Phosphorus speciation and colloidal phosphorus responses to short-term cessation of fertilization in a lime concretion black soil
2023-12-21ShanshanBAIJinfangTANZeyuanZHANGMiWEIHuiminZHANGandXiaoqianJIANG
Shanshan BAI,Jinfang TAN,Zeyuan ZHANG,Mi WEI,Huimin ZHANG and Xiaoqian JIANG,
1School of Agriculture,Sun Yat-sen University,Guangzhou 510275(China)
2Shenzhen Campus of Sun Yat-sen University,Shenzhen 518107(China)
3Modern Agricultural Innovation Center,Henan Institute of Sun Yat-sen University,Zhumadian 463400(China)
4Institute of Agricultural Resources andRegional Planning,Chinese Academy of Agricultural Sciences/National Engineering Laboratory for Improving Quality of Arable Land,Beijing 100081(China)
ABSTRACT Long-term excessive application of mineral fertilizer has led to soil acidification and phosphorus(P)accumulation,increasing the risk of P loss and environmental pollution,and cessation of fertilization is widely considered as a cost-effective management strategy to relieve this situation;however,how such cessation influences P speciation and concentrations in a bulk soil and colloidal fractions and whether decreasing P concentration might maintain soil fertility remain unclear.In this study,the effects of long-term fertilization(ca.40 years)and short-term cessation of fertilization(ca.16 months)on inorganic,organic,and colloidal P in lime concretion black soil were investigated using P sequential fractionation and 31P nuclear magnetic resonance spectroscopy.After long-term fertilization,available P,dicalcium phosphate,iron-bound P,orthophosphate monoesters,and orthophosphate diesters increased significantly,but soil pH decreased by ca.2.8 units,indicating that long-term fertilization caused soil acidification and P accumulation and changed P speciation markedly.In contrast,short-term fertilization cessation increased soil pH by ca.0.8 units and slightly reduced available and inorganic P.Available P after fertilization cessation was 22.9—29.8 mg kg-1,which was still sufficient to satisfy crop growth requirements.Additionally,fertilization cessation increased the proportions of fine colloids(100—450 nm,including nontronite and some amorphous iron oxides)and drove a significant release of iron/aluminum oxide nanoparticles(1—100 nm)and associated P with orthophosphate and pyrophosphate species.In summary, short-term fertilization cessation effectively alleviated soil acidification and inorganic P accumulation,while concomitantly maintaining soil P fertility and improving the potential mobilization of P associated with microparticles.
Key Words: Fe/Al oxide nanoparticles,fine colloids,P accumulation, 31P nuclear magnetic resonance,P sequential fractionation,soil acidification
INTRODUCTION
As an essential macronutrient for crop plants (Westheimer, 1987), phosphorus (P) participates in metabolic processes in various ways and plays a significant role in the overall function of agricultural ecosystem(Vanceet al.,2003).Lime concretion black soil is a typical medium-to low-yield soil in the Huang-Huai-Hai Plain of China,classified as silty texture according to World Reference Base(IUSS Working Group WRB,2014)and characterized by low organic matter content and nutrient imbalance(Liet al.,2011).The low availability and insufficient supply of P in this soil severely restrict agricultural production in this area(Sunet al.,2001;Maet al.,2019).
Excessive application of mineral fertilizers over a long period of time to increase crop production in this area has led to soil acidification(Chenet al.,2014)and a significant accumulation of soil P(Caoet al.,2012),which in turn has increased the risk of soil P loss and environmental pollution(Pahalviet al.,2021).Indeed,P loss from agricultural soils has been recognized as one of the major sources of non-point pollution and contributes to the eutrophication of nearby water bodies(Liet al.,2015).Various management strategies have been implemented to relieve the situation, among which the cessation of fertilization is widely considered to be a cost-effective measure acceptable to farmers(Cade-Menunet al., 2017).Additionally, concerns with respect to the possibility for decreasing soil P concentration to maintain soil fertility during the cessation of fertilization have been raised.Therefore,information regarding the change of soil P in response to long-term fertilization and short-term cessation of fertilization is urgently needed to understand soil P supply capacity under different management strategies and to provide an insight into the sustainability of agricultural eco-environment.
Accumulated P in soils comprises various inorganic and organic species,with different potentials for bioavailability(Turneret al., 2007; Audetteet al., 2016).It is reported that inorganic P includes aluminum (Al)-bound P (Al-P),iron (Fe)-bound P (Fe-P), calcium (Ca)-bound P (Ca-P),and occluded phosphate(O-P)fractions,among others(Rodrigueset al., 2016).Previous studies have indicated that accumulated P in lime concretion black soil is mainly transformed into Al-P,Fe-P,O-P,and apatite(Ca10-P),whereas the transformation into dicalcium phosphate(Ca2-P)and octacalcium phosphate(Ca8-P)occurs to a minor extent(Wanget al.,2009).Xuet al.(2022)found that mineral fertilization significantly increased the concentrations of Ca2-P,Al-P,and Fe-P,concomitant with a slight decrease in the concentration of Ca10-P,indicating that mineral fertilization promoted the transformation of low-activity inorganic P into high-activity inorganic P in lime concretion black soil.Soil organic P has a variety of species that differ in terms of bioavailability(Shenet al.,2011;Sulieman and Mhling,2021),including phosphate monoesters,phosphate diesters,phosphonates,and organic polyphosphates(Condronet al.,2005).Phosphate monoesters are more strongly absorbed by metal oxides due to their large charge density(Murphyet al.,2009),whereas weak sorption makes phosphate diesters more susceptible to transport and degradation(Turneret al.,2002).
Long-term mineral fertilization in Haplic Kastanozem soil is reported to increase soil carbon (C), nitrogen (N),and Olsen-P contents, as well as the concentrations of NH4Cl-extracted P(soluble and loosely bound P)and NH4Fextracted P (Al-P), while the cessation of fertilization reduced inorganic P and orthophosphate concentrations but had no significant effect on organic P concentrations(Chenet al.,2021;Cade-Menun,2022).It is expected that longterm fertilization in lime concretion black soil leads to the accumulation of inorganic and organic P,as well as to the transformation of P fractions and species,whereas the concentrations of high-activity inorganic P fractions and orthophosphate decrease after short-term cessation of fertilization.Thus,it is necessary to study the dynamic changes in different P fractions and species to understand the underlying processes,by which different fertilization management strategies affect soil P dynamics.
Phosphorus availability and mobility depend on the concentration and speciation of P in soil solution, which contains both dissolved and colloidal P (Rick and Arai,2011;Hartlandet al.,2013).Recent studies have shown that colloidal P,especially in<450 nm soil fraction,comprised a high proportion of P in soil solution,which contributes to plant available P after its release by dissolution or desorption or by diffusion near plant roots(Montalvoet al.,2015;Bollynet al.,2017).Bollynet al.(2019)demonstrated that P bound to Fe oxide nanoparticles increased crop growth and P uptake.Jianget al.(2015)reported that colloidal P was present in association with fine clay,Fe/Al oxide minerals,and organic matter.Previous studies(Squariset al.,2013;Fresneet al.,2021)have shown that colloidal P content and composition are affected by the changes in soil properties(e.g.,soil pH and organic C) and mineral forms (e.g., Ca minerals and Fe/Al oxide minerals).For example,a decrease in soil pH might increase the dissolution of Ca minerals and promote the release of Fe/Al oxides(Condron and Newman,2011).However, an increase in soil pH might generate repulsive forces that drive colloid release(Ryan and Elimelech,1996).It is hypothesized that the concentrations and compositions of colloidal and dissolved P would be affected under long-term mineral fertilization and short-term cessation of fertilization,considering that different fertilization strategies might change soil properties such as soil pH and organic C (Zhuet al.,2014;Chenet al.,2020).However,little is known about P speciation and the concentrations of colloidal and dissolved P fractions under different fertilization strategies in soil,which is of great significance for understanding the potential bioavailability and mobility of soil P.
The objective of the present study was to investigate the effects of long-term fertilization and short-term cessation of fertilization on properties, element concentrations in different soil fractions, inorganic P fractions, organic P species,and colloidal and dissolved P in a lime concretion black soil.For this purpose,P sequential fractionation,31P nuclear magnetic resonance(NMR)spectroscopy analysis,and soil fractionation were conducted.Our study could provide important new insights for the development of P fertilization management strategies in lime concretion black soil and contribute to agroecosystem sustainability.
MATERIALS AND METHODS
Study sites andsoil sampling
The study sites were located in Pingyu County of Henan Province,China,where the annual mean temperature and precipitation are 15◦C and 904.3 mm, respectively (Fig.S1, see Supplementary Material for Fig.S1).The soil at these sites is a lime concretion black soil with silty texture,according to World Reference Base for Soil Resources(IUSS Working Group WRB, 2014).Three sampling sites, 15—46 km apart, were chosen for investigation (Table SI, see Supplementary Material for Table SI).At the three sites,soil samples(1-F,2-F,and 3-F)were collected from the topsoil(0—20 cm)in arable winter wheat fields under long-term(ca.40 years)fertilization.Adjacent to the crop field at site 1,control soil sample (1-CKL) was collected from a fallow field that had not been cropped or fertilized forca.40 years.Additionally,control soil samples(2-CKSand 3-CKS)were also collected from winter wheat fields under short-term(ca.16 months) cessation of fertilization at sites 2 and 3,respectively.The particle size and texture classes of lime concretion black soil samples are listed in Table SII (see Supplementary Material for Table SII).We collected soil samples from three plots for each fertilization treatment at each site.In each plot, two soil cores were collected and then pooled to form a composite sample.The plots were distributed into the field diagonally with a distance of at least 30 m from each other.Then, the soil samples were all air-dried,ground,homogenized,passed through a 2-mm sieve,and stored at room temperature until analysis.
Winter wheat and summer maize rotation were adopted as the local planting system.The fertilizer application dose and methods for winter wheat were 750 kg ha-1compound fertilizer(N:P2O5:K2O=15:15:15),150 kg ha-1urea as basal fertilizer,and 150 kg ha-1urea as topdressing in the reviving-jointing period.Summer maize was planted under no tillage after wheat harvesting.Fertilizer application for summer maize included 600 kg ha-1compound fertilizer(N:P2O5:K2O=28:6:6)as basal fertilizer and 225 kg ha-1urea as topdressing in the flare opening period.Crop straw was crushed and rototilled after harvest.In addition,weeds,disease,and insect control were uniformly managed during the winter wheat and summer maize growing seasons.
Analysis of soil properties
The distribution of particle size(soil texture)was determined by the pipette method after sieving,and the texture classes were determined according to World Reference Base for soil resources(IUSS Working Group WRB,2014).Soil pH was measured with a pH meter in aqueous soil suspension(a soil to water ratio of 1:2.5).Soil total C and N were determined by an elementary analyzer (Vario MAX CNS,Elementar,Germany).Soil cation exchange capacity(CEC)was determined by the hexaamminecobalt chloride solution-spectrophotometric method(Aranet al.,2008).The total concentrations of Ca,magnesium(Mg),Al,Fe,and P were analyzed by inductively coupled plasma optical emission spectroscopy(ICP-OES)(Avio 500,PerkinElmer,USA)after microwave digestion with HNO3and HF.There was no residue left after digestion.Soil available P was extracted with 0.5 mol L-1NaHCO3at pH 8.5(Olsen and Sommers,1982)and determined by the molybdate blue colorimetric method(Murphy and Riley,1962).
Soil particle separation was achieved by the method of Squaris and Lewandowski(2003)according to Stokes’Law.Briefly, approximately 100 g of dried soil was dispersed in 200 mL ultrapure water (1:2, weight:weight) in a 1-L bottle.The resultant suspension was shaken for 6 h at 150 r min-1,and then 600 mL ultrapure water was added and mixed.After standing for 6 min, the particles> 20 µm were obtained by removing the supernatant, which was subsequently settled for 12 h and then the new supernatant was removed to obtain soil particle size fraction from 2 to 20µm.The newly collected supernatant was subsequently centrifuged(4-TK,Hengnuo,China)at 3 500×gfor 4 min to separate particle size fraction of 0.45—2 µm.The time for sedimentation and centrifugation was calculated based on Stokes’ Law, which assumed a spherical geometry of particles, with a mean particle density of 2.65 g cm-3and the media density of 1.00 g cm-3(Hendersonet al.,2012).The supernatant was further centrifuged at 3 500×gfor 54 min to obtain particle size fraction of 100—450 nm.The<100 nm particle size fraction was obtained using a pipette and contained the electrolyte phase as well as nanoparticles.Finally,the<3 kDa fraction(nominally 1 nm)(Erickson,2009)was subsequently isolated by passing through a 3-kDa filter(Amicon Ultra,Millipore,USA)by centrifugation.Determinations of Ca, Mg, Al, Fe, and P in the<100 nm and<3 kDa particle size fractions were performed by ICP-OES.The separated particle size fractions were frozen and subsequently lyophilized.An X-ray powder diffractometer(XRD)(Empyrean,PANalytical,Holland)was used to identify minerals for various particle size fractions in the 2θrange from 3◦—90◦.Scan step size and scan rate were 0.026◦and 10◦min-1,respectively.In this study,the particle size fractions of 100—450 nm,1—100 nm,and<3 kDa in the supernatant were defined as fine colloids,nanoparticles,and dissolved fraction,respectively.
Inorganic P fractions were analyzed following the sequential fractionation method(Jiang and Gu,1989;Adhamiet al.,2006;Audetteet al.,2016).Briefly,1.25 g of soil sample was mixed with 25 mL of 0.25 mol L-1NaHCO3(pH 8.0)for 1 h and centrifuged to obtain Ca2-P.This extraction procedure was then repeated sequentially with 0.5 mol L-1NH4Ac (pH 4.2) for Ca8-P, 0.5 mol L-1NH4F(pH 8.2)for Al-P,0.1 mol L-1NaOH-Na2CO3(pH 12)for Fe-P,0.3 mol L-1sodium citrate-dithionite-sodium hydroxide (pH 13)for O-P,and 0.25 mol L-1H2SO4(pH 1.0)for Ca10-P(Audetteet al.,2016).The concentration of each P fraction was measured by the molybdate blue colorimetric method(Murphy and Riley,1962).
Solution 31P NMR spectroscopy
Solution31P-NMR spectroscopy of alkaline extracts has been widely used for elucidating species and quantification of soil organic P (Turner, 2008).Bulk soil samples (4 g)were extracted with 40 mL mixture solution containing 0.25 mol L-1NaOH and 0.05 mol L-1Na2EDTA for 4 h.After shaking for 4 h,extracts were centrifuged for 30 min at 10 000×g.An aliquot of 2 mL supernatant was used to measure total extracted P content by ICP-OES,and the remaining solutions were frozen at-80◦C,subsequently lyophilized,and ground.One hundred milligrams of freeze-dried extract and<100 nm particle size fraction without NaOH-Na2EDTA treatment were separately dissolved in 0.1 mL deuterium oxide(D2O)and 0.9 mL solution containing 1.0 mol L-1NaOH and 0.1 mol L-1Na2EDTA.Then,an aliquot of 20µL NaOD was added to the<100 nm particle size fraction to adjust the pH(Jianget al.,2017b).The reconstituted suspension was centrifuged again for 20 min at 13 000×gand the supernatant was subjected to NMR measurement on a Bruker 500-MHz spectrometer(Germany)equipped with a 5-mm NMR tube.
The NMR parameters for bulk soil samples and the<100 nm particle size fraction were as follows:3.93 and 2.85 s relaxation delay,respectively,0.68 s acquisition time,90◦pulse width, and 8 000 scans.The longest relaxation time was estimated according to the correlation between P/(Mn+Fe)and spin-lattice relaxation times(McDowellet al.,2006).Compounds were identified by their chemical shifts after the orthophosphate peak in each spectrum was standardized to 6.0 ppm during processing (Table SIII,see Supplementary Material for Table SIII)(Cade-Menunet al.,2010).Peak assignments were based on spiking experiments withmyo-inositol hexakisphosphate(myo-IHP),α-/β-glycerophosphate,and adenosine monophosphate(Fig.S2,see Supplementary Material for Fig.S2)and previous reports(Cade-Menunet al.,2017;Jianget al.,2017a).Previous studies have shown that,by31P NMR spectroscopy analysis,the degradation of orthophosphate diesters resulted inαandβ-glycerophosphates and mononucleotides(Gly+Nuc)(Dooletteet al.,2009),which were detected in the orthophosphate monoester region.These compounds were categorized as orthophosphate diesters rather than monoesters(Younget al., 2013; Liuet al., 2018).Peak areas were calculated manually by integration on spectra processed with 7 and 2 Hz line broadening.The concentrations of P species were determined by multiplying peak areas by the concentration of NaOH-Na2EDTA-extracted P for each sample.All spectra were processed with Mestrenova 10.0.2 software.
Statistical analysis
Data were assessed with Shapiro-Wilks tests to meet the criteria of normal distribution and were transformed as needed.Data for the proportions of soil particle fractions to bulk soil were normalized using centered log-ratio transformation.One-way analysis of variance (ANOVA)was conducted to determine differences in soil properties,element concentrations,total P,available P,inorganic P,and organic P among different samples.Differences among mean values were calculated using Tukey’s honestly significant differences(HSD)test at a significance level of 0.05.The analysis was conducted by SPSS 18.0(SPSS Inc.,USA)and Excel software.Figures were created using OriginPro 2021(OriginLab Corp.,USA).
RESULTS
Soil properties
Long-term fertilization at site 1(1-F)significantly increased soil total C, total N, CEC, and available P, while significantly reducing pH by 2.80 units,from 7.96 to 5.16,compared to the sample without long-term fertilization and crop cultivation(1-CKL)(Table I).Conversely,fertilization cessation significantly increased soil pH by 0.81—0.87 units,indicating that it effectively relieved soil acidification in the short term.Additionally,there was no significant difference in total C, total N, and CEC.However, available P decreased by 4.6—11.3 mg kg-1after short-term cessation of fertilization.
Inorganic P fractions
The concentrations and proportions of each inorganic P fraction are shown in Table II and Fig.S3(see Supplementary Material for Fig.S3).The highest concentration of inorganic P (424.0 mg kg-1) was observed in 1-CKL,which contained the highest proportion of Ca10-P(69.2%of inorganic P)as well.After long-term fertilization at site 1,the concentrations of Ca2-P,Al-P,and Fe-P increased by 13.2,36.8,and 11.0 mg kg-1,respectively,whereas that of Ca10-P decreased by 140.1 mg kg-1(Table II).In contrast,total inorganic P concentrations decreased by 45.3—49.6 mg kg-1after short-term cessation of fertilization.Among these,the concentrations of Al-P, Fe-P, and Ca10-P slightly decreased; furthermore, there was no significant difference among fertilization cessation and long-term fertilization(Table II).

TABLE I Chemical properties of bulk soil in lime concretion black soil samples
Solution 31P NMR spectroscopy analysis of bulk soil and<100 nm particle size fraction
According to NMR results(Fig.S4,see Supplementary Material for Fig.S4),orthophosphate accounted for 59.5%—100.0%of extracted P in bulk soil samples.Pyrophosphate(0.7%—2.2% of extracted P) was observed in all samples.Pyrophosphate is derived from soil fauna,flora,and microbes and becomes bioavailable following hydrolysis(Turneret al.,2005).Jiang and Arai(2018)noted that the concentration of pyrophosphate as per NMR spectroscopy analysis was slightly underestimated due to the degradation during extraction.The proportion of organic P relative to extracted P was 24.6%—38.7%,among which the orthophosphate monoesters and orthophosphate diesters accounted for 19.8%—33.0%and 4.8%—6.4%of extracted P,respectively.
Organic P was not detected in 1-CKL.In turn,the organic P concentration in 1-Fwas 85.0 mg kg-1and accounted for 32.6%of extracted P(Table III,Fig.S4).The concentration of orthophosphate monoester in 1-Fwas 69.5 mg kg-1(Table III).These findings indicated that the organic P concentrations in bulk soil samples were significantly increased by long-term fertilization.

TABLE II Concentrations of inorganic P fractionsa) in bulk soil of lime concretion black soil samples

TABLE III Concentrations of P species in bulk soil of lime concretion black soil samples evaluated by solution 31P nuclear magnetic resonance spectroscopy analysis
The NaOH-Na2EDTA-extracted P in bulk soil samples decreased by 44.4—53.3 mg kg-1after short-term cessation of fertilization(Table III).Among P species,the concentrations of orthophosphate decreased by 21.3—34.3 mg kg-1.For organic P, orthophosphate monoesters were the dominant group of organic P compounds(>80%of organic P),which was consistent with the results of a previous study(Turneret al., 2005).Indeed,myo-IHP was considered as a predominant species of organic P (Turneret al., 2003) and accounted for 8.4%—15.6% of organic P across all samples.Furthermore,Gly+Nuc were the dominated orthophosphate diesters in bulk soil.For these degradation products of orthophosphate diesters,α- andβ-glycerophosphates were derived from the degradation of phospholipids,while mononucleotides were derived from RNA and were usually associated with living soil microbial biomass(Turneret al.,2007;Vestergrenet al.,2012).The typical orthophosphate monoester(i.e.,myo-IHP)and orthophosphate diester degradation products(i.e.,Gly+Nuc)did not change significantly after short-term cessation of fertilization.
In<100 nm particle size fraction,no P was detected in 1-CKL, while the concentration of water-extracted P was 1.87—11.76 mg kg-1for other samples (Table IV).Different from bulk soil,< 100 nm particle size fraction mainly contained inorganic P species,including orthophosphate (97.1%—100.0% of extracted P) and pyrophosphate(0.0%—2.9%of extracted P)according to NMR results(Fig.S5,see Supplementary Material for Fig.S5).Furthermore,the opposite trend was observed for orthophosphate and pyrophosphate in<100 nm particle size fraction after shortterm cessation of fertilization, which tended to increase compared to long-term fertilization(Table IV).
Soil particle size fractions
Long-term fertilization had no significant effects on the proportions of sand(>20µm)and silt(2—20µm)fractions,but increased the proportion of clay particles (< 2 µm)significantly(Fig.1).The clay fractions for 1-CKLand 1-F accounted for 0.34%and 6.94%of bulk soil,respectively.Short-term fertilization cessation had no significant effects on the proportions of sand,silt,and 0.45—2µm soil particle size fractions,but increased the proportion of 0.1—0.45µm particle size fraction.
Based on the powder X-ray diffraction patterns,minerals in 2—20 µm soil particle size fraction were dominated by quartz,illite,nontronite,and chlorite(Fig.2).For 0.45—2µm fraction,we observed phyllosilicates represented by illite,montmorillonite,and nontronite(Fig.2).In turn,minerals in 0.1—0.45µm soil particle size fraction contained mainly illite and nontronite(Fig.S6,see Supplementary Material for Fig.S6).

TABLE IV Concentrations of P species in < 100 nm particle size fraction of lime concretion black soil samples evaluated by solution 31P nuclear magnetic resonance spectroscopy analysis

Fig.1 Proportions of soil particle size fractions in bulk soil in lime concretion black soil samples.1-CKL is the control soil sample collected from a fallow field(adjacent to the crop field at site 1)that has not been cropped or fertilized for ca.40 years;1-F,2-F,and 3-Fare the soil samples collected from winter wheat fields under long-term(ca.40 years)fertilization at sites 1,2,and 3,respectively;2-CKS and 3-CKS are the control soil samples collected from winter wheat fields under short-term(ca.16 months)cessation of fertilization at sites 2 and 3,respectively.Vertical bar indicates standard deviation of the mean(n=3).Bars with the same letter for each soil particle size fraction are not significantly different at P <0.05.
Element concentrations in bulk soil, fine colloids, nanoparticles,anddissolvedfraction
Element concentrations in bulk soil and fine colloids are shown in Fig.3.The concentrations of Ca and Mg(18.85 and 8.68 g kg-1,respectively)in 1-CKLwere significantly higher than those in other soil samples,while no significant differences in the concentrations of Al and Fe were detected among bulk soil samples.The P concentrations in bulk soil ranged from 407.3 to 595.5 mg kg-1.
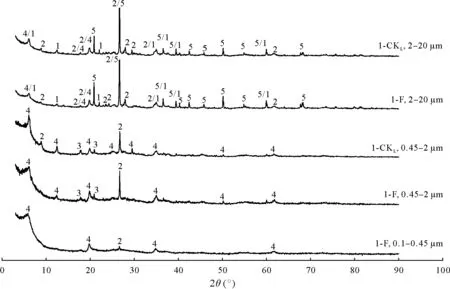
Fig.2 Powder X-ray diffraction patterns of soil particle size fractions in lime concretion black soil samples at site 1.1-CKL is the control soil sample collected from a fallow field(adjacent to the crop field at site 1)that has not been cropped or fertilized for ca.40 years,1-Fis the soil sample collected from winter wheat field under long-term(ca.40 years)fertilization at site 1,and 1,2,3,4,and 5 represent chlorite,illite,montmorillonite,nontronite,and quartz,respectively.

Fig.3 Element concentrations in bulk soil and fine colloids(100—450 nm fractions)of lime concretion black soil samples.1-CKL is the control soil sample collected from a fallow field(adjacent to the crop field at site 1)that has not been cropped or fertilized for ca.40 years,1-F,2-F,and 3-Fare the soil samples collected from winter wheat fields under long-term(ca.40 years)fertilization at sites 1,2,and 3,respectively,and 2-CKS and 3-CKS are the control soil samples collected from winter wheat fields under short-term(ca.16 months)cessation of fertilization at sites 2 and 3,respectively.Vertical bar indicates standard deviation of the mean(n=3).Bars with the same letter(s)for bulk soil/fine colloids are not significantly different at P <0.05.
The amount of fine colloids in 1-CKLwas below detection level.There were no significant differences in the concentrations of Ca, Mg, Al, and Fe in fine colloids among other samples(Fig.3).The concentration of Fe in fine colloids,ranging from 37.85 to 57.25 g kg-1,was significantly higher than those of other elements.The concentration of P in fine colloids ranged from 434.2 to 748.8 mg kg-1and decreased slightly after short-term cessation of fertilization.
As shown in Fig.4,the concentrations of Ca,Mg,Al,and Fe in nanoparticles in 1-Fsignificantly increased compared to 1-CKL;however,no P was detected in 1-CKLand the concentration of P in 1-Fwas 0.76 mg kg-1(Fig.4).Notably, the concentrations of Ca, Mg, Al, Fe, and P in nanoparticles increased significantly after short-term cessation of fertilization,with the highest concentrations recorded for Al and Fe.
In dissolved fraction,the concentrations of Ca and Mg(158.5 and 11.9 mg kg-1,respectively)in 1-CKLwere significantly higher than those in other soil samples(Fig.4).No P was detected in 1-CKLand the concentration of P in 1-Fwas 3.7 mg kg-1(Fig.4).After short-term fertilization cessation, the concentrations of Ca and Mg in dissolved fraction decreased by 28.5—40.4 and 3.5—7.4 mg kg-1,respectively,while those of Al and Fe increased by 16.0—24.0 and 11.0—19.3 mg kg-1,respectively.No significant change was observed for the concentration of P in dissolved fraction after short-term fertilization cessation, with the range of 1.0—5.3 mg kg-1(Fig.4).
DISCUSSION
Changes in soil properties andP speciation after long-term fertilization
The lime concretion black soil in 1-CKL(without longterm fertilization and crop cultivation)was slightly alkaline with high concentrations of Ca and Mg in bulk soil(Table I,Fig.3).Inorganic P was dominated by Ca10-P (Table II),which might cause low available P.Liet al.(2011)reported that lime concretion black soil was a typical carbonate concretion near-alkaline soil.The formation of metal complexes such as Ca-P under alkaline soil conditions causes low P availability for crops(Westermann,1992;Iyamuremye and Dick,1996).Organic P was not detected in 1-CKL(Table III),which might be explained by the low organic matter content in the lime concretion black soil(Table I).
Long-term fertilization enhanced total C and N concentrations,while reducing soil pH significantly,which in turn resulted in soil acidification.These results were in accordance with previous studies on the lime concretion black soil under mineral fertilization(Zhuet al.,2014;Chenet al.,2020).The increase in soil total C and N concentrations might be related to the increased organic matter by biomass inputs from crops and the application of mineral N fertilizer.Soil acidification may be attributed to the increased release of protons from nitrification processes that occurs as a result of excessive application of mineral fertilizers and the continuous removal of base cations by crop harvest(Guoet al.,2010).
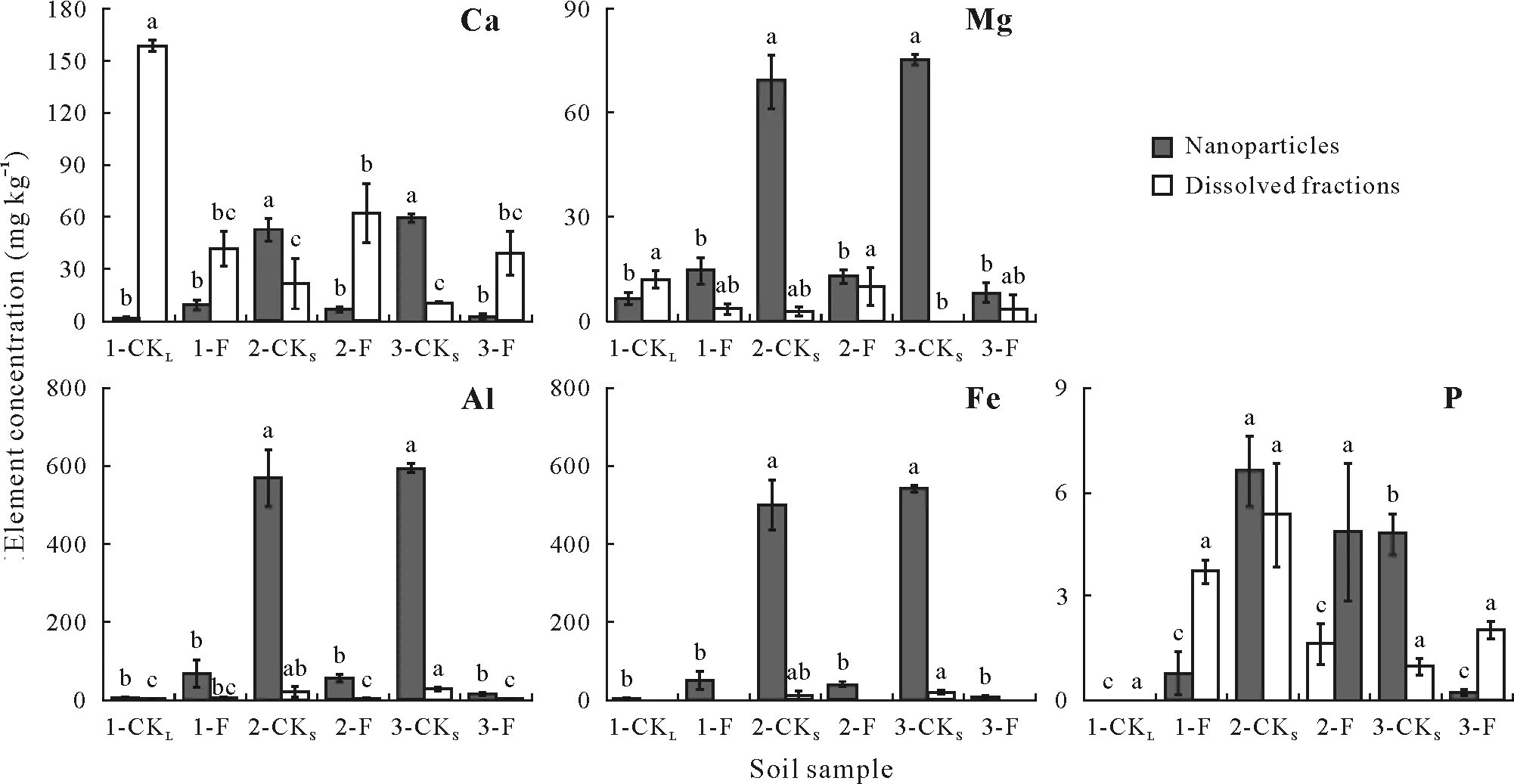
Fig.4 Element concentrations in nanoparticles(1—100 nm)and dissolved fraction(<3 kDa)of lime concretion black soil samples.1-CKL is the control soil sample collected from a fallow field(adjacent to the crop field at site 1)that has not been cropped or fertilized for ca.40 years,1-F,2-F,and 3-Fare the soil samples collected from winter wheat fields under long-term(ca.40 years)fertilization at sites 1,2,and 3,respectively,and 2-CKS and 3-CKS are the control soil samples collected from winter wheat fields under short-term(ca.16 months)cessation of fertilization at sites 2 and 3,respectively.Vertical bar indicates standard deviation of the mean(n=3).Bars with the same letter(s)for nanoparticles/dissolved fraction are not significantly different at P <0.05.
Long-term fertilization caused a significant increase in the proportion of clay particles (Fig.1), which might be explained by mineral weathering, dissolution of mineral phases with a decrease of soil pH,and increase of organic matter.The decrease of soil pH accelerated weathering,dissolution,and transformation of Ca mineral phases,which were transformed into secondary mineral forms(Bunnet al.,2002).Moreover, the increased organic C, as well as the interactions among organic C,mineral surfaces,and metal ions,might contribute to the formation of microaggregates,thus affecting the release and stability of clay particles(Jastrow,1996).Previous studies have shown that soil CEC was dependent on the content and type of soil colloids,clay minerals,and soil organic matter(Murray,2007).The significant increase in CEC under long-term fertilization might be attributed to the increase of clay particle and organic matter contents(Fig.1,Table I).
Long-term fertilization slightly increased total P and significantly increased available P(byca.8 times)in bulk soil(Table I).Phosphorus input by mineral fertilization in winter wheat and summer maize rotation system at our study site was 148.5 kg ha-1per year.In turn,phosphorus removal by crop growth and harvest was estimated at about 140 kg ha-1per year according to previous reports(Wanget al.,2017;Zhaoet al.,2021).Thus,P surplus reached approximately 340 kg ha-1over approximately 40 years, which was similar to the P stock(ca.300 kg ha-1)in the 0—20 cm topsoil after long-term mineral fertilization(Table I).Sieberset al.(2021)found that the total P in the topsoil(0—30 cm)was insignificantly affected by fertilization management practice.Similarly, Huaet al.(2016) reported that longterm fertilization increased available P by 6.6—12.0 times.In this study,a remarkable decrease of Ca10-P and a significant increase of Ca2-P and Fe-P were found for 1-Funder long-term fertilization(Table II).These findings might be partly explained by the input of P fertilizer.Furthermore,soil pH was an important factor affecting soil P availability.The decrease in soil pH likely increased the dissolution and transformation of Ca10-P, subsequently increasing P availability(Zhaoet al.,2019).Several studies have shown that the predominant primary Ca-P in a calcareous soil parent material was transformed into secondary adsorbed and mineral forms of inorganic P associated with Al and Fe by weathering(Condron and Newman,2011).Therefore,the increase in available P was promoted by various factors,including fertilization,weathering,and the transformation of bonded P with the decrease of soil pH.Organic P concentrations in bulk soil improved significantly after long-term fertilization(Table III).Long-term application of fertilizers increased plant biomass and parts of underground biomass such as plant residues and root exudates and enhanced the accumulation of soil organic matter and organic P (Tonget al.,2019).
Responses of soil properties andP speciation to short-term fertilization cessation
Cessation of fertilization increased soil pH significantly(Table I),indicating that such cessation relieved soil acidification effectively in the short term.The lower concentrations of Ca,Mg,and Al and the higher concentration of Fe were monitored in fine colloids compared with bulk soil (Fig.3), indicating fine colloids were dominated by Fe minerals.The XRD pattern of fine colloids contained nontronite(Fig.S6),and we hypothesized that there might also be some amorphous Fe oxides in fine colloids(Pronk
et al.,2011).
Notably,the concentrations of Ca,Mg,Al,Fe,and P in nanoparticles were significantly improved after short-term cessation of fertilization compared to long-term fertilization,especially for the concentrations of Al and Fe.The repulsive electrostatic forces between the Fe/Al oxide nanoparticles and soil clay mineral surface increased with increasing soil pH(Ryan and Elimelech,1996),which might drive the release of Fe/Al oxide nanoparticles and P associated with those Fe/Al oxides.Consistently, P associated with fine colloids and nanoparticles generally represented the active P fraction and was of significance for P mobility(Rick and Arai, 2011).After fertilization cessation, the increase of fine colloids and the release of nanoparticles brought the release of P associated with these particles;thus,the potential mobility of P could improve.These results indicated soil microparticles responded strongly to fertilization cessation and the response was weaker for soil particles with larger size.
The short-term cessation of fertilization reduced available and inorganic P fractions due to the utilization by crops (Tables I and II).Orthophosphate decreased, while orthophosphate monoesters and orthophosphate diesters in bulk soil showed no obvious change(Table III),indicating that orthophosphate was directly taken up by plant roots and organic P mineralization was negligible.These results are consistent with those of Cade-Menunet al.(2017),who reported that inorganic P concentrations decreased significantly but only small differences with P decreases in organic P compound classes were noted after fertilizer cessation in a grassland soil.Compared with bulk soil with various inorganic and organic P species,we did not detect any organic P but only inorganic P species including orthophosphate and pyrophosphate in<100 nm fraction(Table IV,Fig.S5).The concentration of water-extracted P in<100 nm fraction was low,ranging from 0.00 to 11.76 mg kg-1.Organic P in<100 nm fraction was not detected and might be below the limit of detection by NMR.The differences in P speciation and distribution between the microparticle fractions and bulk soil have also been reported in other studies of grasslands(Jianget al., 2017b) and forest soils (Wanget al., 2020).Different from bulk soil,orthophosphate and pyrophosphate in<100 nm fraction increased in response to the cessation of fertilization.As mentioned above,the nanoparticles were released after short-term cessation of fertilization,and thus P species attached to the nanoparticles increased accordingly.
The critical value of available P ranged from 10 to 40 mg kg-1,depending on crop species,soil type,and climate(Jordan-Meilleet al.,2012).Crop yields did not respond to P application above the critical value.In China, the available P value of 20 mg kg-1has been considered as a threshold for optimal crop growth(Liet al.,2015).In this study,the concentration of available P(22.9—29.8 mg kg-1)was still high after fertilization cessation(Table I).These results suggested that the accumulated available P in lime concretion black soil after short-term fertilization cessation was sufficient to meet crop growth requirements,whereby organic P mineralization was limited.
CONCLUSIONS
This study focused on the responses of P speciation and colloidal P to short-term cessation of fertilization in lime concretion black soil.Long-term excessive fertilization indeed led to soil acidification and P accumulation.After cessation of fertilization,the concentrations of available P and inorganic P decreased,while soil pH increased significantly,indicating that short-term cessation of fertilization relieved soil acidification and P accumulation.Available P was still sufficient to meet crop growth requirements after short-term fertilization cessation.The< 100 nm soil particle size fraction was dominated by inorganic P with orthophosphate and pyrophosphate,and their concentrations increased after short-term cessation of fertilization.Furthermore,fertilization cessation brought a slight increase of fine colloids dominated by Fe minerals and drove the significant release of Fe/Al oxide nanoparticles in the short term.Accordingly,P associated with these microparticles as the mobile P pool increased significantly.Our results indicated that the increase of P associated with the microparticles might have large effects on mobile P stocks after fertilization cessation,with important implications for the development of sustainable P fertilization management strategies in lime concretion black soil.
ACKNOWLEDGEMENT
This study was financially supported by the National Natural Science Foundation of China(No.41907063)and the Foundation of Modern Agricultural Innovation Center,Henan Institute of Sun Yat-sen University,China(No.N2021-002).
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
杂志排行
Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
- Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
- Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil
