Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
2023-12-21YosefSTEINBERGERDorsafKERFAHITirzaDONIGERChenSHERMANItaiiAPPLEBAUMandGilESHEL
Yosef STEINBERGER,Dorsaf KERFAHI,Tirza DONIGERChen SHERMANItaii APPLEBAUM and Gil ESHEL
1The Mina&Everard Goodman Facultyof Life Sciences,Bar-Ilan University,Ramat Gan 5290002(Israel)
2School of Natural Sciences,Department of Biological Sciences,Keimyung University,Daegu 42601(Korea)
3Soil Erosion Research Station,Ministryof Agriculture&Rural Development,P.O.Box30,Beit-Dagan 5020000(Israel)
ABSTRACT Soil biota play a crucial role in soil ecosystem stability,promoting organic matter decomposition and nutrient cycling.Compared to conventional farming,organic farming is known to improve soil properties such as aggregation.Despite the importance of soil microbial communities in soil biogeochemical processes,our knowledge of their dynamics is rudimentary,especially under different agricultural management practices.Here we studied the effects of vineyard management practices(conventional and organic)and soil aggregate fractions(micro-,meso-,and macroaggregates)on free-living soil nematodes.The abundance,diversity,and ecological indices,such as the Wasilewska index and trophic diversity,of free-living soil nematodes were determined.We found that the abundance of free-living soil nematodes was increased by organic farming.In addition,plant parasites were found to increase in macroaggregates in the organic plot,which may be attributed to the weeds present due to no-tillage and no herbicides.Nematode family network connectivity increased in complexity with increasing aggregate size,highlighting the importance of the interplay between nematodes and soil inter-aggregate pore size and connectivity.
KeyWords: aggregate size,agroecosystem,ecological index,network connectivity,soil habitat,tillage,trophic group
INTRODUCTION
Several studies on the alteration of vineyards have shown changes in soil physical,chemical,and biotic composition under different agro-management conditions (Couloumaet al., 2006; Probset al., 2008; Collet al., 2011).Such changes affect the structural composition and stability of soil aggregates(Benito and Diaz-Fierros,1992;Golchinet al.,1995;Ladoet al.,2004).Soil particle aggregation plays a vital role in soil structure and significantly influences soil biotic and abiotic components.The interaction and feedback between soil biotic and abiotic components may determine the role of soil in supporting the productivity of croplands and other land use types, such as vineyards.The physical and chemical properties of soil aggregates have been studied intensively by Martinet al.(1955),Greenland(1965a,b),Harriset al.(1966),and Sixet al.(2000),who elucidated the relationships between aggregate stability,pore size,and clay content.Agricultural practices, especially tillage, greatly influence soil organic matter(SOM)content and turnover,soil aggregate morphology,and aggregate stability(Diacono and Montemurro,2011;Zhanget al.,2014).Any changes in agricultural practices can therefore affect soil aggregate size distribution, providing a heterogeneous microhabitat that may facilitate interactions between biota communities.Alteration in soil communities mainly results from temporal changes in soil physical and chemical properties caused by mechanical tillage (Lenz and Eisenbeis, 1998; Neher,1999; Yeates and Bongers, 1999; Garcıa-Álvarezet al.,2004; Tenuta and Ferris, 2004; Donget al., 2008; Pen-Mouratovet al.,2008).In vineyards,management type has been shown to affect the structure,dimension,size,and shape of inter-aggregate pores(Yılmazet al.,2019).The stability of soil aggregates during wet and dry cycles reflects the local microenvironmental conditions,which influence soil susceptibility to erosion,soil aeration,water infiltration,and nutrient availability(Lehmanet al.,2015).As a result,soil microhabitat affects the composition,occurrence,diversity,and functionality of soil biota(Bachet al.,2018).
Free-living soil nematodes comprise a large part of the soil biota of agro-ecosystems in terms of numbers,potential efficiency,and influences on the system(Neheret al.,2012).They play beneficial roles in soil by serving as biological pest control agents and regulating the natural ecosystem and soil nutrient cycling(Yadavet al.,2019).Because of their range of trophic guilds,they reflect changes in the bacteria and fungi they consume and are strongly related to the soil physical and chemical environment.Thus,free-living soil nematodes have been suggested as bioindicators of soil health(Huntet al.,1987;Neher,2001;Lianget al.,2005;Luet al.,2020).Their presence,relative dominance,high diversity,relatively simple extraction, and life strategies (Bongers,1990; Yeateset al., 1993; Ferriset al., 2001; Ferris and Bongers,2006)are of great importance in relevant fields as bioindicators that respond relatively rapidly to variations in resource diversity and availability(Neher,2001).However,little information is currently available on how free-living soil nematode communities are affected by soil aggregate characteristics and agricultural management practices(Ferris and McKenry,1976;Pinkertonet al.,1999;Griffithset al.,2012;Howlandet al.,2014).
Although many studies have reported the effects of vineyard management practices on the soil free-living nematode community and composition,the patterns of nematode abundance, diversity, trophic composition, and distribution in different soil aggregate fractions and variations as a result of management have been poorly studied.The present study aimed to better understand the effects of different sizes of soil aggregates under different vineyard management practices on the soil free-living nematode community structure and distribution.Specifically,the present study aimed to further understand the effects of two different long-term agricultural management practices(conventional and organic)and soil aggregate sizes on nematode community composition and structure.In addition, the soil free-living food web composition was assessed to evaluate how aggregate sizes and management regimes affected the validity of free-living soil nematodes as bioindicators.Four hypotheses were tested in this study.First,long-term organic agricultural management practices that include full soil cover with herbaceous vegetation will positively affect soil aggregation,which will in turn positively affect the abundance and diversity of free-living soil nematodes as a result of increased resource availability.Second,larger soil aggregate size will lead to higher abundance and diversity of free-living nematodes.Third, vineyard management practices and soil sampling location will affect the abundance and diversity of free-living nematodes and the trophic composition of the community.Fourth,the abundance and diversity of soil free-living nematodes will increase with increasing soil aggregate size,leading to higher network complexity.
MATERIALS AND METHODS
Vineyard management,soil sampling,and aggregate fractionation
The two adjacent vineyard plots, one organically managed(ORG)and the other conventionally managed(CON),were each about one hectare in area and located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel(32◦32′12′′N,34◦57′11′′E).The ORG plot is a Carignan vineyard.It has been planted since 1978 and managed based on biodynamic/organic principles since 2013, with no herbicide or tillage for weed management.The weeds in the vineyard are mowed approximately three times a year during the dry season.For plant protection, a combination of biological means and organic certified pesticide and fungicide materials are used.Since 2013,almost every year,homemade compost amended with biodynamic additives has been applied to the vine rows at a rate of 10 L vine-1.The last application of compost before soil sampling was in November 2018.In June 2019, approximately 60 L of red worm humus liquid (“Liquid Worm Juice”) was applied per plot through a dripping system.In the CON plot,weeds are controlled with herbicides twice a year combined with shallow tillage(cultivator)in the spring.As a result,95%—99%of the vineyard floor is bare and free of any herbaceous vegetation.Pests and diseases are controlled using a combination of biological means (mating disruption) and commercial pesticides and fungicides as needed.
The soil in the vineyard plots is a fluvial Vertisol.Fivepoint composite topsoil(0—10 cm)samples(approximately 500 g)were collected between(BR)and within vine rows(WR)in the autumn of 2019.Three replicates were collected for each location and plot.All the collected soil samples were transported to the laboratory in an isolated box to prevent overheating.
The soil samples were sieved in the field to obtain the aggregate fractions of microaggregates(MIA,<500µm),mesoaggregates(MEA,500—1 000µm),and macroaggregates(MAA,1 000—8 000µm)using a set of three sieves(0.5, 1, and 8 mm) according to Jianget al.(2018) with modification.Soil moisture(SM),SOM,and soil pH were determined as described by Fitoussiet al.(2016).
Soil free-living nematode community determination and ecological indexcalculation
To determine the community composition of soil freeliving soil nematodes, 100 g of each soil aggregate size fraction was used for nematode extraction following Cairns(1960).The recovered nematodes were transferred to an Eppendorf tube before being centrifuged for 10 min at 15 000 r min-1to reduce the remaining water.The pellets were used for DNA extraction using a PureLink®Genomic DNA mini kit.
The eluted DNA was stored at-20◦C until being used as template in the polymerase chain reaction(PCR)amplification of the 3’part of the small subunit(SSU)ribosomal DNA (rDNA).The forward primer NF1 (5′-GGTGGTGCATGGCCGTTCTTAGTT-3′)and reverse primer 18Sr2b(5′-TACAAAGGGCAGGGACGTAAT-3′)were used for PCR in a 25-µL final volume including 9.5 µL ultrapure water, 12.5 µL PCRBIO HS Taq Mix Red, 1 µL forward primer,1µL reverse primer,and 1µL DNA template.The PCR program consisted of an initial denaturation step at 98◦C for 30 s,20 cycles of denaturation at 98◦C for 10 s,annealing at 58◦C for 30 s, and extension at 72◦C for 1.5 min,and a final extension step at 72◦C for 10 min.
All PCR products were subjected to Illumina™sequencing.The recovered DNA sequences were processed in QIIME version 1.7.0(Caporasoet al.,2010),using the pipeline for analyzing SSU rDNA sequence data.Default settings and the UCLUST algorithm were used forde novoselection of operational taxonomic units (OTUs) at 97%similarity.The Silva 111 release(Yilmazet al.,2014)was used as a reference for taxonomic assignment of OTUs.As the Silva 111 database contains only an essential number of nematode reads,we sought to reclassify the reads assigned to Nematoda in the QIIME classification by BLAST similarity analysis using a custom-made nematode 18S rDNA sequence database.The database allowed a better insight into the nematode community.All measurements were assessed using data for OTUs with at least five reads in the overall dataset.
The characteristics of the nematode communities were described with ecological indices,including nematode density,relative abundances of omnivores-predators(OP),plantparasitic (PP), fungal-feeding (FF), and bacterial-feeding nematodes(BF)(Yeateset al.,1993),the FF/BFratio(Twinn,1974),Wasilewska index(WI)(Wasilewska,1994),trophic diversity (TD) (Heipet al., 1988; Neher, 2001), the nematode channel ratio(NCR)(Yeates,2003),and Simpson’s dominance indexλ(Simpson,1949).Of these indices,WI,TD,NCR,andλwere calculated as follows:
where FF,BF,and PP are the relative abundances of FF,BF,and PP nematodes,respectively.
wherePiis the proportion of individuals in theith trophic group(Heipet al.,1988;Neher,2001).
Statistical analyses
The data obtained were subjected to analysis of variance(ANOVA) using the SAS software, followed by Tukey’s honest significant difference test to establish the significance of differences between sampling locations in Statistica 4.3 software.Differences were considered statistically significant atP< 0.05.Principal coordinate analysis (PCoA) and canonical correspondence analysis(CCA)were performed using the ampvis2 R package(Andersenet al.,2018).Plots were generated using the ggplot2 R package.
Network analysis was performed using the R software to understand the relationships between the different nematode families detected(Faust and Raes,2012).Though all possible Spearman’s rank correlation coefficients were calculated,only correlations withr>0.5 andP<0.05 were selected to build networks.Network topology was described by average clustering coefficients, average path length, and modularity.The interactive platform Gephi was used to explore and visualize network structure.A thousand random networks of equal size (the same numbers of nodes and edges)were generated as each of the original networks,and their topological properties were compared with the observed network to determine whether the generated networks were random or not.
RESULTS
Soil properties
The SM contents of MIA,MEA,and MAA were 93—144,88—142,and 91—141 g kg-1,respectively(Fig.1).No significant differences in SM were found between aggregate fractions in each plot,but SM of WR was significantly higher than that of BR (P< 0.05).Soil organic matter contents of MIA and MAA were 40 and 38 g kg-1, respectively,in CON,and 56 and 62 g kg-1,respectively,in ORG.No significant differences were found between management practices,sampling locations,and aggregate fractions.No significant differences in soil pH were observed between the three aggregate fractions in each plot.In addition,the pH values were not significantly different between the two sampling locations.
Densityand relative abundances of nematode families and trophic groups
Soil aggregate size significantly affected the density of free-living nematodes in the two vineyard plots(Fig.2).The density of free-living nematodes increased with increasing aggregate size,from a minimum of 132 individuals kg-1in MIA collected from BR in CON to 5 864 individuals kg-1in MAA collected from ORG.The density of free-living nematodes increased 16- and 17-fold in WR and BR in CON,respectively,and it increased 7-and 3-fold in WR and BR of ORG,respectively.The densities of free-living soil nematodes were similar between MIA and MAA fractions in BR of CON,whereas a 6-fold increase was obtained in the MAA faction compared to the MIA fraction in ORG.

Fig.1 Boxplots of moisture contents,organic matter contents,and pH values of aggregate fractions of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm);ns=not significant.The asterisks(*)indicate significant differences at P <0.05.
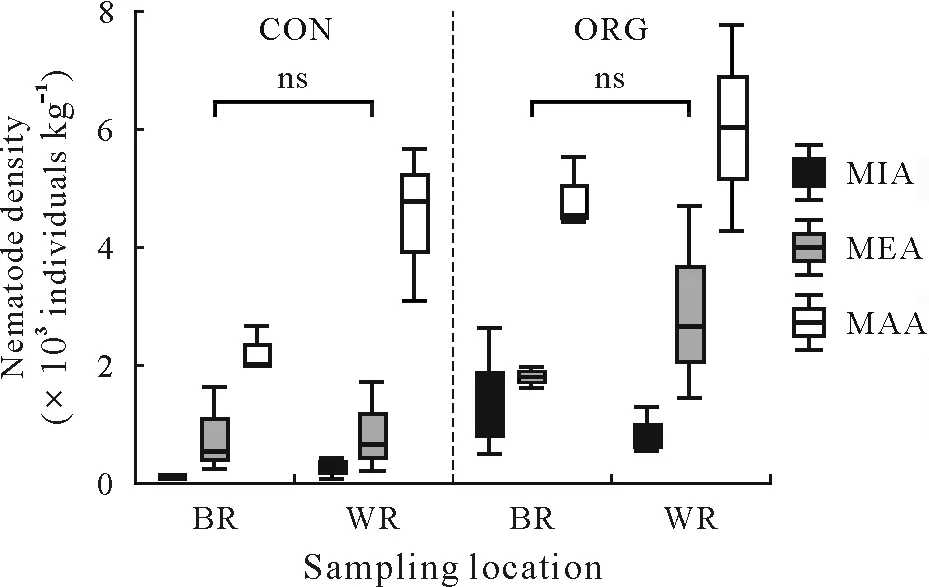
Fig.2 Boxplots of nematode density in different aggregate fractions of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm);ns=not significant.
A total of 652 928 quality-filtered sequences were obtained and clustered into 464 OTUs at 97%similarity.Lowabundance OTUs(<0.01%of the total reads in the database)were eliminated,yielding 226 OTUs.Based on these OTUs,a total of 16 families of soil free-living nematodes were identified.Significant differences in free-living soil nematode families were found between aggregate fractions,sampling locations, and vineyard management practices (Table I).Two unique families,Cephalobidae,which are BF,and Dorylaimoidea, which are PP, were present at high relative abundances in all soil samples regardless of aggregate size.Anguinidae, which are PP, were present at a low relative abundance in WR,without any preference for aggregate size.Aphelenchidae, which are FF, were found in all samples and aggregate sizes.Belondiroidea were found only in WR of ORG.Neodiplogasteridae and Rhabdolaimidae, which are OP and BF, respectively, were present in low relative abundances in CON only.None of the 16 families showed preference for aggregate size.
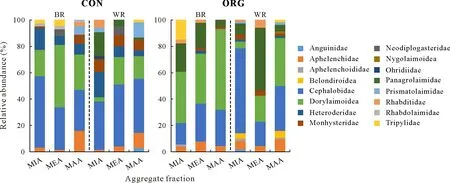
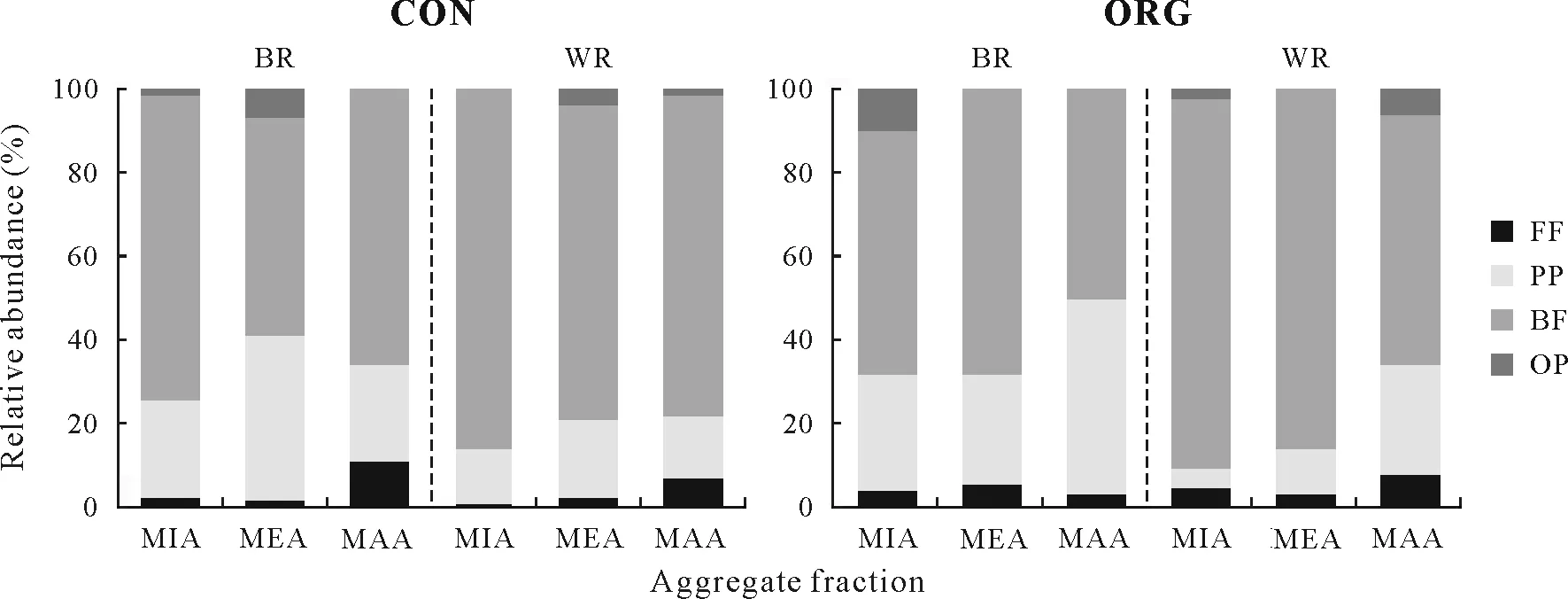
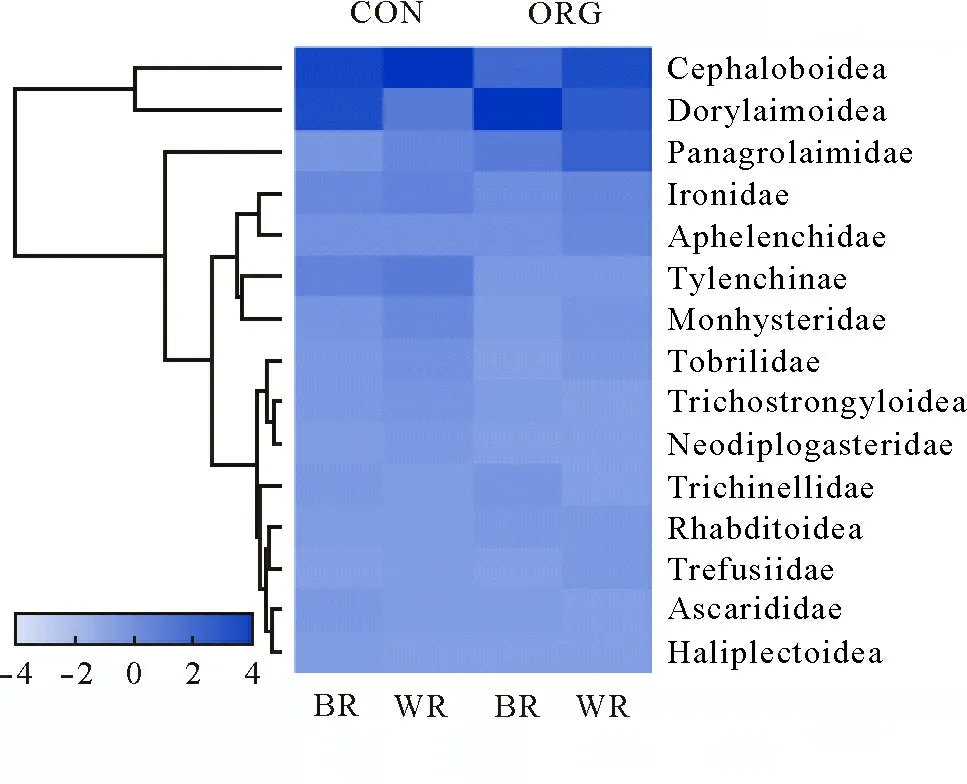
The taxonomic classification of soil free-living nematodes showed a high diversity (Fig.3).Cephalobidae and Dorylaimoidea reached high relative abundances in both plots and sampling locations.The relative abundances of trophic groups revealed that BFwas the main trophic group present in WR and BR of both plots,whereas OP was only found in some samples, without any correlation with aggregate fraction or sampling location(Fig.4).The heatmap constructed for the major OTUs at the family level showed a close relation between Cephalobidae and Dorylaimoidea(Fig.5).
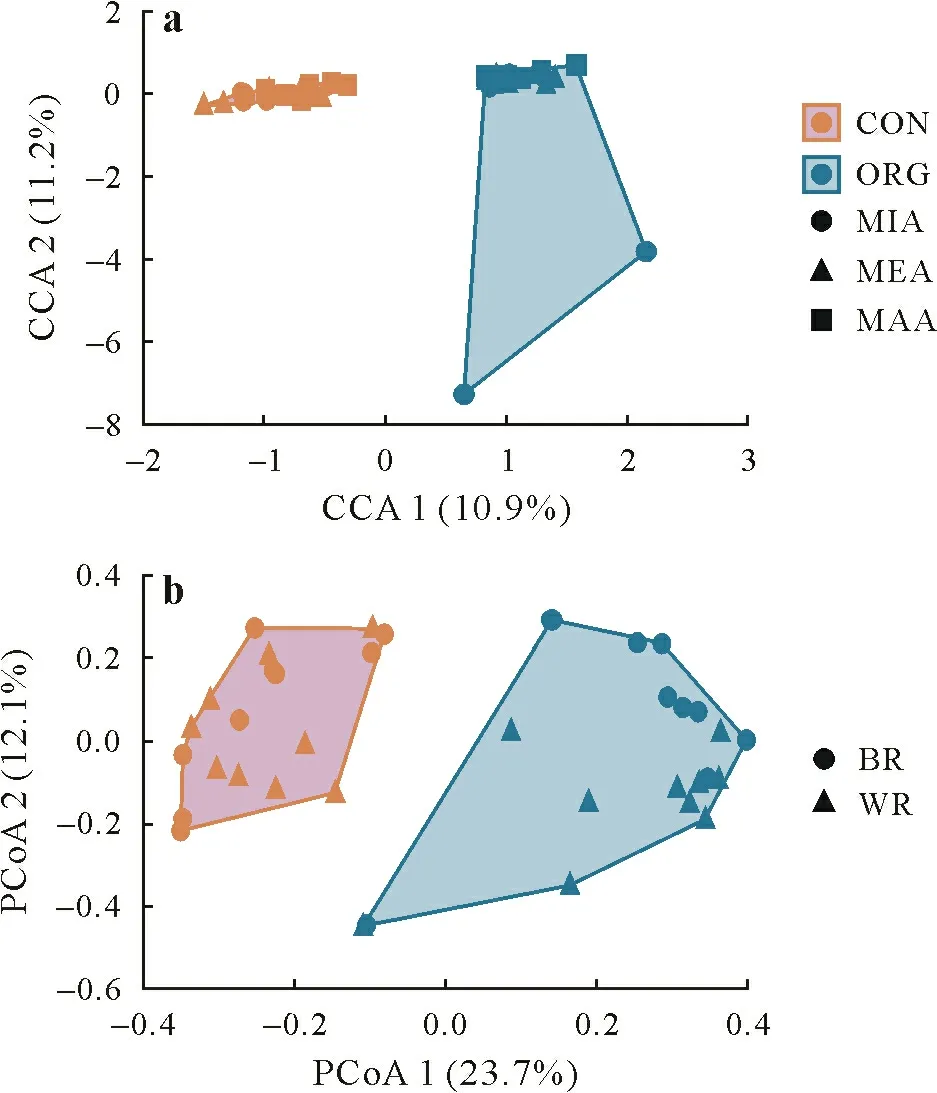
The CCA analysis(Fig.6a)showed that management practice had a stronger effect on the nematode communities than aggregate size.The PCoA analysis (Fig.6b), confirmed that the nematode communities were more affected by management practice than by aggregate size.Distance matrix based on Bray-Curtis similarity suggests a difference between the plots on one hand,and overlap-similarity on the other hand.The main difference was between the CON and ORG plots.
Nematode ecological indices
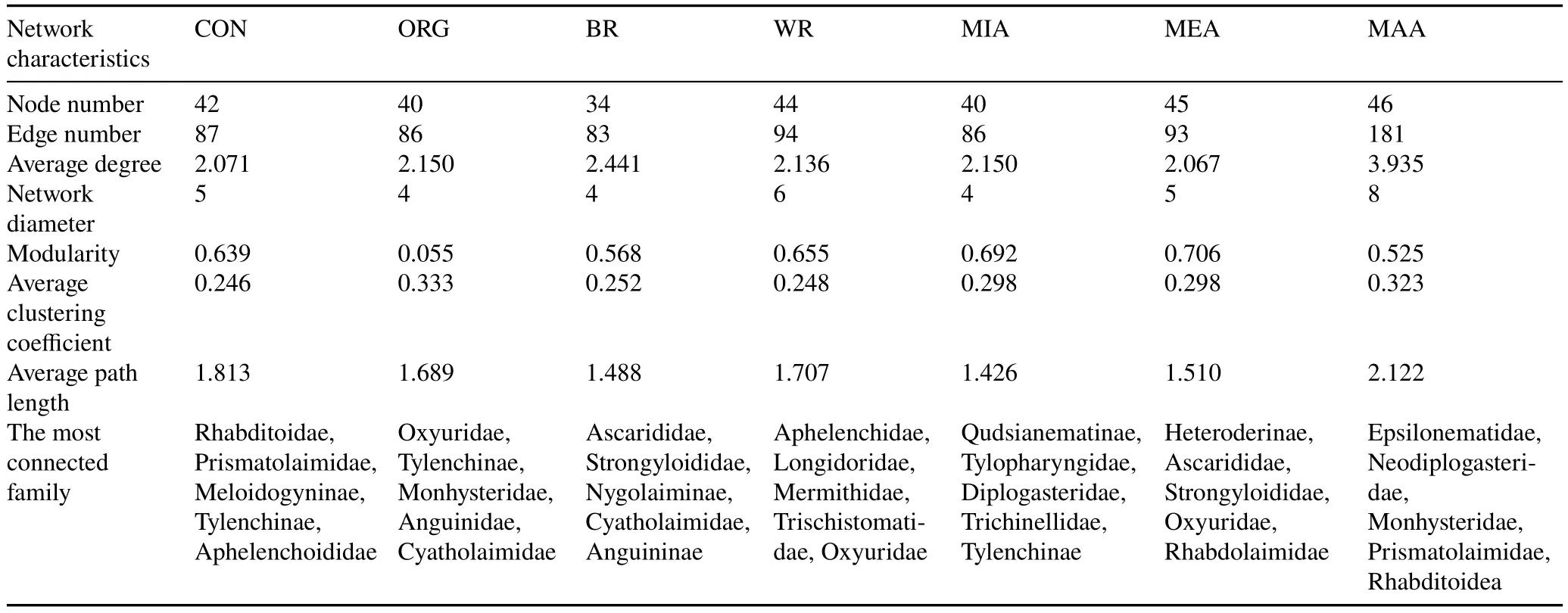
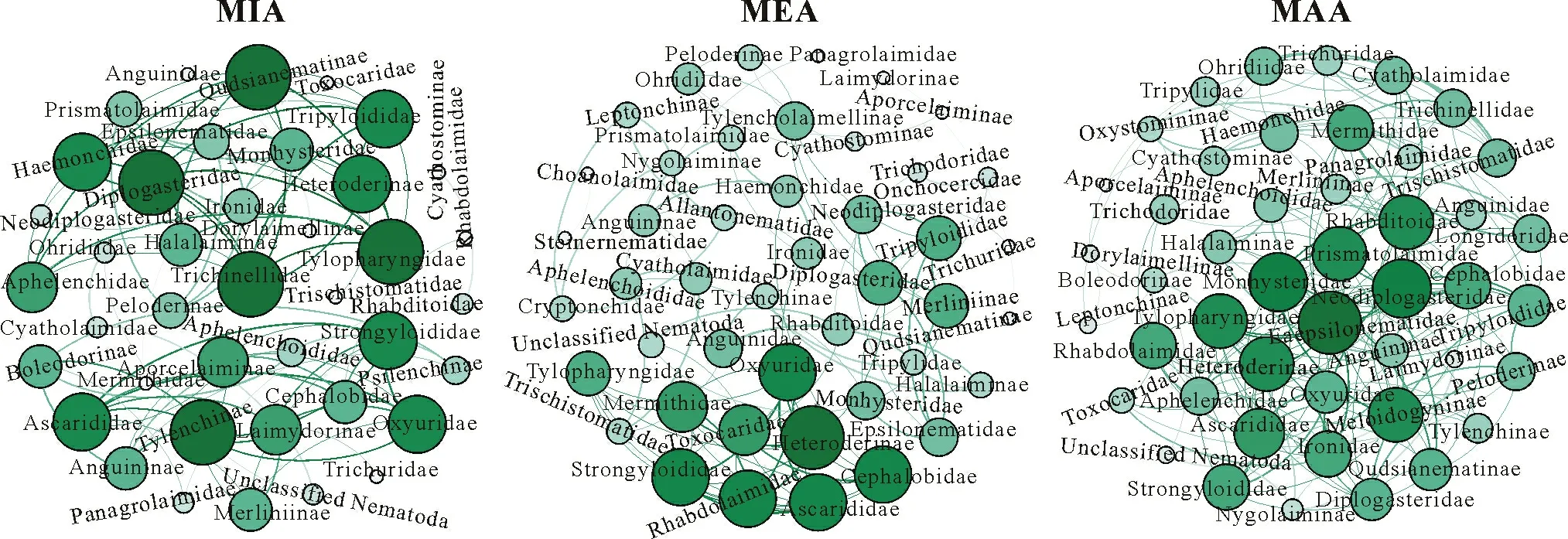
Soil free-living nematode ecological indices were determined and are listed in Table II.The FF/BFratio decreased with decreasing aggregate size(MAA>MIA)in CON,where BFincreased with decreasing aggregate size in both WR and BR.The WI index increased with decreasing aggregate size(MAA TABLE I Relative abundancesa)of free-living nematode families in different aggregate fractionsb)of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel Fig.3 Relative abundances of free-living nematode families in different aggregate fractions of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm). We conducted a correlation-based network analysis to study the relationship between nematode families in different aggregate fractions collected from WR and BR of the two vineyards.The nematode families from the two differently managed vineyards did not show a difference in their connectivity(Fig.7,Table III).The two networks were similar in complexity,with 42 nodes and 87 edges for CON and 46 nodes and 86 edges for ORG.Rhabditoidea and Oxyuridae showed the highest number of correlations with other families in CON and ORG,respectively(Table III).A slightly higher number of correlations between nematode families was found in samples from WR (44 nodes and 94 edges)compared with BR(34 nodes and 83 edges)(Fig.8).For the nematode correlations between different aggregate fractions,the highest number of correlations was found in MAA(46 nodes and 181 edges),followed by MEA and MIA(Fig.9).The nematode families that presented the highest number of correlations with the other nematode families are shown in Table III. Fig.4 Relative abundances of nematode trophic groups in different aggregate fractions of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm);FF=fungal-feeding nematodes;PP=plant-parasitic nematodes;BF=bacterial-feeding nematodes;OP=omnivores-predators. Fig.5 Heatmap of the major operational taxonomic units of free-living nematodes at the family level of soil samples from between(BR)and within vine rows (WR) in the conventionally (CON) and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel. The main driving force of soil aggregate formation is soil biota(Sixet al.,2004),combined with plant roots,inorganic binding agents,and abiotic components(Bronick and Lal,2005).The influence of these factors on soil particle aggregation and the interplay between them is one of the leading forces determining soil biota composition.The sources of SOM are diverse.They can be plants, root and soil biota residues,root and soil biota exudates,and external inputs,such as compost or manure.All these sources are affected by the combination of climatic conditions and anthropogenic activities.The colonization of soil aggregates by bacteria and fungi as primary consumers of SOM magnetizes free-living soil nematodes, which are among the most common consumers in soil biota,with a marked ability to move through the pores in and between aggregates.Due to the inconsistency in aggregate shape and size,soil biota communities are not expected to be evenly and homogeneously distributed within the soil. Fig.6 Canonical correspondence analysis(CCA)(a)and principal coordinate analysis(PCoA)(b)showing the effects of management practice,sampling location,and aggregate fraction on free-living nematodes at the family level of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm). The present study found that vineyard management prac-tice affects aggregate formation,ultimately leading to different community and trophic compositions, as reported in previous studies (Wardleet al., 2004; Darbyet al., 2010;Cesarzet al., 2013).The abundance and diversity of soil free-living nematodes were affected by vineyard management practice,aggregate size,and sampling location.These results supported our hypotheses regarding the important influence of agricultural management, soil aggregation, and sampling location on soil nematode community.Our results also showed that nematode abundance and trophic groups are affected by soil aggregate size and agricultural management.These findings mirror those of Zhanget al.(2019), who found differences in the abundance and trophic composition of soil nematodes in different soil aggregate fractions and differently managed plots. TABLE II Ecological indices of free-living nematodes in different aggregate fractionsa) of soil samples from between (BR) and within vine rows (WR) in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel Fig.7 Network analysis of free-living nematode families of soil samples from the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.The edges stand for strong(Spearman’s correlation coefficient r >0.8)and significant(P <0.01)correlations.The size and darkness of each node are proportional to the number of correlations. However, our results are not in agreement with those of Goldammer(2018),who reported that agricultural management is a critical factor in controlling pest problems in vineyards.The two most common PP nematode families in the soil samples were Dorylaimoidae and Heteroderidae.Dorylaimoidae are persisters(K-strategists)(Bongerset al.,1997),and they were present in all samples,whereas Heteroderidae were present in lower numbers or were missing in the aggregate samples collected from ORG.Moreover,the WI index,which is based on the ratio of the total BFand FF to PP,indicates the efficiency of conventional management in controlling the PP population.Cephalobiadae, which are BFand general opportunistic nematodes, were found in large numbers in all aggregates without any preference for aggregate size and management (Yeates, 2003).Briaret al.(2012) did not find consistent differences between organic and conventional management for nematode trophicabundance in their study on the response of soil free-living nematodes to crop management.Moreover,they showed a high abundance of FFin their samples, which is contrary to our findings.The primary FFidentified in this study belonged to Aphelenchidae(colonizers)(Yeates and Bongers,1999),which are known to be present in the advanced stages of decomposition.This family was found in most samples,without any preference for aggregate size and management.Crop management also affected nematode community abundance,composition,and diversity.The changes in trophic composition yield moderated changes in the different indices,thus explaining the importance of the responses of BF and FF,opportunistic nematode components(colonizers),to changes in management.Soil aggregate fractionation was found to be a very useful tool for differentiating between management practices. TABLE III Characteristics of the networks of free-living nematode families in different aggregate fractionsa) of soil samples from between(BR)and within vine rows(WR)in the conventionally(CON)and organically managed vineyard plots(ORG)located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel Fig.8 Network analysis of free-living nematode families of soil from between(BR)and within vine rows(WR)in the vineyards located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.The edges stand for strong(Spearman’s correlation coefficient r >0.8)and significant(P <0.01)correlations.The size and darkness of each node are proportional to the number of correlations. Fig.9 Network analysis of free-living nematode families in different aggregate fractions of soil samples from the vineyards located near Shuni Park,Binyamina,in the northern part of the coastal plain of Israel.MIA=microaggregates(<500µm);MEA=mesoaggregates(500—1 000µm);MAA=macroaggregates(1 000—8 000µm).The edges stand for strong(Spearman’s correlation coefficient r >0.8)and significant(P <0.01)correlations.The size and darkness of each node are proportional to the number of correlations. Co-occurrence patterns of microorganisms offer a useful tool for assessing soil ecosystem functions by understanding microbial community functions and their potential interactions with each other and with the environment,in addition to revealing the niche structure in the soil(Maet al.,2016,2018; Banerjeeet al., 2018).Soil aggregate size affected nematode connectivity(Fig.4).In the association networks based on the correlation between nematode families and soil aggregate fractions,network patterns were different between aggregate fractions(Table III),with MIA having the lowest number of correlations compared to MAA and MEA.Our results mirror the findings of Jianget al.(2015),who found that aggregate fractions affected the connectivity of nematodes,with the average connectivity complexity increasing with increasing aggregate size.We found that the total number of free-living nematodes increased with aggregate size(Fig.2).Therefore,the distribution of soil nematodes is likely controlled by the aggregate size and availability of resources associated with different soil structures(Neher,2010).Tisdall and Oades(1982)showed that plant roots and fungal hyphae stabilize MAA by supplying decomposable organic matter to the soil and supporting large microbial populations(including nematodes)by providing more feeding sites.It was also shown that BFand PP are more common in MAA than in MIA,suggesting that pore spaces in the MAA are more suitable for the survival of nematodes (Jianget al.,2013;Zhanget al.,2013).Although network analysis allows evaluating the interactions between nematodes,it remains a theoretical framework.Thus,there is a need for experimental evidence and further exploration of how nematodes interact with other taxa and the overall environments.Our results indicate the importance of soil aggregate size in shaping nematode abundance and networks. CONCLUSIONS Organic vineyard management tends to improve soil properties compared to conventional vineyard management(Collet al., 2012).Our study showed differences in the abundance of nematode communities and trophic groups in differently managed vineyard plots and different soil aggregate fractions.Soil free-living nematodes increased in abundance and diversity in the organically managed vineyard plot,probably because of the presence of weeds.In addition,nematode connectivity became more complex with increasing soil aggregate sizes.Further studies using soil free-living nematodes as bioindicators of soil processes should focus on the effects of agricultural practices(conventionalversusorganic)on nematode distribution across different aggregate fractions.Furthermore,it would be interesting to understand how nematodes react to other types of land use in different environments.In the present study,we examined only one example of land use,vineyard,in a Mediterranean climate.Further studies on other types of land use are needed to better understand the importance of nematodes in soil ecosystem functioning. ACKNOWLEDGEMENTS The authors thank Ms.Efrat Shakartchy for her contribution at the beginning of the project and her assistance during the laboratory analysis of samples,and Ms.Yael Laure for her careful reading, valuable comments, and preparation of the manuscript for publication.We gratefully acknowledge Mr.Yossi Yodfat and Mr.Bark Somek,the vineyard owners,for allowing us to sample their plots and providing information on vineyard management.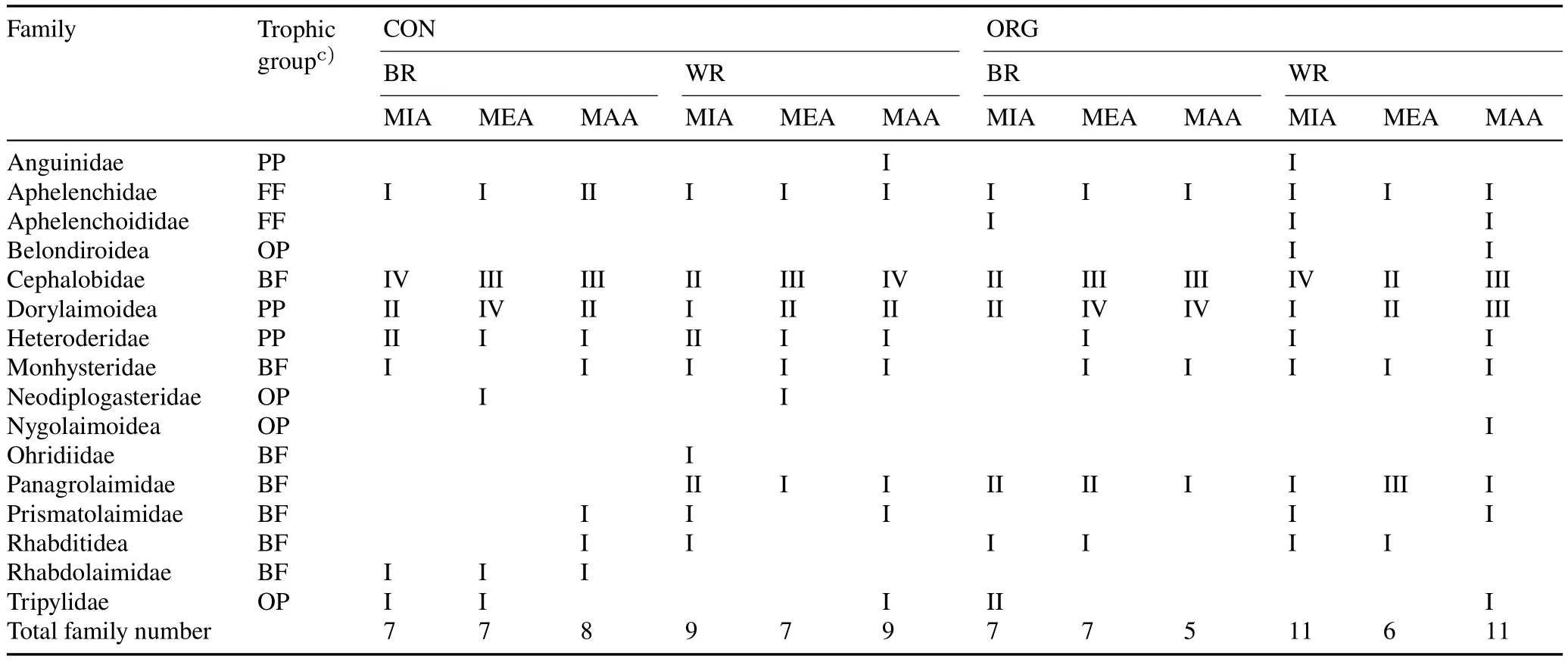
Nematode familynetworks
DISCUSSION



杂志排行
Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
- Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil
- Environmental similarity is more important than distance in the community structuring processes of ammonia-oxidizing archaea in agricultural soils
