatabai, 1982).Alkaline(AlP)and acid phosphatase(AcP)activities were measured by determining thep-nitrophenyl(PNP)produced fromp-nitrophenyl phosphate disodium photometrically at 420 nm(Tabatabai and Bremner,1969).Urease(UA)activity was assayed by quantifying the ammonium(NH+4)released from urea hydrolysis photometrically at 527 nm(Douglas and Bremner,1970).Soil microbial counts were estimated using the serial dilution technique and pour plate method at 10-3and 10-4dilutions(Rajet al., 2015).The media used for bacteria, fungi, actinobacteria,and phosphate-solubilizing bacteria(PSB)were nutrient agar, potato dextrose agar, Kenknight’s medium,and Pikovskaya’s agar,respectively.For bacteria and PSB,the plates were incubated at 37◦C,whereas for fungi and actinobacteria, at 28◦C.The counts were recorded after 24—48 h of incubation for bacteria and 48—72 h of incubation for fungi,actinobacteria,and PSB and expressed as number of colony-forming units(CFUs)per gram of dry soil.
Halosulfuron methyl residue extraction and quantification
For halosulfuron methyl extraction, 10 g soil was extracted with 40 mL acetonitrile in an ultrasonic bath at 30◦C for 3 min and filtered through activated charcoal(3 mg)and anhydrous sodium sulfate(2 g).The solvent was removed using a rotary vacuum evaporator.The residue was reconstituted in 2 mL acetonitrile and analyzed using HPLC(Waters,USA).A Spherisorb C18column (5 µm octadecyl silica,4 mm internal diameter×250 mm length)(Waters,USA)was used to separate the analytes.The HPLC conditions for quantification of halosulfuron methyl and determination of the metabolites halosulfuron(M1),methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate(M2),4,6-dimethoxy-2-pyrimidinamine(M3),and methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate(M4)are summarized in Table II.The metabolites of halosulfuron methyl in soil were identified using liquid chromatography tandem mass spectrometry(LC-MS/MS)(Alliance 2495,Waters,USA).Mass spectrometry analysis was performed in positive electron spray ionization mode.The optimized parameters were as follows:desolvation gas flow 600 L h-1, collision gas flow 35 L h-1, desolvation temperature 350◦C,cone voltage 30 kV,and collision voltage 50 kV.
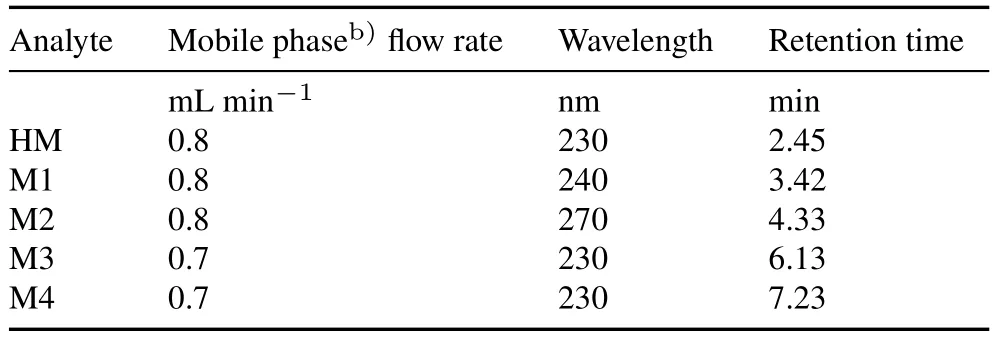
TABLE II High-pressure liquid chromatography conditions for the quantification of halosulfuron methyl(HM)and its metabolites M1—M4a)
Data analysis
Halosulfuron methyl degradation kinetics was fitted by zero-,first-,and second-order kinetic equations(Eqs.1,2,and 3,respectively):
whereCtandC0are the halosulfuron methyl concentration(µg g-1)at timetand 0(d),respectively,andkis the rate constant(d-1).For zero-,first-,and second-order degradation kinetics,the DT50(d)was calculated using Eqs.4,5,and 6,respectively:
As the coefficient of determination(R2)is not sufficient for measuring the goodness of fit, the standard error of estimation(SEE)was calculated as:
whereXis the observed residue concentration (µg g-1),X′is the predicted residue concentration(µg g-1),nis the number of measurements,andmis the number of parameters used in degradation model.
The extent of the dependence of halosulfuron methyl degradation on temperature was determined using the Arrhenius equation:
whereEais the activation energy (kJ mol-1),Ris the universal gas constant(kJK-1mol-1),Ais the empirical constant(d-1),andTis the absolute temperature(K).The temperature coefficientQ10,which represents degradation rate changes with 10◦C increase in temperature,was calculated as:
wherek2andk1are the rate constants(d-1)at temperaturesT2andT1(◦C),respectively,andT2=T1+10.
The effects of application rate, soil type, and temperature on the degradation of halosulfuron methyl and its influence on soil enzymatic and microbial activities were statistically analyzed using analysis of variance in the SPSS statistics software.The responses of soil enzymatic activities and microbial counts to halosulfuron methyl were statistically analyzed through principal component analysis(PCA)performed using the STAR (version 2.0.1.0) software of International Rice Research Institute,Philippines.
RESULTS AND DISCUSSION
Method validation
The calibration curve of halosulfuron methyl was linear in the range 0.008—5 µg mL-1withR2> 0.99 (data not shown).The limit of detection and limit of quantification were 0.002 6and 0.008µg mL-1,respectively.A recovery experiment was carried out at five spiking levels(1,0.5,0.1,0.05,and 0.01µg g-1),and the mean percentage recoveries varied from 88.33%to 99.43%(Table III).To assess intraand inter-day precision,the relative standard deviation(RSD)of spiked samples was calculated by analyzing samples three times a day (RSDr) and three times for three consecutive days (RSDR), respectively.The RSDrand RSDRranged from 2.87% to 8.50% and 3.15% to 9.20%, respectively.The developed method was sensitive and selective for the determination of halosulfuron methyl in soils.

TABLE III Method validation parameters for halosulfuron methyl extraction from three differently textured soils
Halosulfuron methyl degradation
Effect of application rate and soil type.Halosulfuron methyl concentration at day 0(3 h after application)ranged from 0.032 to 0.039 and 0.062 to 0.069 µg g-1in the studied soils at R1 and R2 application rates, respectively,whereas,at the higher application rates of R3 and R4,the concentration at day 0 varied from 0.091 to 0.097 and 0.122 to 0.129µg g-1,respectively(Fig.1).Halosulfuron methyl concentration decreased significantly with time.Irrespective of soil type,halosulfuron methyl concentration was below detectable limit(<0.008µg g-1)at 45 and 60 DAA at R1 and R2,respectively,whereas it was<0.008µg g-1at 90 DAA when applied at R3 and R4.The slower degradation at higher application rates might be due to inhibition of microbial activity at higher application rates.Kalsi and Kaur(2019)and Kaur H and Kaur P(2018)observed a similar slower degradation of bispyribac sodium and penoxsulam at higher application rates.In contrast,flufenacet undergoes faster degradation at higher application rates (Gupta and Gajbhiye, 2002), probably because of the capacity of the microbial community to use it as a carbon source.
Different kinetic models were used for the determination of the kinetic parameters for halosulfuron methyl degradation in soils.TheR2and DT50values obtained from the different kinetic models are listed in Tables IV,SI,and SII(see Supplementary Material for Tables SI and SII).Based onR2,the degradation of halosulfuron methyl fitted well into the first-order kinetic equation,withR2ranging 0.97—0.99.AsR2is not sufficient for the comparison of different kinetic models,in addition toR2,SEE was assessed for different kinetic models.The lower value of SEE for the first-order kinetic model(0.001 0—0.008 5)as compared to the zeroand second-order kinetic models indicated a better fit of the data to first-order kinetics.A linear regression model was applied,and the variations between the observed and predicted residue concentrations are shown in Fig.1.The accuracy of the residues detected in the studied soils was assessed by the residual error between the observed and predicted values (Juraskeet al., 2012).The residual errors were in the ranges of-0.002 3%—0.003 0%,-0.002 0%—0.002 9%,and-0.0068%—0.004 4%for LS,SL,and CL,respectively,whereas the residual plots showed rather random patterns,indicating that the applied regression models provide good fits to the experimental data (Fig.S1, see Supplementary Material for Fig.S1).
Similar results for the better fit of a first-order kinetic model for the degradation of mesotrione,metribuzin,andS-metolachlor were reported by Kaczynskiet al.(2016),Mehdizadehet al.(2019),and Longet al.(2014).In contrast,Noshadi and Homaee(2018)and Swarcewicz and Gregorczyk(2012)observed deviations in the degradation kinetics of metribuzin and pendimethalin from first-order to biexponential degradation.This deviation was probably because,immediately after application,herbicides were in an available compartment where they were degraded rapidly.However,with time,herbicides moved into the protective compartment after adsorption,where they were no longer available or less accessible to soil microorganisms.According to Zimdahl and Gwynn(1977),the invalidity of the first-order kinetics is because the associated equilibria (e.g., adsorption and leaching)are not considered in the assumption.Additionally,there are a number of other variables,such as water,catalysts(e.g., clay), organic surfaces, and microbes that affect the rate of reaction.These variables are not constant and do not appear in the equation.

Fig.1 Observed(OC)and predicted concentrations(PC)of halosulfuron methyl in the days after application(DAA)to three differently textured soils at different incubation temperatures(5,15,and 25 ◦C)and application rates(R1—R4).Error bars are standard deviations of the means(n=3).LS=loamy sand;SL=sandy loam;CL=clay loam;R1=67.5 g ha-1;R2=135 g ha-1;R3=202.5 g ha-1;R4=270 g ha-1.

TABLE IV Statistical parametersa) estimated using a first-order kinetic model for halosulfuron methyl degradation in three differently textured soils during the 120-d incubation at different application rates and incubation temperatures
In this study,DT50for halosulfuron methyl varied from 9.38 to 23.32,10.49 to 26.16,and 12.29 to 33.77 d in LS,SL,and CL,respectively(Table IV).Halosulfuron methyl was more persistent in CL because of its higher organic matter(OM) and clay contents, which resulted in comparatively greater adsorption of halosulfuron methyl in soil by electrostatic interactions,hydrogen bonding,π→πinteractions(charge transfer),and van der Waals forces,thereby reducing its availability in soil solution(Deviet al.,2019;Sainiet al., 2022).Faster degradation in SL might also be due to the larger particles and pore size of the soil,which provide comparatively more favorable conditions for oxidative degradation and chemical hydrolysis (Deviet al., 2019).Similar increase in the persistence of halosulfuron methyl with increase in soil clay and OM contents was observed by Deviet al.(2019).
However,Rajasekharam and Ramesh(2014)and Wanget al.(2020)reported comparatively faster degradation of halosulfuron methyl owing to rapid chemical hydrolysis in acidic soils(pH 5.0—6.0).The observed DT50of halosulfuron methyl was significantly lower than that reported for other sulfonylurea herbicides,such as pyrazosulfuron,chlorsulfuron,and imazosulfuron,although they have very similar structures (Zhenget al., 2008; Wang Y Set al., 2010).This was probably due to the enhanced electrophilicity of halosulfuron methyl in the presence of a strong electronwithdrawing chlorine group on the pyrazole ring, which favors the formation of a five-membered transition state in basic soils,thereby facilitating bridge contraction and faster degradation(Zhenget al.,2008).
The metabolites of halosulfuron methyl in the soils were identified,along with literature data(EFSA,2012).As this study was performed under dark conditions,photodecomposition did not play a major role in degradation,and chemical and microbial degradation were the major degradation pathways of halosulfuron methyl in soil.A plausible degradation pathway in soil is shown in Fig.2.As shown in the figure,halosulfuron methyl undergoes ester hydrolysis(Pathway 1)to form the corresponding acid metabolite M1.The bridge contraction and rearrangement of halosulfuron methyl (Pathway 2) results in the formation of the ester metabolite M2, which further undergoes the cleavage of sulfonylurea bridge to form metabolites M3 and M4.Halosulfuron methyl can also undergo direct cleavage(Pathway 3) to form the metabolite 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylic acid (M5).The metabolites of halosulfuron methyl are ultimately mineralized to carbon dioxide (EFSA, 2012).Among the plausible metabolites,M1, M2, M3, and M4 were detected in this study.These metabolites could not be quantified because of a lack of certified analytical standards.Detector responses to these metabolites in different treatments are shown in Fig.3.

Fig.2 Degradation pathways of halosulfuron methyl(HM)in soil.M1=halosulfuron;M2=methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate;M3=4,6-dimethoxy-2-pyrimidinamine;M4=methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate;M5=3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylic acid.
The appearance and persistence of the metabolites in the soil varied with application rate and soil type.Metabolites M1 and M2 appeared at 7 DAA in all three soils(Fig.3).They persisted up to 60 DAA in LS and SL and 90 DAA in CL.Metabolite M3 appeared at 15,21,and 30 DAA in LS,SL,and CL,respectively,whereas M4 appeared at 10,15,and 21 DAA in LS,SL,and CL,respectively.Metabolite M3 persisted up to 45 DAA in LS and SL and 60 DAA in CL,whereas M4 persisted up to 21,30,and 45 DAA in LS,SL,and CL,respectively.Representative HPLC chromatograms of metabolites detected at different DAA in LS are shown in Fig.4.The presence of these metabolites was confirmed by MS/MS(Fig.5).Metabolite M1 showed a molecular ion peak at mass/charge(m/z)421.8(M++1)with fragment ions atm/z230.9 and 198.9.Metabolite M2 showed a molecular ion peak atm/z328.7(M++1)with fragment ions atm/z175.1 and 154.6.Metabolite M3 showed a molecular ion peak atm/z254.7(M++1)with fragment ions atm/z238.1.Metabolite M4 showed a molecular ion peak atm/z156.15(M++1)with fragment ions atm/z139.9.
Effect of temperature.
The degradation of halosulfuron methyl increased with increasing temperature,andkvaried from 0.020 to 0.048,0.023 to 0.062,and 0.027 to 0.073 d-1at 5,15,and 25◦C,respectively(Table IV).The DT50of halosulfuron methyl ranged from 14.39 to 33.77, 11.05 to 28.94, and 9.38 to 25.41 d at 5,15,and 25◦C,respectively.The enhancement in degradation with temperature was due to the increase in microbial growth and activity,water solubility,and the rate of abiotic reactions such as oxidation and hydrolysis.Additionally, an increase in temperature decreases the attractive forces between herbicide and soil surface.This will increase desorption, and more herbicide material will be available in soil solution for degradation (Cessnaet al.,2017).A similar effect of temperature on degradation due to increased solubility and decreased adsorption of herbicides was reported by Kalsi and Kaur (2019), Suet al.(2019),Chowdhuryet al.(2021),Kauret al.(2022),and Kaur and Kaur (2022).However, Kaur P and Kaur P (2018), Kauret al.(2018), and Kumar and Singh (2020) observed an increase in the adsorption of pretilachlor, pendimethalin,and sulfosulfuron with increasing temperature, which did not explain the enhancement of dissipation rate.Conversely,enhancement of degradation with increasing temperature in these studies was due to an increase in solubility or the stimulation of microbial activity with an increase in temperature, as some ecological groups dominate within certain temperature range.

Fig.3 Detector responses to the degradation metabolites (M1—M4) of halosulfuron methyl in the days after application (DAA) to three differently textured soils at different application rates(R1—R4).The metabolites were detected using high-pressure liquid chromatography.M1=halosulfuron;M2=methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate;M3=4,6-dimethoxy-2-pyrimidinamine;M4=methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate;LS=loamy sand;SL=sandy loam;CL=clay loam;R1=67.5 g ha-1;R2=135 g ha-1;R3=202.5 g ha-1;R4=270 g ha-1.

Fig.4 High-pressure liquid chromatograms of halosulfuron methyl(HM)and its degradation metabolites(M1—M4)detected at 0,7,15,and 30 d after application(DAA)in loamy sand.CK=control with no HM application;M1=halosulfuron;M2=methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate; M3 = 4,6-dimethoxy-2-pyrimidinamine; M4 = methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate.

Fig.5 Mass spectra of halosulfuron methyl degradation metabolites (M1—M4) and their respective fragment ions (marked with asterisks). m/z =mass/charge; M1 = halosulfuron; M2 = methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl) amino)-1-methyl-1H-pyrazole-4-carboxylate; M3 = 4,6-dimethoxy-2-pyrimidinamine;M4=methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate.
The two parameters,EaandQ10,provide measures for determining the changes inkwith temperature.Increasing the temperature from 5 to 15◦C and from 15 to 25◦C did not increasekfor the degradation of halosulfuron methyl to the same extent (Table IV).A greater rate of degradation was observed when the temperature was increased from 15 to 25◦C than when it was increased from 5 to 15◦C.The averageQ10values for halosulfuron methyl indicated that the rate of degradation increased by 1.1—1.9 folds with 10◦C increase in temperature.SimilarQ10values in the range of 1.3—1.9 have been reported for metachlor,atrazine,and flufenacet(Jaikaewet al.,2017;Marín-Benitoet al.,2019).However,these values are lower than the default value of 2.58 proposed by the Forum for the Co-ordination of Pesticide Fate Models and Their Use.This could be due to variations in soil physicochemical properties including soil hydrology,herbicide structural configuration,experimental conditions(e.g., moisture and temperature), and the subtle interplay between these factors.
The values ofEawere in the ranges of 6.83—13.77,7.87—14.80,and 9.74—16.46kJ mol-1in LS,SL,and CL,respectively(Table V).A lower value ofEaindicates that halosulfuron methyl is more sensitive to temperature(Purnamaet al., 2014; Easonet al., 2022).TheEadecreased in the order of CL>SL>LS,which is related to the clay and OM contents of the soils.Due to the high clay and OM contents of CL,halosulfuron methyl is strongly adsorbed to the organo-minerals of the soil,and comparatively greater energy is required to desorb it from the soil and increase its availability in soil solution for degradation.Similarly,lowEavalues have been reported for clopyralid (5.5—28.4 kJ mol-1)and azoxystrobin(7.48 kJmol-1)by Purnamaet al.(2014),and Šojićet al.(2009).However,previous studies have reported higherEavalues for various sulfonylurea herbicides, including mesosulfuron methyl (79.1 kJ mol-1),metsulfuron methyl(54.7—82.4 kJmol-1),and rimsulfuron(65.1—92.0 kJmol-1)(EFSA,2007;Wang H Zet al.,2010).Additionally,temperature did not affect the degradation of these compounds to the same extent, although they share the same structural functionality.This difference was probably due to the degradation process operating for individual components, which can be either biotic, abiotic, or both,depending on the physicochemical properties of the soil and the experimental and environmental conditions.
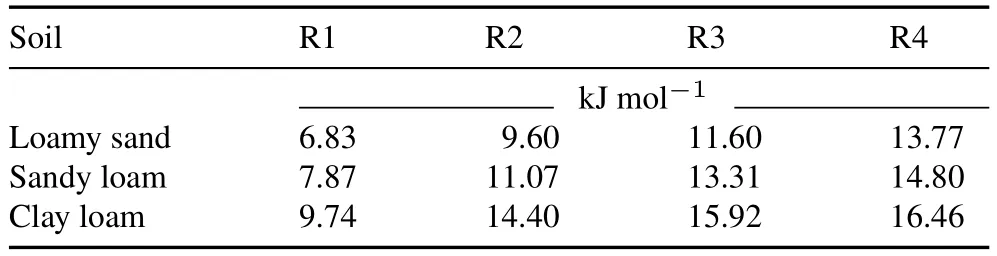
TABLE V Activation energy values for halosulfuron methyl degradation in three differently textured soils at different application rates(R1—R4)a)
Effect of halosulfuron methyl on soil enzymatic and microbial activities
The activities of DH, AlP, AcP, and UA are reliable indicators of soil changes induced by herbicide application.Heat maps showed that application rate, soil type, and temperature exerted variable effects on the activities of DH, AlP, AcP, and UA in the soils (Figs.6and S2, see Supplementary Material for Fig.S2).In the soils with no halosulfuron methyl application (R0), DH, AlP, and AcP activities increased continuously with time and were in the ranges of 10.62—19.79 µg TPFg-1d-1, 21.09—38.14 µg PNP g-1h-1,and 8.27—17.41µg PNP g-1h-1,respectively,at 5◦C.With the application of herbicide, DH, AlP, and AcP activities decreased significantly (P< 0.05) during the first 30 and 45 DAA at application rates of R2 and R3,respectively.However,at the application rate of R4,the initial inhibition in DH,AlP,and AcP activities was observed for 60 DAA.The decrease in DH,AlP,and AcP activities after application of halosulfuron methyl was attributed to its lethal action on soil microorganisms,which in turn affected enzymatic activity(Jyotet al.,2015).After initial inhibition,DH, AlP, and AcP activities increased significantly (P<0.05) in the studied soils owing to a decline in the toxic effect of halosulfuron methyl because of its degradation.Alternatively,it might be due to the adaptation of microbes to the applied herbicide and increase in the capacity of utilizing the herbicide as a carbon source.
Similar effects of halosulfuron methyl application on DH, AlP, and AcP activities were observed at 15 and 25◦C.However,all these activities were comparatively higher at 25◦C, followed by 15 and 5◦C.This might be due to the increase in enzymatic activity caused by the increase in microbial population with increasing temperature.Although DH,AlP,and AcP activities varied significantly(P<0.05)with soil type,they displayed similar trends in all soils after halosulfuron methyl application,which was probably because all the studied soils were alkaline in nature.The highest activities were observed in CL due to its comparatively high organic carbon content,which acts as an energy source for soil microorganisms.

Fig.6 Heatmaps depicting the effects of halosulfuron methyl application at different rates(R0—R4)on dehydrogenase(DH),alkaline phosphatase(AlP),acid phosphatase(AcP),and urease(UA)activities at different days after application during the 120-d incubation in three differently textured soils,loamy sand(LS),sandy loam(SL),and clay loam(CL).The color codes indicate the magnitude of effects.R0=0 g ha-1;R1=67.5 g ha-1;R2=135 g ha-1;R3=202.5 g ha-1;R4=270 g ha-1.
Most reports on the effects of herbicides on DH and AlP activities in soil are in agreement with our results,where transitory effects on these activities have been observed after the application of herbicides,depending upon the type and amount of herbicide, exposure time, and the intrinsic properties of soils(Daset al.,2017;Ataikiruet al.,2019).However, Singh and Ghoshal (2013) and Tomkielet al.(2019)observed stimulatory effects of butachlor and flufenacet on DH and AlP activities, whereas Sahuet al.(2019)and Zhanget al.(2014)observed that the application of chlorantraniliprole and fomesafen initially increased and then decreased DH and AlP activities in herbicide-treated soils.In contrast,Heet al.(2007)reported that herbicides had no significant influence on soil enzymatic activities.The variability in the results is because the observed response is a result of numerous factors,and microbial community structure and soil type and conditions contribute to divergent research findings.
The AlP activity was higher than AcP activity(Figs.6and S2).This was probably because the studied soils were alkaline(pH 8—9)in nature,resulting in the predominance of AlP.These results were consistent with those previously reported by Greaveset al.(1976)for 2-methyl-4-chlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid, suggesting that alkaline soils predominantly contain AlP.Urease activity(2.85—2.97µg NH+4g-1min-1)was not significantly(P>0.05)affected by halosulfuron methyl application(Fig.6),likely because UA is an extracellular enzyme bound to organic and inorganic colloids and does not mediate halosulfuron methyl degradation(Baruah and Mishra,1986;Gianfredaet al.,1993).
Our results suggest that halosulfuron methyl application disturbs soil homeostasis by interfering with the metabolic profile of the soil, which affects soil respiration and the activities of enzymes that participate in the transformation of carbon and phosphorous.Both DH and AlP activities can be considered as sensitive bioindicators capable of describing the response of soil microorganisms to halosulfuron methyl.The inhibitory effect of herbicides on soil enzymatic activities decreases with time as the soil ecosystem becomes stable after exposure to environmental stressors, owing to the formation of an adequate defense mechanism responsible for maintaining the biological equilibrium of the soil.
Different microbial populations, including bacteria,fungi, actinobacteria, and PSB, were examined.Microbial activity increased with time in the control soils.The abundances of bacteria,fungi,actinobacteria,and PSB based on CFU values initially decreased in the first 30 and 45 DAA at the application rates of R2 and R3,respectively,and in the first 60 DAA at the application rate of R4(Figs.7 and S3,see Supplementary Material for Fig.S3)due to the toxic effect of herbicides on soil microorganisms,which reduces their abundance and activity(Priyaet al.,2017).After the initial inhibition, microbial counts increased significantly(P<0.05)due to the adaptation of microbes to halosulfuron methyl or its degradation by soil microbes.
The overall microbial counts of bacteria, fungi, actinobacteria,and PSB were in the ranges of 99—220,80—189,53—199,and 18—102×104CFU g-1,respectively(Fig.S3),indicating the rapid emergence of bacterial colonies as compared to fungi,actinobacteria,and PSB.The results of PSB were corroborated by the AlP activity in soil.Similar effects of halosulfuron methyl application on microbial populations were observed at the other temperatures tested,but microbial populations were comparatively higher at 25◦C,followed by 15 and 5◦C.The microbial populations were the highest in CL.

Fig.7 Heatmaps depicting the effects of halosulfuron methyl application at different rates(R0—R4)on the abundances of bacteria(Bac),fungi(Fun),actinobacteria(AcB),and phosphate-solubilizing bacteria(PSB)at different days after application during the 120-d incubation in three differently textured soils,loamy sand(LS),sandy loam(SL),and clay loam(CL).The color codes indicate the magnitude of effects.R0=0 g ha-1;R1=67.5 g ha-1;R2=135 g ha-1;R3=202.5 g ha-1;R4=270 g ha-1.
The observed results were consistent with those reported in the literature.Tomkielet al.(2019)and Baćmagaet al.(2015) reported that soil contaminated with flufenacet +isoxaflutole at 0.25—160 mg kg-1and diflufenican+mesosulfuron + iodosulfuron mixture at 9.12—36.48 mg kg-1altered the ecophysiological diversity of soil bacteria,fungi,and actinobacteria due to chemical stress on the soil,which exerts an initial toxic effect on soil microbes.The initial inhibitory effects of atrazine,glyphosate(Bonfleuret al.,2015),paraquat,and atrazine on soil-dwelling microorganisms were also confirmed by Sebiomoet al.(2011) and Kucharski and Wyszkowska(2008).In contrast,mesotrione(Pose-Juan
During the inhibition period, herbicide residue was significantly(P<0.05)positively correlated with the activities of DH,AlP,and AcP and the abundances of bacteria,fungi,actinobacteria,and PSB(data not shown),indicating decrease in enzymatic and microbial activities as herbicide degradation occurred.However,after the initial inhibition,herbicide residue was significantly(P<0.05)negatively correlated with the activities of DH,AlP,and AcP and the abundances of bacteria, fungi, actinobacteria, and PSB,indicating that herbicide residue at 0.008—0.011µg g-1was non-toxic to soil enzymes and microbes(Pose-Juanet al.,2015).Previous studies by Carpioet al.(2020), García-Delgadoet al.(2019), Barbaet al.(2019), and Jones and Ananyeva(2001)also reported a positive correlation between herbicide residue and soil enzymatic and microbial activities.
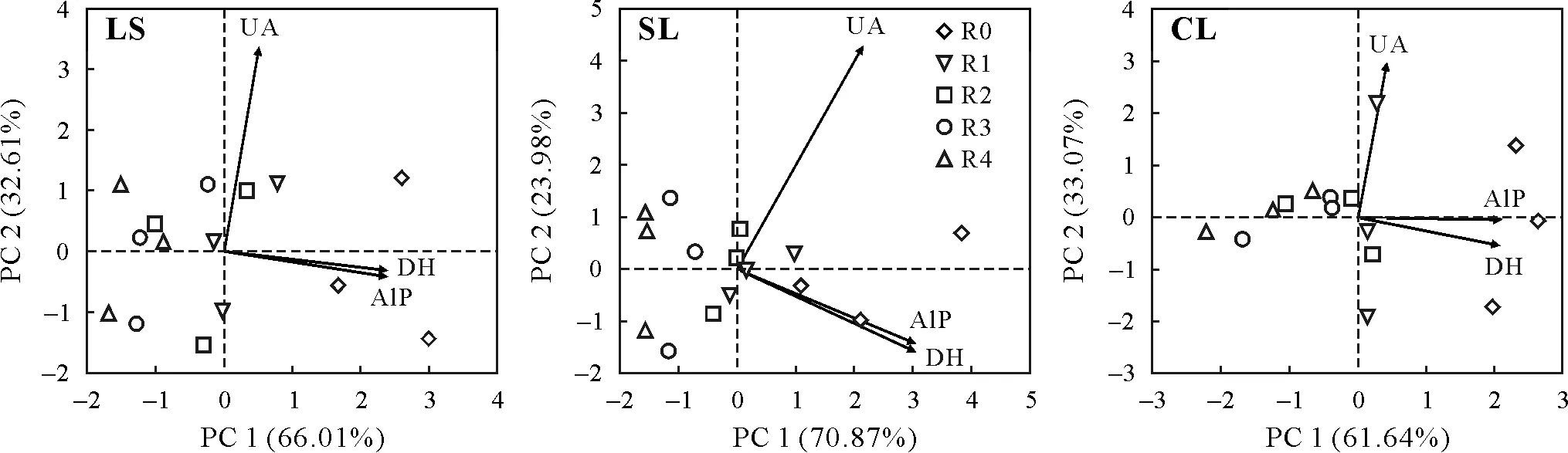
Changes in soil enzymatic and microbial activities in response to herbicide application were studied using PCA.The resistance of soil enzymes and microbes to halosulfuron methyl contamination confirmed the significant impact of the examined factors.The first(PC1)and second principal components(PC2)explained 61.64%—70.87%and 23.98%—33.07%of the total variance in soil enzyme activities in the three soils,respectively(Fig.8).The PC1 and PC2 explained 90.16%—93.27%and 3.32%—5.43%of the total variance in microbial populations in the three soils,respectively(Fig.9).The loading values of DH,AlP,and UA activities were 0.979,0.965,and 0.031,respectively,which showed that DH andAlP activities were the major contributors to the relationship between enzymatic activity and herbicide application rate(Fig.8).The loading values of bacteria,fungi,actinobacteria,and PSB were 0.983,0.102,0.975,and 0.811,respectively,indicating that bacteria,actinobacteria,and PSB were the major contributors to the relationship between microbial activity and herbicide application rate(Fig.9).Similar but not identical representations were observed at all studied temperatures.
CONCLUSIONS
Halosulfuron methyl degradation followed first-order kinetics,and degradation rate was influenced by soil physicochemical properties, application rate, and temperature.Faster degradation was observed in LS, followed by SL and CL.Increased temperature had a positive effect on degradation.The appearance and persistence of halosulfuron methyl metabolites varied with soil type,and halosulfuron, methyl 3-chloro-5-((4,6-dimethoxy-2-pyrimidinyl)amino)-1-methyl-1H-pyrazole-4-carboxylate,4,6-dimethoxy-2-pyrimidinamine,and methyl 3-chloro-1-methyl-5-sulfamoyl-1H-pyrazole-4-carboxylate metabolites were detected in the studied soils.The application of halosulfuron methyl showed short-lived transitory effects on DH,AlP,and AcP activities and soil microbial activity,whereas UA remained almost stable at all herbicide application rates.These results suggest that judicious use of herbicides will not disrupt the existing balance of soil biological processes,which otherwise may potentially alter the reaction chains pertinent to vital soil processes.However,the validity of this study needs to be ascertained through field experiments.
ACKNOWLEDGEMENT
The authors are thankful to the Head of the Department of Agronomy and the Head of the Department of Chemistry,Punjab Agricultural University,Ludhiana,India for providing research facilities.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
- Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
- Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil
