Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
2023-12-21BingXIADanHUANGMaoYEHaoQIUHongfengCHENKeqiangZHAORongliangQIUandRongrongYING
Bing XIA,Dan HUANG,Mao YE,Hao QIU,Hongfeng CHEN,Keqiang ZHAO,Rongliang QIU and Rongrong YING,
1Nanjing Institute of Environmental Sciences of the Ministryof Environmental Protection(NIES),Nanjing 210042(China)
2Research Academyof Environmental Sciences of Anhui Province,Hefei 230071(China)
3College of Environmental and Resource Sciences,Zhejiang University,Hangzhou 310058(China)
4KeyLaboratoryof Soil Environment and Pollution Remediation,Institute of Soil Science,Chinese Academyof Sciences,Nanjing 210008(China)
5Shanghai JiaoTong University,School of Environmental Science and Engineering,Shanghai 200240(China)
6Guangdong Laboratoryfor Lingnan Modern Agriculture,College of Natural Resources and Environment,South China Agricultural University,Guangzhou 510642(China)
ABSTRACT The biosurfactants rhamnolipids and the“soil ecosystem engineers”earthworms are often used to remediate contaminated soils.However,the effects of rhamnolipids on earthworm intestinal flora and microbial community in soil containing earthworms are not clearly understood.In our study,a 21-d microcosm experiment was carried out to reveal the effects of rhamnolipids on microbial abundance,composition,and metabolism,as well as contaminant degradation capacity.Both rhamnolipids and earthworms had positive effects on soil bacteria.Rhamnolipid-amended soil(RT)showed higher bacterial abundance and metabolic activity than earthworm-amended soil(ET),while the improvement in bacterial composition and contaminant degradation capacity by rhamnolipids was lower than that by earthworms.Notably,these effects were further amplified by the combined treatment of rhamnolipids and earthworms(RET).Specifically,the bacterial abundance(log-transferred)increased from 9.5 copies g-1 in the control with no addition to 10.3,10.6,and 11.1 copies g-1 in ET,RT,and RET,respectively.Compared to ET,the relative abundance of the dominant phylum,Proteobacteria,increased from 41.66%to 51.67%in RET,and more pollutant-degrading bacteria were also enriched in RET.Therefore,the increases in bacterial abundance and contaminant-degrading bacteria led to the following ranking of soil dioxin removal rate:RET(77.28%)>ET(59.83%)>RT(24.65%)>control(4.71%).Moreover,the addition of rhamnolipids enhanced the abundance of bacterial functional genes involved in metabolism and environmental information processing.In addition,the composition and diversity of bacteria in the gut of earthworms were conspicuously affected by rhamnolipids,and the relative abundance of Microbacterium and Shewanella increased significantly(P <0.05).Therefore,this study revealed that rhamnolipids remarkably influenced the abundance,composition,and metabolism of the microbial community in earthworm gut,further promoting the degradation rate of dioxin,providing theoretical support for optimizing the combined application of rhamnolipids and earthworms in soil bioremediation engineering and for the assessment of the ecological impact of rhamnolipids.
KeyWords: bioremediation,biosurfactant,contaminant-degrading bacteria,intestinal bacteria,metabolic pathway,metabolism,microcosm experiment,soil pollution
INTRODUCTION
Soil pollution caused by industrial and agricultural activities poses a significant threat to human health and natural ecosystem (Rogowski and Yake,2005).These pollutants are hardly biodegradable,persistent,and migratory in the environment,and can be enriched in the food chain,posing great risks to public safety.Therefore,the remediation of soil pollution has become a rapidly growing global concern.Although a variety of soil remediation technologies have been developed,bioremediation is generally considered a low-cost and environment-friendly technology(Zhang H Yet al.,2020).
Earthworms(Metaphire guillelmi),known as“soil ecosystem engineers”, are the invertebrates with the largest biomass in soil.They have a striking ability to digest organic matter,improve soil physical and chemical properties,and promote biodegradation of exogenous substances,which is conducive to the stability and sustainable development of terrestrial ecosystems(Blouinet al.,2013;Chaoet al.,2022).Therefore,earthworms are widely used for the bioremediation of contaminated soils due to their wide distribution,rapid development and reproduction,high tolerance,and resistance to pollutants(Varedaet al.,2019;Žaltauskait˙eet al.,2022).Earthworm activities can directly or indirectly affect the migration and transformation of organic pollutants in soil.Specifically,there are two important pathways for the degradation of pollutants in the process of earthworm-mediated bioremediation,including the metabolism and degradation of pollutants by intestinal bacteria of earthworms (Chaoet al.,2021;Xiaoet al.,2022)as well as the metabolism of soil bacteria under the disturbance of earthworm activities(de Menezeset al.,2018; Luoet al.,2022).Therefore,to understand the remediation ability of earthworms,it is crucial to explore the composition and ecological functions of earthworm intestinal bacteria and soil microbia affected by earthworm activities(Wanget al.,2019;Linet al.,2021).
Typically,surfactants are used in soil bioremediation engineering to facilitate the desorption of pollutants from soil particles and overcome the limitation to remediation imposed by low bioavailability of pollutants(Higgins and Luthy,2006;Maoet al.,2015).Compared to chemically synthesized surfactants, biosurfactants produced by microorganisms are readily degraded and environment-friendly.Among them,rhamnolipids have attracted extensive attention because of their excellent performance(Weiet al.,2020).On one hand,rhamnolipid addition to a polluted soil can improve the bioavailability of pollutants through solubilization or emulsification(Wenet al.,2009;Liuet al.,2017).On the other hand, as an additional carbon source, rhamnolipids are microbial enhancers that can promote microbial proliferation(Rahmanet al.,2003;Fanaeiet al.,2020).Therefore,rhamnolipids have been widely used in soil remediation.Furthermore,the combined application of rhamnolipids and repairing organisms,such as“ecological engineers”earthworms,has also received increasing attention.However,there is still a knowledge gap on whether and how rhamnolipids affect microorganisms in earthworm gut and the surrounding soil,which is important for evaluating the ecology of rhamnolipids.
In this study,soil samples were collected from an abandoned iron and steel plant contaminated with dioxin.Both separate and combined effects of rhamnolipids and earthworms on the dissipation of dioxin and soil physicochemical properties were explored through a 21-d microcosm experiment.Additionally,16S rRNA gene high-throughput sequencing technology and qPCR fluorescence quantitative analysis were used to explore the effects of rhamnolipids on the abundance,composition,and diversity of bacteria in soil and earthworm gut.This study aimed to provide theoretical support for the combined application of rhamnolipids and earthworms in soil bioremediation engineering and for the assessment of the potential ecological impacts of rhamnolipids on other small indigenous animals.
MATERIALS AND METHODS
Soil samples
Soil samples were collected using the five-point sampling method at 5—20 cm soil depth from an abandoned iron and steel plant in Hefei,Anhui Province,China.After air-drying and grinding,the samples were passed through a 2-mm sieve and mixed evenly for subsequent microcosm experiment.Soil physicochemical properties,including pH,total P,organic matter,total N,available P(AP)and K(AK),NO-3-N,NH+4-N,organic C,and cation exchange capacity,were determined according to the method described by Fanet al.(2022)and are shown in Table SI(see Supplementary Material for Table SI).Potential pollution was also detected,including total Cu and Zn measured by inductively coupled plasma-atomic emission spectroscopy(Optima 8000,PerkinElmer,Waltham,USA) and 16types of polycyclic aromatic hydrocarbons (PAHs) and dioxin measured by the ultra-trace analysis,based on the high-resolution gas chromatography/high-resolution mass spectrometry method(Anezakiet al.,2009).It was found that the contents of heavy metals didn’t exceed the pollution standard,and almost all the PAHs were below the detection limit,while only dioxins were found at high initial concentrations(3.61 ng kg-1dry soil).
Experimental materials
Metaphire guillelmiis a typical earthworm species in the Yangtze River Delta in China.It is widely distributed all over the world and has an important impact on soil ecological functions.Therefore,M.guillelmiwas selected as the model earthworm type for our study,and all earthworms used in this study were obtained from an earthworm farm in Huai’an,Jiangsu Province,China in October 2021.Mature earthworms with evident rings and weighing between 3.0 and 5.0 g were selected.All earthworms used were domesticated in the same experimental soil for 2 weeks before experiment.After domestication, earthworms showing a normal level of activity were selected for the subsequent experiments.The rhamnolipids used were mono-rhamnolipids(CAS No.869062-42-0)purchased from Aladdin Reagent Co.,Ltd.(Shanghai,China).
Microcosm experiment
A 21-d microcosm experiment was conducted with four treatments:the addition of 2.5%(weight/weight)rhamnolipids(RT),10 earthworms(ET),and 2.5%(weight/weight)rhamnolipids+10 earthworms(RET),and a control with no addition(CK).The concentration of rhamnolipids was determined according to the median value of the concentration of rhamnolipids applied in bioremediation projects(Salwaet al.,2009).The microcosms were established by weighing 500 g soil into 1-L tissue culture bottles for each treatment in triplicate.Soil water content in the microcosms was maintained at 70%of the maximum water-holding capacity at room temperature(25±2◦C)during the incubation period.Soil samples(5 g)were collected every 3 d and stored at 4◦C for further biomass analysis.After incubation,soil physicochemical properties and dioxin concentration were measured again.
Extraction of intestinal contents from earthworm
After incubation, the surviving earthworms were selected for dissection according to the method described by Chaoet al.(2020).Earthworms were washed 3 times with phosphate-buffered saline(PBS),placed on ice for 15 min,and then transferred to 50%absolute ethanol for 1-min anesthesia.To obtain complete intestinal contents, dissection began at the male foramen and ended at the anus.The dissection procedure was performed in an anaerobic incubator(Mecaplex,Grenchen,Switzerland).Contents of earthworm gut in ET and RET were stored at-80◦C for subsequent analyses of bacterial communities and functional genes.
DNA extraction,sequencing,and processing
Bacterial communities in earthworm gut and soil were detected using high-throughput sequencing technology(Chaoet al.,2022).Total DNA was extracted from 0.25 g soil using the FastDNA kit (MP Biomedicals,California,USA).Then,1% agarose gel electrophoresis was used to check the integrity and purity of the DNA,and DNA concentration was detected using a NanoDrop ND-1000 UV-visible spectrophotometer(NanoDrop Technologies,USA).Subsequently,DNA samples were sent to Meige Biotechnology Co., Ltd.(Guangzhou, China) for PCR amplification by PremixTaq kit (TaKaRa,Kyoto,Japan),library construction,and high-throughput sequencing on the Illumina Miseq platform(2×250 bp paired-end reads).The quality of the sequences obtained was shown in Table SII(see Supplementary Material for Table SII).All raw sequence data generated were deposited in the National Center for Biotechnology Information(NCBI)under accession No.PRJNA888476and are publicly accessible at https://www.ncbi.nlm.nih.gov/.Raw reads were quality-controlled using FastQC(v0.14.1)and Trimmomatic(v0.39)with default parameters.Usearch(v10.0.240)(Rogneset al.,2016)was used to assemble highquality clean reads,and UCLUST(Velskoet al.,2018)was used to eliminate redundant sequences.The bacterial 16S rRNA assembly sequences obtained were annotated based on the Greengenes v4.10.0 database using QIIME2(2019.4)(Funget al.,2021).Additionally,the metabolic pathways of the bacterial communities in each sample were predicted using PICRUSt2 based on KEGG database(Douglaset al.,2020).
Analysis of soil bacterial biomass
The DNA extracted from soil samples collected during incubation was diluted 10 times with nuclease-free water for quantitative determination of bacterial abundance using qPCR based on 16S rRNA gene V4.The primer sequences were 515F(5′-GTGCCAGCMGCCGCGGTAA-3′)and 806R (5′-GGACTACHVGGGTWTCTAAT-3′).The PCR amplification conditions for 16S rRNA are shown in Table SIII(see Supplementary Material for Table SIII).Each sample was reperforated 3 times.Results were recorded as valid data,if the difference between reperforated holes was less than 1 Ct, and the average value was taken for further analyses.If the difference was greater than 1Ct,the experiment would be repeated once.
Data analysis
Data normalization,Pearson correlation,and all difference tests (T test) were performed in Python (v3.9),and aPvalue< 0.01 was considered statistically significant.The alpha diversity(Chao 1 index and Simpson index)and beta diversity were calculated by R (v3.4.3).Ultimately,the experimental data were visualized using “vegan” and“ggplot2”packages of R(v3.4.3),Cytoscape(v3.9.1),and chiplot(https://www.chiplot.online/).A hierarchical clustering tree for soil and gut microbiota was constructed based on the Bray-Curtis distance,and the principal coordinate analysis(PCoA)diagram was drawn based on average of the Euclidean distance.
RESULTS AND DISCUSSION
Responses of soil physicochemical properties toearthworm and rhamnolipid addition
Considering the physical and biological disturbances caused by earthworms,as well as the effect of rhamnolipids as an exogenous substance on soil, the physicochemical properties of soil were determined in different treatments.As shown in Table SI,soil pH changed from alkaline to neutral in the presence of earthworms,which was potentially caused by earthworm-produced acidic amino acids.Further,soil NO-3-N content increased significantly (P< 0.05)after addition of earthworms.Specifically,NO-3-N content increased nearly 32 times in ET and 17 times in RET.This may be due to the presence of nitric oxide in the earthworm mucus and the entry of many anaerobic metabolites(such as NO2)into soil,which could promote N mineralization(Zhang W Xet al.,2020).Moreover,the addition of earthworms significantly(P<0.05)increased soil AP and AK contents,likely due to the mineralization of organic P in the earthworm gut and the transformation of P and K promoted by related enzymes from earthworms.
Interestingly,the effects of rhamnolipids on soil physicochemical properties appeared to be different from those of earthworms.Specifically,rhamnolipids increased the organic matter content,but significantly reduced the contents of AP and AK,which may be caused by the complexation affinity of rhamnolipids on soil ions,including K+and PO3-4 (Ochoa-Lozaet al.,2001).In addition,microbial growth stimulated by rhamnolipids may also reduce AP and AK contents.Moreover,unlike earthworms,rhamnolipids had little effect on soil NO-3-N.Therefore,the addition of earthworms and rhamnolipids affected soil physical and chemical properties directly related with the living environment of indigenous microbial communities.
Responses of soil and earthworm gut bacterial biomass and alpha diversitytorhamnolipid addition
Microbial biomass is an important indicator for evaluating bioremediation technologies.Therefore,the abundance of the bacterial community was monitored continuously during the microcosm experiment(Fig.1a).Bacterial abundance tended to be stable over time in CK, with a logtransferred value of approximately 9.5 copies g-1,whereas,in RT and RET,it showed an upward trend during 0—12 d.Moreover,bacterial abundance in ET showed a fluctuating growth trend.Specifically,the highest bacterial abundance was observed in RET,followed by ET and RT,with the corresponding log-transferred values of 11.1, 10.6, and 10.3,respectively.This finding proved that rhamnolipids and earthworms both effectively promoted the growth of soil microorganisms,and the improvement of bacterial biomass was further enhanced when rhamnolipids and earthworms were added in combination,which may benefit from nutritional supplementation and improved soil properties by earthworm activities and rhamnolipid addition(Whanget al.,2008;Xiaoet al.,2020;Hanet al.,2021).
Interestingly,although rhamnolipids and earthworms increased soil bacterial abundance,they severely reduced the number of soil bacterial species from 1 137 toca.600(Fig.2a).Additionally,alpha diversity decreased significantly in soil with rhamnolipids and earthworms.Specifically,the Chao 1 value of the bacterial communities in RT,ET,and RET was lower than 1 000,whereas it exceeded 3 000 in CK (Fig.1b).Moreover,the Simpson index for bacterial communities followed the order:RT>ET>RET>CK(Fig.1c),confirming that the addition of rhamnolipids and earthworms resulted in a decrease in soil bacterial species uniformity,likely because the addition of rhamnolipids and/or earthworms provides more suitable conditions for some dominant bacteria by changing soil physicochemical properties(Wolfet al.,2019).Thus,the growth of dominant bacteria occupied more ecological niches,thereby limiting or even totally impeding the growth of some other primitive bacteria.Finally,a sharp reduction in biodiversity of soil bacterial communities was observed(Airaet al.,2019).
Rhamnolipids contributed to the reduction in bacterial diversity in both soil and earthworm gut(Fig.1b,c).There are two possible reasons for this finding.Firstly,decreasing soil bacterial diversity under the addition of rhamnolipids restricted the types of soil bacteria ingested by earthworms.Alternatively,earthworms consuming rhamnolipids would also change the gut nutritional status,potentially stimulating the growth of dominant bacteria,thus further reducing the alpha diversity of bacteria in earthworm gut (Wolfet al.,2019).Additionally,the alpha diversity of bacteria in ET and RET was higher in earthworm gut than that in soil,but lower than that in CK,which may be related to the unique anaerobic and eutrophic conditions of earthworm gut(Sapkotaet al.,2020).
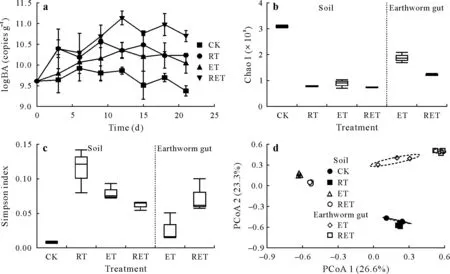
Fig.1 Changes in soil bacterial abundance(BA)with time(a)and box plots of soil and earthworm gut bacterial alpha diversity indices,Chao 1(b),and Simpson index(c),and principal coordinate analysis(PCoA)on soil and earthworm gut bacterial beta diversity(d)in a dioxin-contaminated soil added with rhamnolipids and earthworms alone(RT and ET,respectively)and in combination(RET)at the end of a 21-d microcosm experiment.CK=control with no addition.
Responses of soil bacterial composition toearthworm and rhamnolipid addition
Soil bacterial community composition showed significant differences among treatments(Fig.2).Specifically,only 343 common bacterial species were found in soil and earthworm gut.The hierarchical clustering tree and PCoA diagram also confirmed that the structure of soil bacteria differed from that of gut bacteria,with relatively small differences in ET,while results of CK and RT were closer to each other(Figs.1d and S1,see Supplementary Material for Fig.S1).Therefore,earthworm gut had a stronger impact on bacterial community structure than rhamnolipids.

Fig.2 Soil and earthworm gut bacterial species number(a)and relative abundance(RA)at the phylum level(b),genus level(c),and species level(d)in a dioxin-contaminated soil added with rhamnolipids and earthworms alone(RT and ET,respectively)and in combination(RET)at the end of a 21-d microcosm experiment.CK=control with no addition.
The dominant flora was the main driver of soil ecological functions; thus,clarifying the effects of rhamnolipids and earthworms on dominant bacteria was conducive to understanding their impacts on the ecological function of microbial communities.As shown in Fig.2b,the top 10 soil bacterial phyla were Proteobacteria,Bacteroidetes,Actinobacteria,Verrucomicrobia,Acidobacteria,Fusobacteria,Chloroflexi,Planctomycetes,Gemmatimonadetes,and Firmicutes.Among them,Proteobacteria occupied an absolute dominant position, with a relative abundance of 94.49%in RT,followed by RET(51.67%),CK(49.20%),and ET(41.66%),indicating that rhamnolipids significantly promoted the growth of Proteobacteria(P<0.01).This may be related to the increases in soil pH and organic matter content caused by rhamnolipid addition,as confirmed by the significant positive correlations of the relative abundance of Proteobacteria with pH and organic matter content(Fig.3a).Notably,the composition of bacteria at the phylum level in ET was close to that in RET,with Bacteroides being the second most dominant(relative abundance=43.24%and 29.06%in ET and RET,respectively).Interestingly,Verrucomicrobia and Fusobacteria were not detected in CK or RT,but were the dominant bacteria in ET and RET,indicating that they were both closely related to earthworm activities (Honget al.,2011).Moreover,rhamnolipids increased the relative abundance of Verrucomicrobia from 7.88%in ET to 11.77%in RET and increased that of Fusobacteria from 4.17%in ET to 5.49%in RET.Correlation analysis further confirmed that these phyla,as dominant bacteria,were closely related to increased NO-3-N,TN,and AK and AP contents,and to the decreased pH caused by earthworm activities(Fig.3a)(Depkat-Jakobet al.,2010;Liuet al.,2013).

Fig.3 Correlations between dominant bacteria at the phylum level in soil and soil physicochemical properties(P <0.05)(a),correlations between dominant bacteria at the genus level in earthworm gut(P <0.05)(b),and co-occurrence network analyses of dominant bacteria at the family level in soil(c)and earthworm gut(d).In a and b,larger circle sizes indicate larger absolute values of R.In c and d,red and blue lines indicate positive and negative correlations,respectively.Asterisks*,**,and***indicate significant differences at the 0.05,0.01,and 0.001 probability levels,respectively.R =correlation coefficient;TP=total P;OM=organic matter;TN=total N;AP=available P;AK=available K;OC=organic C;CEC=cation exchange capacity.
Consistently,earthworms and rhamnolipids also caused significant changes in dominant bacteria at the genus and species levels.Specifically, the total relative abundance of the top 20 soil bacterial genera increased substantially to 70%, 84%, and 78% in RT, ET, and RET, respectively, compared to CK (< 10%) (Fig.2c).In soils without earthworms and rhamnolipids(CK),Acinetobacterwas the bacterial genus with the highest relative abundance,while in RT,Pseudomonasaccounted for 37.15%,followed byStenotrophomonas(8.14%),Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium(6.94%),andPseudoxanthomonas(6.87%).Moreover,the total relative abundance ofPseudomonasandFlavobacteriumin RT(close to 40%) were just equivalent to that ofPseudomonasin ET and RET.The occurrence ofFlavobacterium,Aeromonas,Shewanella,Cetobacterium,andAlgoriphagusclosely correlated with earthworms,implying that they may be from earthworm feces(Yanget al.,2019;Zhuet al.,2021).
As shown in Fig.2d,Pseudomonas alcaligenesshowed a high abundance in RT,ET,and RET,reaching 15.75%in RET,indicating that rhamnolipids are conducive to the proliferation of this group.Similarly,Stenotrophomonas maltophiliashowed marked advantages in soil amended only with rhamnolipids.Consistently,Kumariet al.(2018)reported thatPseudomonas alcaligenesandStenotrophomonas maltophiliahad an outstanding ability to degrade organic pollutants,such as PAHs after rhamnolipid addition.The relative abundance of a type of uncultured Bacteroidetes bacterium reached dominance at 18.75%in ET,which decreased to< 8% in RET.These results indicate that the dominant soil bacteria in four treatments are significantly different.Notably,compared with rhamnolipids,earthworm activities showed more conspicuous impact on bacterial abundance and diversity,which may be closely related to the earthworm gut environment and earthworm fecal transmission.
Responses of earthworm gut bacterial composition torhamnolipid addition
Rhamnolipids and soil bacteria affected by rhamnolipids may enter the gut of earthworms through ingestion, and thus,influence earthworm gut microbial structure and the corresponding ecological functions.The analyses of dominant bacteria at the phylum,genus,and species levels were performed to explore the effect of rhamnolipid addition on the intestinal bacteria of earthworms.Proteobacteria,Actinobacteria,and Verrucomicrobia were the three most dominant bacterial phyla in earthworm gut (Fig.2b).As Actinobacteria was almost absent in soil,we inferred that its growth must be closely related to the anaerobic conditions of earthworm gut(Passet al.,2015).Moreover,rhamnolipids increased the abundance of Proteobacteria from 30.88%in ET to 38.92% in RET,and the same effect was observed on Verrucomicrobia.In contrast,Chloroflexi and Planctomycetes were more abundant in earthworm gut in the absence of rhamnolipids,suggesting that rhamnolipids inhibit the growth of related bacteria.
Furthermore,the analysis of dominant bacterial genera in earthworm gut confirmed that rhamnolipids reduced earthworm gut bacterial diversity.Specifically,the total relative abundance of the top 20 dominant genera was higher in the presence of rhamnolipids(exceeding 65%)than that in the absence of rhamnolipids(<40%)(Fig.2c).In addition,there were obvious differences between ET and RET with respect to the composition of the dominant genera.Luteolibacter(9.51%)was the genus with the highest relative abundance in ET,followed byAgromyces(4.59%),Ensifer(4.02%),andPseudomonas(3.76%).Dominant species in ET werePseudomonas alcaligenes,Sinorhizobiumsp.ZJB1101,andAgromyces ulmi,but their relative abundance was all less than 4%(Fig.2d).However,in the presence of rhamnolipids(RET),the relative abundance ofMicrobacterium,dominated byMicrobacteriumsp.148,exceeded 20%.Moreover,
Chitinibacter(11.63%),Microbacterium(8.87%),andEnsifer(6.06%) were the other three genera whose relative abundance was higher in RET than in ET.In RET,dominant species mainly includedSinorhizobiumsp.ZJB1101(5.37%),Rhodococcus degradans(4.51%),andRoseimicrobium gellanilyticum(2.89%).These bacteria reportedly have an enhanced ability to transform pollutants and a preference for a weakly alkaline environment(Xueet al.,2013),which may be beneficial for the removal of organic pollutants in RET.Therefore,in addition to affecting soil bacterial communities,rhamnolipids have significant effects on bacteria in earthworm gut,including altering community composition and reducing diversity.
Co-occurrence network analyses of bacterial communities in soil and earthworm gut
The co-occurrence networks of dominant bacteria at the family level with a relative abundance greater than 2% in soil and earthworm gut were constructed to analyze the interrelationships among different bacterial genera.There were significant positive correlations (P< 0.01)between Microbacteriaceae,Nocardioidaceae,Streptomycetaceae,Mycobacteriaceae,and other families belonging to Actinobacteria(Fig.3c).Furthermore,most of them were significantly negatively correlated with other phyla (P<0.01).Therefore,in RT and ET,Actinobacteria decreased significantly, while bacteria from other phyla increased.Moreover, negative correlations were found between the families of Proteobacteria and other families.Interestingly,negative correlations also existed among Pseudomonadaceae,Enterobacteriaceae,and most Proteobacteria families,largely because bacteria of the phylum Proteobacteria have a more restrictive niche-adaptation width.
Compared with soil,there was a simple co-occurrence network in earthworm gut (Fig.3b, d).Significant positive correlations were found among these bacteria whose relative abundance was higher in the gut environment in the absence of rhamnolipids, includingLuteolibacter,Cetobacterium,Stenotrophomonas,Pseudoxanthomonas,Shewanella,Flavobacterium,Acinetobacter,Aeromonas,Pseudomonas,andAlgoriphagus.However,when rhamnolipids were added,Chitinibacter,Sphingomonas,Ensifer,Agromyces,Rhodococcus,Microbacterium,Microvirga,Chthoniobacter, andAllorhizobium-Neorhizobium-Pararhizobium-Rhizobiumshowed higher relative abundance and significant positive correlations were also found among them (P< 0.05).As expected,the relative abundance of these two dominant bacterial groups in the treatments with and without the addition of rhamnolipids appeared to be negatively correlated.Therefore,dominant bacteria driven by rhamnolipids tend to be negatively correlated with other genera.
Responses of metabolic pathways of bacteria in soil and earthworm gut toearthworm and rhamnolipid addition
In addition to diversity,biomass,and composition,functional genes of bacterial communities were also predicted herein.As shown in Fig.4a,the abundance of functional genes involved in metabolism was the highest, followed by that involved in environmental information processing,genetic information processing,and cellular processes,with abundance ranging from 100 000 to 500 000.In soil,the addition of rhamnolipids and/or earthworms caused a significant upregulation(7.5%—37.9%)of metabolism-related genes.Meanwhile,the addition of rhamnolipids alone caused the most significant effect(5.7%)on bacterial metabolism in soil and earthworm gut.This was explained by the fact that,as additional nutrients,rhamnolipids can increase microbial metabolic activity.Interestingly,the metabolic activity of bacteria in earthworm gut was superior to that of microorganisms in soil in the same environment,likely due to earthworm gut eutrophication.
The abundance of genes involved in amino acid metabolism, carbohydrate metabolism, metabolism of cofactors and vitamins,metabolism of terpenoids and polyketides,xenobiotic biodegradation and metabolism,and lipid metabolism is higher(Fig.4b).Notably,almost all metabolic pathways of soil bacteria were upregulated in RT compared with other treatments.Dominant pathways included xenobiotic biodegradation and metabolism and lipid metabolism(Fig.S2,see Supplementary Material for Fig.S2).The abundance of related metabolic genes also increased significantly when earthworms and rhamnolipids co-existed.Consistently,xenobiotic biodegradation and metabolism and carbohydrate metabolism of earthworm gut bacteria in RET increased compared with ET,suggesting that rhamnolipids not only affect the metabolic activity of soil bacteria but may also indirectly affect earthworms by directly affecting their intestinal bacteria,which may result from the utilization of rhamnolipids by bacteria.
Bacterial pathways related to microbial responses to polluted environments mainly included functional genes involved in environmental information processing,whose abundance followed the order: RT> RET> ET> CK in soil and RET> ET in earthworm gut (Fig.4c).This may be because rhamnolipids increase the bioavailability of pollutants such as dioxin and stimulate the stress response of bacteria,resulting in a distinct increase in the abundance of functional genes involved in environmental information processing(Linet al.,2021).Notably,the abundance of related functional genes in RET was lower than that in RT,but higher than that in CK probably because the dominant bacteria carried by earthworms are efficient pollutant transformers that rapidly degrade pollutants into a bioavailable state(Hanet al.,2021).Therefore,pollutants will not be sufficient to elicit a strong response to environmental stress.Overall,the addition of rhamnolipids and earthworms changed the metabolic pathways of soil bacteria,particularly those involved in metabolism and environmental adaptation.
Response of dioxin degradation in soil toearthworm and rhamnolipid addition
The purpose of using rhamnolipids and earthworms in bioremediation is to promote the degradation of pollutants in soil.In this study,contaminants that may be present in iron and steel plants were detected,and the contents of PAHs and heavy metals(Zn and Cu)didn’t exceed the pollution standard(Table SI).The initial concentration of dioxin with severe biological poisoning risks was 3.61 ng kg-1of soil,which exceeds the WHO control standard for soil dioxin(0.60 ng kg-1).Therefore, the degradation efficiency of dioxins after cultivation was analyzed.As expected, the addition of rhamnolipids and earthworms significantly(P<0.05) reduced the concentration of dioxin in soil,with a descending order of CK(3.44 ng kg-1)>RT(2.72 ng kg-1)>ET(1.45 ng kg-1)>RET(0.82 ng kg-1).Compared with CK,rhamnolipids slightly improved dioxin degradation rate from 4.71%to 24.65%(Fig.4d).However,in soil amended only with earthworms(ET),dioxin degradation rate increased to 59.83%.Interestingly,dioxin content decreased to 1.00 ug kg-1in RET,with the degradation rate exceeding 77%,indicating that the simultaneous presence of rhamnolipids and earthworms further promoted pollutant degradation.
Pearson correlation analysis showed that the residual amount of dioxin was significantly negatively correlated with the abundance of phyla Verrucomicrobia and Fusobacteria(Fig.4a).The existence of these bacteria was directly related to the activities of earthworms and was further enriched in soil amended with both rhamnolipids and earthworms(Fig.2b).In addition,there was a significant (P< 0.05)negative correlation between the residual amount of dioxin and the abundance of generaMicrobacterium,Shewanella,Chthoniobacter,Microvirga,andRhodococcus.The relative abundance of these bacterial genera also increased in earthworm gut and soil where earthworms and rhamnolipids were added in combination.Previous studies have shown that these genera have strong pollutant tolerance and can convert pollutants into nitrates(Torreset al.,2018;Guoet al.,2022).Therefore,we speculate that earthworm activity will promote the colonization of pollutant-degrading bacteria,while the addition of rhamnolipids can further promote the proliferation of related bacteria in soil and earthworm gut by providing more nutrient substance(Fig.1a).In addition,as biosurfactants,rhamnolipids can improve the desorption of pollutants from the surface of soil particles,further enhancing the degradation rate of dioxin in soil(Rahmanet al.,2003).
CONCLUSIONS
Compared with earthworms, rhamnolipids increased soil and earthworm gut bacterial abundance and metabolic activity more significantly;however,there were strong differences in bacterial composition and contaminant degradation, mainly driven by earthworms.Notably, bacterial community abundance and pollutant metabolism were further enhanced when rhamnolipids were added in combination with earthworms.Moreover,rhamnolipids influenced earthworm gut bacterial composition and metabolism,leading to the degradation and removal of pollutants.However,the rapid proliferation of dominant bacteria under appropriate conditions influenced by rhamnolipids and earthworms led to a decrease in bacterial diversity in soil and earthworm gut.Therefore,our study provides new theoretical support for the application and ecological risk evaluation of biosurfactants used as a tool for bioremediation of soil pollution.
ACKNOWLEDGEMENT
This study was financially supported by the National Key Research and Development Program of China (No.2018YFC1803100) and the Youth Innovation Promotion Association of Chinese Academy of Sciences(No.2018350).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
- Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil
- Environmental similarity is more important than distance in the community structuring processes of ammonia-oxidizing archaea in agricultural soils
