Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil
2023-12-21JinfengWANGHuanyuBAOYuBonMANJunCAIJiaLIBenhuaSUNandFuyongWU
Jinfeng WANG,Huanyu BAO,Yu Bon MAN,Jun CAI,Jia LI,Benhua SUN and Fuyong WU,
1College of Natural Resources and Environment,Northwest A&F University,Yangling 712100(China)
2Engineer and Technology Academy of Ecology and Environment,Shanxi Province Key Laboratory of Soil Environment and Nutrient Resources,Shanxi Agricultural University,Taiyuan 030031(China)
3Key Laboratory of Plant Nutrition and the Agri-environment in Northwest China,Ministry of Agriculture,Yangling 712100(China)
4State Key Laboratory of Urban Water Resource and Environment,Harbin Institute of Technology,Harbin 150090(China)
5Consortium on Health,Environment,Education and Research,Department of Science and Environmental Studies,The Education University of Hong Kong,Tai Po,Hong Kong 999077(China)
ABSTRACT For years,biochar has been successfully used for the remediation of polycyclic aromatic hydrocarbons(PAHs)in contaminated soils,not only for improving their removal from soil but also for reducing their uptake by crops.However,the underlying mechanism of biochar application reducing PAH uptake and accumulation in winter wheat remains unclear.Pot trials were conducted on a PAH-contaminated soil amended with bamboo biochar,coconut shell biochar,and maize straw biochar(MSB)for an entire growth period of winter wheat.Compared with no biochar control(CK),application of the three types of biochar significantly(P <0.01)reduced grain PAH concentration,total equivalent concentration(TEC),and incremental lifetime cancer risk(ILCR),indicating that biochar application,especially MSB,reduced the risk of exposure to PAHs in wheat grain.Furthermore,all three types of biochar significantly(P <0.05)reduced PAH uptake and accumulation in wheat roots and stems,probably because biochar application enhanced the degradation of PAHs in the rhizosphere soil.Compared with CK,application of the three types of biochar significantly(P <0.05)reduced the concentration of PAHs in the rhizosphere soil by 15.9%—33.7%.It was found that the degradation rate of high-molecular-weight(HMW)PAHs(5-and 6-ring PAHs)was significantly(P <0.05)higher than that of low-molecular-weight(LMW)PAHs(2—4-ring PAHs)regardless of the type of biochar used.Additionally,all three types of biochar significantly increased the relative abundance of the dominant bacterial phyla and genera in soil.Redundancy and correlation analyses also showed that there was a strong correlation between the removal rate of PAHs and dominant bacteria in the rhizosphere soil.This study indicated that biochar effectively reduced the health risk from dietary exposure to PAHs in wheat grains by increasing the abundance of bacteria related to PAH degradation,promoting the biodegradation of PAHs in the rhizosphere soil,and consequently reducing PAH uptake by wheat.
Key Words: bamboo biochar,coconut shell biochar,degradation,incremental lifetime cancer risk,maize straw biochar,rhizosphere soil,total equivalent concentration,toxicity equivalency factor
INTRODUCTION
Polycyclic aromatic hydrocarbons(PAHs)are a class of refractory organic compounds primarily derived from natural(e.g.,volcanic activities and forest fires)and anthropogenic(e.g.,industrial processes,incomplete combustion of fossil fuels,and coke production)activities(Weiet al.,2014;Usmanet al.,2016;Dat and Chang,2017).They are ubiquitous in the environment and have been the focus of environmental research for many years.In 2016,ca.32 720 t PAHs were emitted in China, accounting for 21% of the global PAH emission (Hanet al., 2019).The main pool of PAHs was detected in soil, including agricultural soils (Wanget al.,2020a).The total concentration of PAHs in topsoil layers of farmland in eastern China ranged from 8.8 to 3 880µg kg-1(Sunet al.,2017).According to the number of rings in PAHs,they are usually divided into high-molecular-weight PAHs(HMW PAHs,5-and 6-ring PAHs)and low-molecularweight PAHs(LMW PAHs,2—4-ring PAHs).Due to their high distribution coefficient,PAHs can be strongly adsorbed on the surface of particles and stored in soil,making their degradation very difficult(Pinget al.,2007).Available PAHs in farmland soil can freely pass through cell membranes of living organisms (Barnieret al., 2014), thus entering the food chain after absorption by crops and posing a potential threat to human healthviathe ingestion of crop products(Wang Jet al., 2018).According to China National Standard for Food Safety(GB 2762—2022),the concentration of benzo[a]pyrene(BaP)in cereals and their products should be below the food contamination limit of 5.0µg kg-1.Consequently,reducing PAHs in agricultural soil is essential for effective mitigation of the risk of human exposure to PAHsviaagricultural products,and thereby solving the problem at the root.Additionally,PAHs can interfere with soil N cycle and affect crop growth(Guoet al., 2020).Therefore, it is crucial to reduce both uptake and accumulation of PAHs in crops for food security.
Biochar is obtained by high temperature pyrolysis of biomass material.As it is inexpensive,environmentally friendly, and effective, biochar has been widely applied as soil amendment for environmental remediation(Duttaet al.,2017).Biochar can improve gas transport and soil properties,including soil fertility,bulk density,and water-holding capacity (Farkaset al., 2020).Biochar can reduce PAH bioavailability in soil throughπ—πelectron interactions and surface adsorption due to its loose and porous structure,rich functional groups,and large specific surface area(SSA)(Wanget al.,2011).Further,biochar generally reduces plant PAH uptake,although this effect depends on both biochar properties and plant species (Ahmadet al., 2014).Khanet al.(2013) found that the application of sludge biochar significantly reduced PAH accumulation in lettuce mainly by reducing PAH bioavailability through biochar adsorption.Niet al.(2017)found that the application of bamboo and corn biochar decreased PAH accumulation in tuberous vegetables (e.g., carrot) and that bamboo biochar produced at 700◦C(BB700)reduced PAH bioavailability mainly by immobilization,while corn straw biochar produced at 300◦C(CSB300)reduced soil PAH level mainlyviapromoting PAH degradation by soil bacteria.Under a paddy-upland rotation cropping system, the application of CSB300 reduced the enrichment of PAHs in rice grains mainly by improving the biodegradation of PAHs in rhizosphere soil(Niet al.,2021).Nonetheless,although previous studies have reported that biochar can reduce PAH accumulation in crops, the underlying mechanisms are still not fully understood.
Winter wheat(Triticum aestivumL.)is one of the main food crops in China, and approximately 70% of this crop is planted in the Northern China Plain,which is suffering from high PAH emission(Ma and Harrad,2015;Tianet al.,2018).This is also a serious health threat to the humans who rely on wheat as a staple food.Therefore,investigating the effect of biochar on the uptake and accumulation of PAHs in winter wheat and the underlying mechanisms will provide a potential strategy for safe crop production.
Rhizosphere serves as a gateway for the transfer of soil contaminants into the food chain(Storeyet al.,2014).Thus,reducing PAH availability in rhizosphere soil is considered an effective method to alleviate the risk of PAHs in crops and humans(Liuet al.,2013).Generally,biochar can alleviate the risk of PAHs in plants in two ways.Firstly, biochar reduces PAH accumulation in plants by decreasing PAH bioavailability in soil(Wanget al.,2011).Secondly,biochar can enhance the biodegradation of PAHs in soil by promoting microbial activity and community structure,which is attributed to its physical and chemical properties, such as porosity, pH, SSA, and nutritional (N and P) characteristics(Enniset al.,2012;Anyikaet al.,2015;Zhanget al.,2018).Konget al.(2018) demonstrated that the relative abundance(RA)of PAH-degrading microbes increased in a soil amended with wheat straw biochar, further promoting microbiological metabolism.However,few studies have focused on the effects of biochar on microbial community structure in PAH-contaminated rhizosphere soils.
Therefore, the main purposes of this study were to explore: i) the effect of biochar application on the uptake and accumulation of PAHs in winter wheat,ii)the effect of biochar application on PAH bioavailability in the rhizosphere soil of winter wheat, and iii) the responses of bacterial communities in the rhizosphere soil to different types of biochar.
MATERIALS AND METHODS
Experimental soil
Soil samples were collected from the 0—20 cm topsoil layer of a farmland field near the Weihe Power Plant located in Xi’an City in Shaanxi Province, China (34◦25′55′′N,108◦54′26′′E).After removing debris such as rocks and plant roots,the soil samples were dried and passed through a 2-mm sieve.Partial basic soil properties were as follows:pH,8.51;organic matter,20.6 g kg-1;total N,1.31 g kg-1;and total P,0.40 g kg-1.The soils are composed of 58.76%silt,36.27%sand,and 4.97%clay and sandy loam,which are derived from loess material,and classified as Eumorthic Anthrosols (FAO,2014).The concentration of the 16 US Environmental Protection Agency(EPA)priority PAHs in soil was 4 156µg kg-1,which reached a heavy pollution level, according to the soil PAH-pollution classification(Maliszewska-Kordybach,1996).
Preparation and characterization of biochar
Bamboo,coconut shell,and maize straw were collected from the corresponding main production areas.The three materials were air-dried and crushedviaa mesh sieve(2 mm).The details for the preparation of bamboo biochar(BB),coconut shell biochar(CB),and maize straw biochar(MSB)were previously described by Yuanet al.(2011).Briefly,the three materials were placed in a ceramic crucible,covered with an air-tight lid,pyrolyzed under a limited oxygen supply, heated to 500◦C in a muffle furnace for 2 h at a temperature increase rate of 20◦C min-1, cooled, and ground through a 2-mm screen.The SSA was characterized using the Brunauer-Emmett-Teller(BET)test(Micromeritics Nove 2000e apparatus, Quantachrome Corp., USA).The SSA of BB,CB,and MSB were 250,300 and 1 050 m2g-1,respectively.The elemental compositions of the three types of biochar were determined using an elemental analyzer(Euro-Vector EA3000,QES Group Berhad,Malaysia)(Table SI,see Supplementary Material for Table SI).The pH of BB,CB,and MSB(measured at a biochar:water ratio of 1:2.5,weight/volume)were 9.67,8.94,and 8.65,respectively.
Pot trials
The PAH-contaminated soil samples(5.0 kg)were mixed with BB, CB, or MSB (2%, weight/weight) and placed in a plastic pot (diameter, 32 cm; height, 28 cm).The PAH-contaminated soil without biochar was used as a control (CK).Each treatment was repeated three times.Basic fertilizers, including 200 mg kg-1N (CH4N2O and NH4H2PO4),200 mg kg-1K2O(KCl),and 100 mg kg-1P2O5(NH4H2PO4),were mixed with the soil as described in our previous study(Wuet al.,2019).To reach equilibrium,the experimental soil was maintained at 60% of the field water-holding capacity for three weeks before planting.
Seeds of winter wheat(cv.Xiaoyan22),purchased from the Longfeng Seed Company of Shaanxi Province,China,were disinfected with 3% H2O2for 30 min, washed with distilled water,and placed in an incubator(25◦C)for one day.Fifteen pre-germinated seeds were planted in plastic pots on October 28, 2021.Pot trials were conducted in a rainproof mesh greenhouse located at Northwest A&F University, China.Seedlings were thinned to 10 pot-1at the three-leaf stage and watered daily to maintain 60% of the field water-holding capacity during the entire cultivation period.
Soil and plant sampling
Rhizosphere soil of winter wheat was collected at maturity using the shaking method.Briefly,the root was dug layer by layer with a knife,gently shaking offthe non-rhizosphere soil after the root was removed,and then soil adhered to the root was collected with a small brush and stored as rhizosphere soil.One part of the rhizosphere soil was wrapped with Al foil and stored at-80◦C for DNA extraction.The remaining portion was air-dried and used for PAH determination.Plant samples were harvested at maturity, cleaned with distilled water,air-dried in the dark,and divided into four parts:root,stem,leaf,and grain.All plant samples were ground and sieved using a 2-mm sieve for further analyses.
Determination of PAHs in soil and plant samples
The methods used for determination of PAHs were previously described(Tianet al.,2018).Briefly,plant or soil samples were extracted with dichloromethane and acetone(HPLC grade,1:3,volume/volume)for 18 h using the Soxhlet Method based on US EPA Standard Method 3540C (US EPA, 1996).A rotary evaporator (RV8, IKA, Germany)was used to concentrate the extract to 2 mL, which was then passed through a silica column with a lower layer of approximately 2 g silica gel (100—200 mesh) and an upper layer of approximately 2 g anhydrous sodium sulfate(Na2SO4).After all the extracted solutions passed through the column,a 60-mL mixture solution of dichloromethane and hexane(1:1,volume/volume)was applied to wash the column.All eluents were rotated and evaporated to dryness and then dissolved in methanol to 2 mL.High performance liquid chromatography with fluorescence detector(LC-20 A,Shimadzu,Japan)was used to determine the concentration of PAHs in the solution.Detailed procedures and conditions of the instrumentation were described in our previous studies(Wanget al.,2020b).
Quality control
The specific methods to determine the limits of detection(LoD)have been previously stated(Wanget al.,2020b)as shown in Text S1(see Supplementary Material for Text S1).The recovery rate and LoD of the 16 US EPA priority PAHs ranged from 76.7% to 138.4% and from 0.06 to 1.41 µg kg-1,respectively.The removal rate(RR)(%)of PAHs in each treatment was calculated by the following formula:
whereC0(µg kg-1)is the initial soil PAH concentration andCt(µg kg-1)is the final soil PAH concentration at maturity of winter wheat.
Analysis of bacterial community structure
The bacterial community in the rhizosphere soil was analyzed using 16S rDNA sequencing technology.The detailed analytical method was previously described by Wanget al.(2021).Briefly, DNA was extracted from the soil samples using an E.Z.N.A.®Soil DNA kit(D4015,Omega Inc., USA).The V3—V4 variable region of the bacterial 16S rRNA was amplified using the universal primers 338F(5′-ACTCCTACGGGAGGCAGCA-3′)and 806R(5′-GGACTACHVGGGTWTCTAAT-3′).The PCR products were detected using 2%agarose gel electrophoresis,and the target fragment was recovered using the AxyPrep PCR Cleanup kit(Corning,USA).Finally,the PCR products were purified using AMPure XT beads(Beckman Coulter Genomics,USA)and quantified by a Qubit assay(Invitrogen, USA).The amplicon pools were used for sequencing,and the size and quantity of the amplicon library were evaluated using an Agilent 2100 bioanalyzer (Agilent, USA) and Library Quantification kit for Illumina (Kapa Biosciences, USA),respectively.Libraries were sequenced using the NovaSeq PE250 platform.
Bioconcentration factors (BCFs) and risk assessment of dietary exposure to PAHs via winter wheat
Bioconcentration factors for PAHs in winter wheat were calculated using the following formula:
whereCplantandCsoil(µg kg-1) indicate the PAH concentrations in wheat tissues and rhizosphere soil,respectively.
Owing to the different carcinogenicity of each PAH,the toxicity equivalency factor(TEF)of BaP was set as 1.0 to convert other PAHs into relative toxicity levels.The carcinogenicity of PAH-intake from wheat grain was quantified using total equivalent concentration(TEC).Only 11 PAHs were detected in wheat grain in this study,and the TEF of each PAH is shown in Table SII(see Supplementary Material for Table SII).Based on a previous study by Jiaet al.(2018),TEC(µg kg-1)of PAHs was calculated as follows:
whereCirepresents the individual concentration of theith PAH in wheat grain (µg kg-1) and TEFirepresents the corresponding TEF of theith PAH based on the TEF of BaP.
The total level of exposure to PAHs in daily dietary wheat grain for adults(ED)(ng d-1)was calculated as follows:
where IR indicates the daily intake of wheat grain(172.87 g d-1) by an adult (FAO, 2013).Human health-risk was assessed by calculating the incremental lifetime cancer risk(ILCR)from PAH-contaminated wheat grain based on the following formula:
where EF represents the exposure frequency in 1 year(365 d),SF is the slope factor of BaP against oral cancer,which was 7.3 mg d kg-1(Wang Jet al., 2018), CF represents the conversion factor(10-6mg ng-1),ED is the exposure duration of adults(43 years),AT is the average life span of carcinogens(25 550 d),and BW is the average adult body weight(62.82 kg).
Statistical analysis
SPSS 23.0 was used for one-way analysis of variance,and all bars were performed with Origin 2019b.Unless otherwise specified,all comparisons of means were performed atP<0.05.All data in this study were based on dry weight.
RESULTS AND DISCUSSION
Growth of winter wheat
Compared with CK,the addition of CB and MSB significantly (P< 0.05) increased winter wheat grain yield by 10.6%and 5.43%,respectively,whereas BB had an insignificant effect on grain yield(Fig.1a).Application of the three types of biochar exhibited a slight effect on the biomass of root, stem, and leaf of winter wheat.Both CB and MSB had a higher N content,a lower pH,and a more appropriate C/N ratio than BB(Table SI),which were beneficial to grain yield.Previous studies have demonstrated that biochar can improve soil environment,promote nutrient utilization,and increase crop yield(Van Zwietenet al.,2010;Jefferyet al.,2011;Gereaet al.,2013).Additionally,Schmidtet al.(2014)reported that incomplete carbonization of biochar may enhance plant growth by improving the availability of nutrients in soil.In this study,the higher SSA of CB(300 m2g-1)and MSB(1 050 m2g-1),compared with that of BB(250 m2g-1),may have enhanced the adsorption capacity of biochar to PAHs, while the higher nutritional properties of MSB could increase the biodegradation of PAHs(Table SI),thus alleviating the toxic effects of PAHs on winter wheat.Gellet al.(2011) found that the addition of biochar alleviated plant toxicity due to the adsorption of toxins.
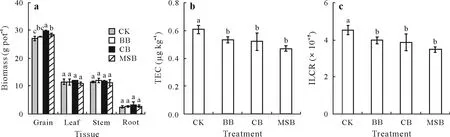
Fig.1 Effects of bamboo biochar (BB), coconut shell biochar (CB), and maize straw biochar (MSB) on the biomass of different tissues (a), toxicity equivalency concentration(TEC)of grain(b),and incremental lifetime cancer risk(ILCR)of grain(c)in winter wheat growing on a polycyclic aromatic hydrocarbon-contaminated soil.Error bars are the standard deviations of means(n=3).Different letters indicate significant differences(P <0.05)among different biochar treatments.CK represents the control with no biochar.
Uptake and accumulation of PAHs in winter wheat
Compared with CK,application of the three types of biochar significantly(P<0.01)reduced PAH concentrations by 15.4%—26.8%in grain and by 12.4%—19.4%in stem(Fig.2a).Both CB and MSB significantly(P<0.01)reduced root PAH concentrations by 26.4%and 21.5%,respectively,but had no effect on leaf PAH concentrations.Similarly,Brennanet al.(2014)and Niet al.(2018)found that biochar addition significantly reduced PAH concentrations in the root and stem of corn and rice.Furthermore,a significant negative correlation was found between the removal rate of total and LMW PAHs in the rhizosphere soil and their concentrations in root(correlation coefficient(r)=-0.554,P<0.05 for total PAHs;r=-0.706,P<0.01 for LMW PAHs),stem(r=-0.643,P<0.01 for total PAHs;r=-0.839,P<0.01 for LMW PAHs), and grain(r=-0.704,P<0.01 for total PAHs;r=-0.613,P< 0.05 for LMW PAHs)(Table SIII, see Supplementary Material for Table SIII).These results suggest that the reduction in the accumulation of PAHs in wheat tissues is partially due to the degradation of PAHs in the rhizosphere soil.Consistently,Niet al.(2021)found that biochar increased the degradation of PAHs in soil and reduced the bioenrichment of PAHs in carrot.However,an insignificant correlation was found between leaf PAH concentrations and PAH removal rate in the rhizosphere soil(Table SIII).Generally,PAH uptake by winter wheat occursviafoliage and root(Taoet al.,2009).Atmospheric PAHs deposited on the surface of leaves can enter plantsviastomata or cuticle(Pullaguralaet al.,2018).Application of the three types of biochar significantly(P<0.05)reduced the BCF of PAHs in grain,but had no significant effect on the BCF of PAHs in leaf(Table SIV,see Supplementary Material for Table SIV).Application of CB and MSB significantly(P<0.05)reduced the BCF of PAHs in root and stem.In addition,although PAH bioavailability in the rhizosphere soil was not determined,biochar addition could reduce PAH bioavailability in soil by adsorbing,consequently reducing uptake of PAHs by wheat root.Similarly,Khanet al.(2015)found that the addition of biochar reduced PAH bioavailability in soil and PAH accumulation in radish.
Human health risk due to PAH accumulation in winter wheat grain
The main route of human exposure to PAHs is dietary intake,which accounts for 70%of total exposure(Ma and Harrad,2015).The food safety standard maximum levels of BaP in wheat grain is 5.0µg kg-1(GB 2762—2017),while BaP was not detected in wheat grain in this study.Thus,TEC and ILCR of PAHs were used to assess the risk of wheat grain exposure to PAHs.The TEC of PAHs in winter wheat grain was generally low (0.47—0.61 µg kg-1) and significantly(P< 0.05) decreased under the three biochar treatments compared with CK(Fig.1b).The ILCR values of PAHs>10-4,10-6—10-4,and<10-6indicate high,low,and no risk to human health,respectively(Wang Jet al.,2018).In our study,wheat grain had a low risk of exposure to PAHs under all treatments,with ILCR values between 4.51×10-5and 3.48×10-5(Fig.1c).Furthermore,the ILCR values of PAHs were significantly (P< 0.01) reduced by 11.8%, 11.4%,and 22.8%under BB,CB,and MSB,respectively,compared to CK.Therefore,the application of biochar reduced the risk of wheat grain exposure to PAHs, especially under MSB,which may be caused by the lower concentration of PAHs in the rhizosphere soil(Fig.2b).Similarly,Niet al.(2021)reported that biochar with different supplemental levels and production temperatures significantly reduced the risk of rice grain exposure to PAHs.

Fig.2 Effects of bamboo biochar(BB),coconut shell biochar(CB),and maize straw biochar(MSB)on the concentration of polycyclic aromatic hydrocarbons(PAHs)in different tissues of winter wheat growing on a PAH-contaminated soil(a)and the concentration of PAHs in the rhizosphere soil(b).Error bars are the standard deviations of means(n=3).Different letters indicate significant differences(P <0.05)among different biochar treatments for winter wheat tissue or PAHs.CK represents the control with no biochar.LMW PAHs=low-molecular-weight PAHs(2—4-ring PAHs);HMW PAHs=high-molecular-weight PAHs(5-and 6-ring PAHs).
Distribution of PAHs in winter wheat tissues
The distribution of 2—6-ring PAHs in winter wheat root,stem, leaf and grain is shown in Fig.3.Among 3- and 4-ring PAHs,only acenaphthylene(ACY)was not detected in all tissues,whereas only benzo[b]fluoranthene(BbF)and benzo[k]fluoranthene(BkF)were detected in 5-ring PAHs.Both CB and MSB treatments significantly (P< 0.05)reduced the proportion of 3-and 5-ring PAHs in root and leaf.This may be explained by the following two reasons.First,the strong fluidity of 3-ring PAHs makes them easily absorbed by biochar,which reduces their bioavailability in soil.Second,HMW PAHs had a higher removal rate than LMW PAHs in the rhizosphere soil after biochar addition(Fig.2b),which further reduced plant uptake of 5-ring PAHs.Similarly,Niet al.(2018)reported that CSB300 and BB700 amendments both reduced the bioavailability of LMW and HMW PAHs in soil.In addition,the CB and MSB treatments both significantly reduced (P< 0.05) the proportion of 2-ring PAHs in wheat stem and grain,whereas increased the proportion of 4-ring PAHs (Fig.3).This may be because 2-ring PAHs were more easily degraded than 4-ring PAHs in soil amended with CB and MSB,resulting in reduced plant uptake and translocation of 2-ring PAHs to the aboveground tissues of the crop.
Removal of PAHs in the rhizosphere soil
Concentrations of PAHs in the rhizosphere soil after winter wheat harvest are shown in Fig.2b.For biocharamended treatments, the ratio of concentrations of PAHs accumulated in wheat tissues to those in the rhizosphere soil was less than 1%,indicating that the reduction of PAHs in soil was mainly due to degradation rather than plant absorption,in line with a previous report on bioremediation of a PAH-contaminated soil cultivated with ryegrass (Maet al.,2022).In the CK treatment,PAH concentrations in the rhizosphere soil decreased significantly over time,which may be related to the presence of indigenous microbes capable of degrading PAHs in long-term contaminated soils(Baoet al.,2020).Compared with CK,the application of all three types of biochar(BB,CB,and MSB)significantly(P<0.05)reduced PAH concentrations in the rhizosphere soil by 15.9%,26.1%,and 33.7%,respectively.Similarly,the addition of straw biochar could significantly reduce soil PAH concentrations (Qinet al., 2013).In our study, the three types of biochar tested showed significant differences in PAH removal efficiency.Compared with BB and CB,MSB was more effective in removing PAHs because the C/N ratio of MSB(22)was close to the microbial optimum C/N ratio(25)(Table SI),which was conducive to the growth of PAH-degrading bacteria.Additionally, the surface microconfiguration of MSB, including its high SSA (1 050 m2g-1),contributed to an increased adsorption of soil PAHs.Due to these two reasons, soil PAHs may be adsorbed or degraded to a greater extent under MSB addition.Moreover,compared with CK,the addition of BB,CB,or MSB significantly(P<0.05)reduced the concentrations of LMW PAHs by 14.4%,23.4%,and 22.6%and the concentrations of HMW PAHs by 17.4%,29.0%,and 45.4%,respectively(Fig.2b).Furthermore,the degradation rate of HMW PAHs was significantly(P<0.05)higher than that of LMW PAHs,regardless of the biochar type.These results agreed with those of Niet al.(2017),who found that the degradation rate of HMW PAHs in soil using corn straw-derived biochar was significantly higher than that of LMW PAHs.
Bacterial community structure in the rhizosphere soil

Fig.3 Effects of bamboo biochar(BB),coconut shell biochar(CB),and maize straw biochar(MSB)on polycyclic aromatic hydrocarbon(PAH)distribution in grain(a),leaf(b),stem(c),and root(d)of winter wheat growing on a PAH-contaminated soil.CK represents the control with no biochar.
High-throughput sequencing was performed to analyze changes in bacterial community in the rhizosphere soil after biochar addition.Specifically, Chao 1 and Shannon index were used to characterize the richness and diversity of bacteria,respectively.The larger their values are,the higher the abundance and biodiversity are (Huanget al., 2019).The present study showed that biochar application apparently increased Chao 1 and Shannon index and operational taxonomic units of soil bacterial communities (Table I),suggesting that the application of biochar increased the diversity and richness of soil bacterial community.Several previous studies have also reported that biochar can increase the richness and diversity of bacteria in soil(Zhanget al.,2019;Zhuet al.,2020).
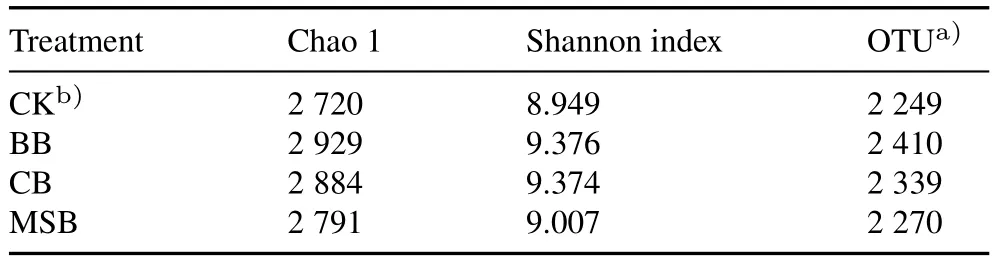
TABLE I Effects of bamboo biochar(BB),coconut shell biochar(CB),and maize straw biochar(MSB)on richness and diversity indices of bacterial communities in the rhizosphere soil of winter wheat growing on a polycyclic aromatic hydrocarbon-contaminated soil
The bacterial communities in the rhizosphere soil belonged mainly to 15 phyla and differed significantly(P<0.05)among different biochar treatments(Fig.4).Six dominant phyla,including Proteobacteria(RA=41.1%—44.1%),Bacteroidetes(RA=27.8%—31.6%),Actinobacteria(RA=9.92%—12.9%),Gemmatimonadetes(RA=5.64%—6.99%),Acidobacteria(RA=2.32%—3.66%),and Firmicutes(RA=1.76%—2.47%),together accounted for over 88%of the total abundance.Similarly,Duanet al.(2021) found that biochar increased the abundance of bacterial communities in soil,with the most predominant groups mainly belonging to the phyla of Proteobacteria,Actinobacteria,Acidobacteria,Firmicutes,and Gemmatimonadetes.Generally,Proteobacteria are considered to be the largest soil bacterial phylum(Martinyet al.,2005).Proteobacteria and Bacteroidetes are abundant in PAH-contaminated soils and are directly involved in PAH biodegradation (Weiet al., 2020; Zhanget al.,2020).Compared with CK,the RAs of Actinobacteria and Firmicutes were significantly increased by all three biochar treatments,especially by MSB(Fig.4).Firmicutes can degrade complex organic compounds,exhibit electrochemical activity,and participate in extracellular electron transfer(Chenet al.,2017).As for Actinobacteria,they can effectively degrade HMW PAHs but cannot remove LMW PAHs from the culture medium(Isaacet al.,2015).In addition,the results of redundancy and correlation analyses(Fig.5,Fig.S1,see Supplementary Material for Fig.S1)showed high correlations of Proteobacteria, Firmicutes, Bacteroidetes,Actinobacteria,Acidobacteria,and Verrucomicrobia with the removal rate of PAHs.Thus,our results clearly indicate that these bacteria play an important role in the degradation and metabolism of PAHs in rhizosphere soil.

Fig.4 Effects of bamboo biochar(BB),coconut shell biochar(CB),and maize straw biochar(MSB)on the relative abundances of the top 15 bacterial phyla(a)and genera(b)in the rhizosphere soil of winter wheat growing on a polycyclic aromatic hydrocarbon-contaminated soil.CK represents the control with no biochar.Phylum levels A—O in a=Proteobacteria,Bacteroidetes,Actinobacteria,Gemmatimonadetes,Acidobacteria,Firmicutes,Fibrobacteres,Nitrospirae,Chlorobi,Verrucomicrobia,JL_ETNP_Z39,Cyanobacteria,Chloroflexi,Saccharibacteria,and Elusimicrobia,respectively;Genus levels A—O in b=Salinimicrobium,Sphingomonas,Acidibacter,Flavisolibacter,Chryseolinea,Devosia,Steroidobacter,Ohtaekwangia,Lysobacter,Bacillus,Bryobacter,Arthrobacter,Nocardioides,Flavobacterium,and Terrimonas,respectively.

Fig.5 Redundancy analyses of the correlations between the removal rate of polycyclic aromatic hydrocarbons(PAHs)and the abundance of bacteria at the phylum(a)and genus(b)levels in the rhizosphere soil of winter wheat growing on a PAH-contaminated soil.LMW PAHs=low-molecular-weight PAHs(2—4-ring PAHs);HMW PAHs=high-molecular-weight PAHs(5-and 6-ring PAHs).
The top 15 bacterial genera(26.7%—31.5%)with RAs greater than 0.1%were selected to compare the changes in bacterial communities under different biochar treatments.The composition profiles are shown in Fig.4.Compared with CK,the application of biochar increased the abundance of 11 genera in the rhizosphere soil,i.e.,Sphingomonas,Flavisolibacter,Steroidobacter,Ohtaekwangia,Lysobacter,Bacillus,Bryobacter,Arthrobacter,Nocardioides,Flavobacterium,andTerrimonas.These results indicate that the application of biochar effectively improved the abundance of dominant bacteria in the rhizosphere soil.Among the 11 genera above,Sphingomonas,Ohtaekwangia,Lysobacter,Bacillus,Arthrobacter,andNocardioideswere found to be the key genera related to PAH degradation (Fig.5b).In addition, the RAs of these six genera were significantly(P<0.05)correlated with the removal rates of LMW(r=0.511—0.890), HMW (r= 0.525—0.800) and total (r=0.611—0.865) PAHs (Fig.S1).These results suggest that these six genera might be PAH-degrading bacterial genera.Numerous previous studies have found that these six bacterial genera are dominant in PAH-contaminated soils and are capable of degrading PAHs(Ferreiraet al.,2013;Eskandaryet al.,2014;Wang Bet al.,2018;Zhaoet al.,2018;Liet al.,2019).
CONCLUSIONS
Compared to no biochar,applications of three types of biochar(BB,CB,and MSB)reduced the concentrations of PAHs in winter wheat stem (by 12.4%—19.4%) and grain(by 15.4%—26.8%);furthermore,CB and MSB significantly reduced the accumulation of PAHs in root(by 26.4%and 21.5%, respectively).Consequently, the ILCR values of PAHs in the BB, BC, and MSB treatments were reduced by 11.8%,11.4%,and 22.8%,respectively.Moreover,the removal efficiency of PAHs was improved in the MSB treatment (33.7%) and was better than that of the other two biochar treatments (BB, 15.9%; CB, 26.1%).Regardless of biochar type, the rate of removal of HMW PAHs was higher than that of LMW PAHs,probably because HMW PAHs were firstly degraded to LMW PAHs during the degradation process and then completely decomposed.In other words,the LMW PAH pools were at least partly supplied by HMW PAH decomposition products.Additionally,biochar significantly increased the abundance of dominant bacterial phyla and genera in the rhizosphere soil,and redundancy and correlation analyses suggested that the dominant bacteria may be partially related to PAH degradation.This study indicated that biochar mainly promoted the biodegradation of PAHs in the rhizosphere soil by increasing the abundance of related degrading bacteria and thus reduced the uptake and accumulation of PAHs in winter wheat,thereby effectively reducing the risk of exposure to PAHs in dietary wheat grain.
ACKNOWLEDGEMENT
This work was financially supported by the National Natural Science Foundation of China(Nos.42077325 and 41571456)and the Natural Science Basic Research Plan in Shaanxi Province of China(No.2019JZ-25).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
CONTRIBUTION OF AUTHORS
Jinfeng Wang and Huanyu Bao contribute equally to this work and share co-first authorship.
杂志排行
Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
- Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
- Environmental similarity is more important than distance in the community structuring processes of ammonia-oxidizing archaea in agricultural soils
