Enhanced initial biodegradation resistance of the biomedical Mg-Cu alloy by surface nanomodification
2023-11-18WenZhngMingChunZhoZhenoWngLiliTnYingweiQiDengFengYinKeYngAndrejAtrens
Wen Zhng, Ming-Chun Zho, Zheno Wng, Lili Tn, Yingwei Qi, Deng-Feng Yin,∗,Ke Yng, Andrej Atrens
a School of Material Science and Engineering, Central South University, Changsha 410083, China
b Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
c School of mechanical and Mining Engineering, University of Queensland, Brisbane, QLD 4072, Australia
Received 5 September 2021; received in revised form 1 November 2021; accepted 17 December 2021
Available online 5 February 2022
Abstract Mg-Cu alloys are promising antibacterial implant materials. However, their clinical applications have been impeded by their high initial biodegradation rate,which can be alleviated using nanotechnology by for example surface nanomodification to obtain a gradient nanostructured surface layer. The present work (i) produced a gradient nanostructured surface layer with a ∼500 μm thickness on a Mg-0.2 Cu alloy by a surface mechanical grinding treatment (SMGT), and (ii) studied the biodegradation behavior in Hank’s solution. The initial biodegradation rate of the SMGTed samples was significantly lower than that of the unSMGTed original counterparts, which was attributed to the surface nanocrystallization, and the fragmentation and re-dissolution of Mg2Cu particles in the surface of the SMGTed Mg-0.2 Cu alloy. Furthermore,the SMGTed Mg-0.2 Cu alloy had good antibacterial efficacy. This work creatively used SMGT technology to produce a high-performance Mg alloy implant material.
Keywords: Mg-Cu alloy; Gradient nanostructure; Biodegradation; Surface mechanical grinding treatment.
1. Introduction
Biodegradable magnesium (Mg) alloys are promising for applications as biomedical metallic implants, because they offer a promising, practical alternative in bone regeneration and other orthopedic applications [1–3]. However, the postoperative infection associated with the implant is a severe problem. A countermeasure occurs if the implant itself prevents the infection. Mg alloys naturally have an antibacterial effect after implantation in the body because of the local alkaline environment with a high pH value that is produced by Mg biodegradation due to the low solubility of Mg(OH)2[4–5].However, the adjacent pH value is limited in vivo (i) because Mg alloys have a lower degradation rate in vivo, (ii) because of the buffer capacity of the body fluid, and (iii) the degradation products deposited on the surface production inhibit further degradation. Moreover, the surface degradation products also provide favorable sites for bacterial adhesion. Therefore,the antibacterial effects obtained from the alkalinity would be expected to be effective only at the early implantation stage.This weakness of the antibacterial effect was substantiated by Brooks et al. [6] and Hou et al. [7], who found that the antibacterial effects of Mg alloys was either small or nonexistent in body fluid environments. Therefore, there is an urgent need for increasing the antibacterial ability of Mg alloy implants in clinical medicine because of the risk of bacterial infection.
The antibacterial ability of an Mg alloy can be increased by alloying with an antimicrobial metal such as copper (Cu).Cu has good antibacterial and antifungal effects and also is a necessary trace element for human health and has many biological functions and benefits in the human body [8]. The release of Cu ions from a corroding Mg-Cu alloy can provide the desired antibacterial effects. Therefore, Mg-Cu alloys are a promising type of antibacterial implant material for clinical application. Our previous work demonstrated that the Mg-Cu alloys presented a strong antibacterial ability and excellent cytocompatibility [9–10].
However, Mg-Cu alloys tend to corrode quickly in the physiological environments because the Mg2Cu intermetallic particles cause to micro-galvanic corrosion acceleration of the alloy matrix [8,10]. The high degradation rate also causes a large amount of gas production. The high biodegradation rate and high gas production were previously believed to be the main factors that prevented application of biodegradable Mg-based orthopedic implants.However,recent measurements have found that the actual survival period of Mg-based implantsin vivowas sufficiently long, making them feasible as bone implant materials for clinical applications. For example, Zhao et al. found that the Mg screw was still present after 12 months, when vascularized bone grafting was fixed by a biodegradable magnesium screw for treating osteonecrosis of the femoral head [11]. Witte et al. demonstrated that the average in vivo biodegradation rate was four orders of magnitude lower than the in vitro biodegradation of Mg alloys [12]. Therefore, the main obstacle to clinical application of Mg alloys is their rapid initial degradation at the initial stage of implantation rather than throughout the implantation cycle. It is of crucial importance to have a lower biodegradation rate for the first few days and especially for the initial few hours, because the biodegradation rate in the body typically decreases with implant time. The promising future of Mg-Cu alloys as biomedical implant materials thus requires a lower biodegradation rate at the initial stage of implantation,i.e. a lower initial biodegradation rate.
The biodegradation of Mg-Cu alloys in body fluid environments starts at the surface. Therefore, controlling the surface performance may be effective in decreasing the biodegradation rate, especially in decreasing the initial biodegradation rate. Surface modification has been widely explored to enhance the biodegradation resistance of Mg alloys, using protective polymer, ceramic or composite surface coatings [13–14].An alternative approach to control the Mg biodegradation rate is to refine the surface microstructure. A finer grain size leads to a lower biodegradation rate [15]. Therefore, surface grain refinement of Mg-Cu alloys may decrease their initial biodegradation rate.
The surface mechanical grinding treatment (SMGT) is an advanced severe plastic deformation technique for grain refinement, and is becoming more attractive for producing high performance metals by inducing a gradient nanostructure in the metallic surface layer, resulting in higher mechanical strength and oxidation resistance [16–17]. However, never before has SMGT been used to decrease the initial biodegradation rate by refining the surface grain size for the promising antibacterial biodegradable Mg-Cu alloys. SMGT is different to conventional surface modification using coating technology.The SMGT surface grain refinement method is a self-coating to decrease the initial biodegradation rate of Mg-Cu alloys,which is congruent with the currently highly-popular strategy of plain materials processing, a plain approach without changing the chemical composition [18].
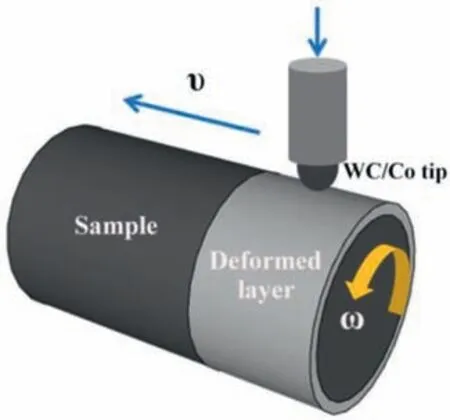
Fig. 1. Schematic illustration of the surface mechanical grinding treatment(SMGT).
On the other hand,the remarkable surface grain refinement by SMGT produces a nanocrystalline surface layer containing a high volume fraction of grain boundaries and a great deal of structural defects [16–17], resulting in an increase of stored-energy that could increase reactivity and accelerate biodegradation. It is thus necessary to explore the biodegradation of the SMGT layer on Mg-Cu alloys.
This work produced a gradient nanostructured surface layer on a Mg-0.2 Cu alloy by SMGT, and investigated the microstructure, hardness and biodegradation behavior of the SMGTed alloy in comparison with the unSMGTed original counterpart.
2. Experiments
The binary Mg-0.2 Cu alloy was composed of 99.8 wt.%Mg and 0.2 wt.% Cu, and was produced as a laboratoryscale vacuum-melted ingot. The as-cast Mg-0.2 Cu alloy was prepared by melting pure copper (99.9 wt.%) into pure magnesium (99.99 wt.%) in a high-purity graphite crucible protected by SF6 (1 vol%) and CO2(balance), was homogenized at 750 °C for 30 min, and was poured into a high-purity graphite mold that was preheated to 200 °C [19]. Specimens with a diameter of 10 mm for SMGT were cut from the as-cast ingot, mechanically ground with SiC papers, ultrasonically cleaned using acetone, ethanol and distilled-water, and blow-dried using hot flowing air.
Preliminary experiments optimized the SMGT process parameters as follows. A cylindrical specimen was rotated at 200 rpm. A WC/Co tool tip was moved along the specimen circumference at 10 mm/min, and was pressed 25 μm into the sample surface, as is illustrated in Fig. 1 schematically.The gradient nanostructured surface layer was produced using multiple passes (6 times) with the same sample rotation speed, the same tip sliding speed and a stepwise increased pressing depth (25 μm per pass).
The microstructure of the SMGTed layer was evaluated using transmission electron microscopy(TEM).The TEM specimens of the top surface layers were extracted using an electric discharge machine and were mechanically polished and ionbeam milled from the un-SMGTed side. Cross-section specimens were mounted into epoxy resin and polished to produce a section through the treated surface. The micro-hardness values along the depth were measured on a cross-sectional specimen using a Dynamic Ultra Microhardness fitted with the standard indenter, with a 20 g load used for a duration of 10 s. The adjacent indentations were separated by 50 μm to minimize interference between measurements. The qualitative phase analysis before and after SMGT used by X-ray diffraction (XRD) with Cu-Kαradiation in the range of 10 - 90° at 40 kV and 30 mA using a scan rate of 8°/min.
Potentiodynamic polarization curves were measured with a scan rate of 0.5 mV/s at 37 °C in the Hank’s solution. A saturated calomel electrode was the reference electrode (SCE),a platinum electrode was the counter electrode, and the specimen was the working electrode. Specimens for potentiodynamic polarization curves were sealed using silicone rubber so that only a semicircle of the surface was exposed.The platinum counter electrode was formed into a semicircular surface in the same arc as the specimen, ensuring that the surface of the specimen remained parallel to the platinum electrode in a localized region. All potentials were referred to the SCE.The biodegradation rate was evaluated from corrosion current density using Eq. (1) [20–21].
wherePiis the biodegradation rate, andicorris the corrosion current density.
Immersion tests were conducted at 37 °C for different times by immersing the samples in Hank’s solution.Untreated sides were sealed with epoxy resin, and only the SMGTed surface was exposed to the Hank’s solution. The ratio of surface area/solution volume was set as 1.25 cm2/mL according to ISO10993. After the immersion test, the biodegradation products were removed using the cleaning solution containing 200 g/L CrO3,10 g/L AgNO3and 20 g/L Ba(NO3)2in an ultrasonic cleaning bath for 1 min at room temperature.Biodegradation rates were evaluated from hydrogen evolution and weight loss using Eq. (2) and Eq. (3) [20–21], respectively:
where pH is biodegradation rate, andVHis H2evolution rate.
wherePWis biodegradation rate, andΔWis mass loss rate.
Biodegradation morphologies were documented using a camera. Topographic maps were obtained using 3D laser microscopy. Every biodegradation test was performed at least 4 times.
For the measurement of Cu2+ion release,the samples were immersed in the Hank’ s solution for 24 h at 37 ± 0.5 °C according to ISO Standard 10,993–12: 2002 and ISO Standard 10,993–15: 2000. The released Cu2+ion content was measured by high frequency plasma spectrometry.
Staphylococcus aureus(S. aureus, ATCC 25,923) is one of the most common bacteria causing infection and was used as a model bacterium. After disinfection by ultraviolet radiation,all the specimens and control groups (pure Mg) were cultured in the Hank’s solution with S. aureus in three replications and placed in a 12-well untreated tissue culture polystyrene plate. Each well contained the specimen and the S. aureus suspension with a concentration of (1∼10) × 105CFU/ml prepared using sterile Hank’s solution to which 1.25 cm2/mL was added. The plates were kept at constant temperature for the required time at 37±0.5°C.The specific culture methods and experimental operations have been presented elsewhere[19,22].
3. Results
Fig. 2 presents the original as-cast microstructureal characteristics of the un-SMGTed specimens. The morphology of grains was large columnar grained structures in the optical micrographs, which mainly consisted of irregular grains and presented the varying grain size. The average grain size was approximately 100 μm (Fig. 2a). The primaryα-Mg matrix and the second phase particles were clear in the backscattered electron(BSE)micrograph. The granular intermetallic phase formed during the solidification appeared as the bright particles and distributed along grain boundaries or in the grain interiors(Fig.2b).The chemical compositions of theα-Mg matrix and the granular intermetallic phase were identified using EDS. Theα-Mg matrix mainly consisted of Mg(Fig.2c),while the granular intermetallic phase contained Mg and Cu with a Mg-Cu atomic ratio of 2 (Fig. 2d). EDS mapping revealed that the granular intermetallic phase was rich in Cu compared to theα-Mg matrix (Figs. 2e, 2f and 2g). The binary Mg-Cu phase diagram indicates an eutectic reaction during cooling, which producesα-Mg and the Mg2Cu intermetallic phase. This indicated that the granular intermetallic phase was the intermetallic Mg2Cu. Therefore, the as-cast microstructure contained large amount of Mg2Cu precipitates with lengths larger than a few to dozens of microns as shown in Fig. 2.
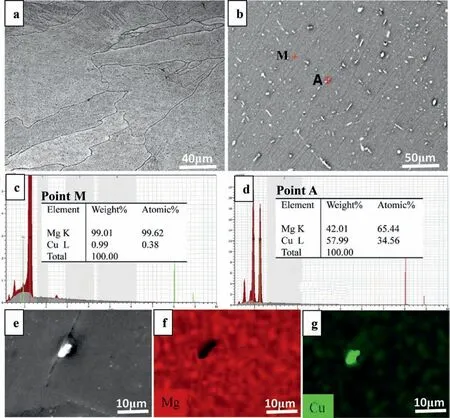
Fig. 2. As-cast microstructure of the un-SMGTed specimen. (a) optical micrograph; (b) BSE image; (c) and (d) EDS spectra for point M (Mg matrix) and point A (intermetallic phase), respectively; (e), (f) and (g) SEM-EDS mapping of the intermetallic phase: (e) SEM micrograph, (f) Mg, (g) Cu.
Fig. 3a shows the cross-sectional SEM morphology of the SMGTed specimen. Remarkable plastic deformation and the microstructure refinement had been produced in the deformed surface layer. The initial microstructure was broken into a nanocrystalline structure, so that the microstructure was difficult to discern in the SEM micrograph. Figs. 3b and 3c show the nanocrystalline microstructure of the top surface layer of the SMGTed specimens. The grains were almost equiaxed with an average size of ∼100 nm, much smaller than that of the as-cast counterparts (∼100 μm) [19]. The corresponding selected area electron diffraction (SAED) pattern that was embedded in Fig. 3b indicated random crystallographic orientations of the nano-sized grains. SMGT had produced significant grain refinement.In addition,the distribution and amount of intermetallic Mg2Cu particles had also been significantly changed by the SMGT processes.TEM examination indicated no precipitates. The distribution of the intermetallic Mg2Cu particles was explored by EDS mapping of the distribution of Mg and Cu. This mapping revealed that these two elements were dispersed throughout the whole matrix. There were no Cu-rich zones or Mg-deficient zones (Figs. 3d, e and f). The XRD analyses before and after SMGT, Fig. 3g, indicated that the Mg2Cu second phase was not detected in the SMGTed specimens, consistent with TEM and EDS mapping. This indicated that the intermetallic Mg2Cu particles had essentially disappeared in the SMGTed nanocrystalline surface layer.Fig. 3h presents the micro-hardness with distance from the topmost surface into the substrate for the SMGTed specimens.The micro-hardness on the topmost surface (∼1.15 GPa) was almost double that of the substrate (∼0.65 GPa) at the depth of ∼500 μm, which was attributed to the significantly refined grains in the nanostructured surface layer. The decrease of hardness with increasing depth agrees with the increase of grain size with increasing depth according to Hall-Petch relation [23]. Therefore, the thickness of the gradient hardened layer was identical to that of the refined surface layer determined by microstructure observations, i.e. the thickness of the microstructure-refined (i.e. hardness-enhanced) layer was∼500 μm.
Fig. 4a shows the open circuit potential (OCP) as a function of immersed time. The OCP values of the un-SMGTed and the SMGTed specimens initially increased and were relatively stable after 400 s. The SMGTed specimens had a stable value more positive than that of the un-SMGTed specimen.This indicated that the SMGTed specimens might produce more stable films.Fig.4b shows potentiodynamic polarization curves.The data from the potentiodynamic polarization curves were listed in Table 1.The corrosion potential of the SMGTed surface was a little more positive than the un-SMGTed surface. The corrosion current density of the SMGTed specimen was significantly lower than that of un-SMGTed alloy.Fig. 4c presents the EIS result of the un-SMGTed and SMGTed specimens. The characteristic of their Nyquist plots was similar. SMGT thus did not change the characteristic of the surface processes during corrosion. The SMGTed specimen had an enlarged capacitive loop. A larger diameter capacitive loop represents a better degradation resistance [24].Fig. 4d presents the pH variation during immersion. The pH values of the un-SMGTed and SMGTed specimens increased rapidly from pH 7.4 to pH 10.3 and pH 9.6, respectively,in the initial 3 h of immersion. The lower pH value of the SMGTed specimens indicated lower biodegradation rate.Thereafter, with increasing immersion time, the pH value of the un-SMGTed specimens increased slowly while that of the SMGTed specimens was relatively stable.

Fig. 3. (a) cross-section SEM image of the SMGTed specimen; (b) and (c) a bright-field TEM image with corresponding SAED pattern inserted and a dark field TEM image of the nanocrystalline microstructure on the SMGTed top surface layer; (d), (e) and (f) SEM-EDS mapping: (d) SEM micrograph, (e) Mg,(f) Cu; (g) XRD patterns before and after SMGT; (h) microhardness distribution with depth from the SMGTed top surface to the substrate.

Table 1 Corrosion rate data in Hank’s solution.

Fig. 4. (a) OCP curves, (b) potentiodynamic polarization curves, (c) EIS curves and (d) pH variation during immersion of the specimens before and after SMGT.
The cathodic partial reaction and the anodic partial reaction are given by Eq. (4) and Eq. (5), respectively. The overall corrosion reaction is given by Eq. (6) [25–26].
The biodegradation rates from the independent measurement of hydrogen evolution and weight loss for 12 h immersion are also listed in Table 1. The biodegradation rates determined by independent measurements of hydrogen evolution and weight loss were in good agreement.This agreement indicated that the hydrogen evolution data reproduced the weight loss measurements, which was expected from Eq. (6), in that one molecule H was evolved for every atom of corroded Mg and was in agreement with previous research [27]. This provides confidence in the experimental measurements. The biodegradation rates evaluated from the polarization curves were significantly lower than measured from the immersion tests as often observed, but showed the same trends [28–29],i.e. SMGTed Mg-0.2 Cu alloy presented a lower biodegradation rate. The polarization curves give a corrosion rate related to the corrosion onset, while the immersion tests give a corrosion rate related to average corrosion during a considerable period after corrosion onset. Therefore, the biodegradation rate from the immersion test is more practical. It is noteworthy that the SMGTed specimens had a significantly lower biodegradation rate (by ∼a factor of ten times lower)than the un-SMGTed specimens.

Fig. 5. Biodegradation rate during immersion of specimens before and after SMGT.
Fig. 5 presents the biodegradation rate at different immersion times. The un-SMGTed specimens had a high initial biodegradation rate that decreased with increasing immersion time. In contrast, the SMGTed specimens had a much lower biodegradation rate for all the times studied. The biodegradation rate of SMGTed specimens did increase with increasing immersion time, but was nevertheless lower than that of the un-SMGTed specimens for 96 h immersion, which indicated that nanocrystalline surface layer acted like a protective film and retarded the initial corrosion of the alloy. This indicated that the SMGTed gradient nanostructured surface layer could effectively decrease the initial biodegradation rate, although there might not be much improvement of biodegradation resistance during long time exposure when the specimen was completely corroded.
As stated above, a main obstacle to clinical application of Mg alloys is their rapid initial degradation at the initial stage of implantation rather than throughout the implantation cycle. Therefore, it is of crucial importance to have a lower biodegradation rate for the initial period produced by the SMGTed gradient nanostructured surface layer,which provides a promising future for the Mg-Cu alloys as biomedical implant materials.This is also one reason why surface protective coating technology for corrosion protection of Mg alloys is widely used, although more work is needed for the corrosion protection of Mg-Cu alloys by surface protective coatings.This SMGT surface grain refinement method is expected to act as a self-coating to improve the initial biodegradation resistance of the Mg-Cu alloys.
Figs. 6a through f present the corrosion surface morphologies and topographic maps after removing the biodegradation products for specimens immersed for the initial 12 h. The SMGTed specimens only had some localized corrosion areas and the corrosion areas were much smaller than those of the un-SMGTed specimens. The surface of un-SMGTed specimens had suffered severe corrosion attack, showing a rough surface with irregular ridges and localized corrosion. The SMGTed specimens remained smooth and bright, their surface was corroded gently with some scattered pitting, and the corrosion damage was mostly horizontally distributed. Fig. 6g shows typical depth distributions.The maximum depth of corrosion pits of the SMGTed specimens was ∼20 μm. In contrast,the orientation of the corrosion of the un-SMGTed specimens was random, demonstrated by the presence of a high contrast in color(yellow and green),in which the depth of the localized corrosion pit was up to 100 μm.The biodegradation degree from the surface morphologies was in agreement with that from the topographic maps and with the biodegradation rate from the immersion tests.
Fig. 7 presents the number of S. aureus colony forming units (CFU) /ml at different intervals in extracts without adjusting pH. The colonies of S. aureus in the extract of the SMGTed Mg-0.2Cu specimens decreased from 494 to 73 after 6 h and then disappeared to 0 after 12 h. This indicated that after 12 h the antibacterial ability of the SMGTed Mg-0.2Cu alloy reached 100%. The results confirmed that the SMGTed Mg-0.2Cu alloy possessed strong antibacterial properties. Usually, if the alloy contains elements that are antibacterial, then the alloy also has antibacterial ability. The magnesium matrix as anode was corroded and then the copper ions around the magnesium matrix were released into solution.The good antibacterial ability of the SMGTed Mg-0.2Cu alloy is an expected and desired result because Cu is antibacterial.
4. Discussion
4.1. Nano crystallization and Mg2Cu redissolution
The microstructural information of the surface layer in Fig. 3 indicated that nano-sized grains were produced at the topmost surface layer for the SMGTed Mg-0.2Cu sample,which was attributed to the surface severe plastic deformation induced by the SMGT. Huang et al. [30] found that the formation mechanism of the nanocrystalline surface layer was dominated by dislocation activities according to dislocation formation and interaction under the depth dependent strain and strain rate. In simple terms, a mass of dislocation tangles, dislocation cells and dislocation walls were formed by the accumulative strains in the deeper position of the surface layer, which were gradually changed into sub-grain boundaries with smaller spaces because of the intensive interaction with the more dislocations under the increased strains at the subsurface layer. Finally the grain size was decreased into the nanoscale under the high strain and strain rate at the topmost surface layer. Grain refinement typically results in an enhancement in hardness and strength for metals according to Hall-Petch relation [23], i.e. the decrease of hardness with increasing depth agrees with the increase of grain size with increasing depth. Therefore, the microhardness gradient distribution with the depth as shown in Fig. 3h was consistent with the in-depth microstructure evolution.

Fig. 6. Corrosion surface morphologies, topographic maps, and typical height fluctuation of the surface of the topographic maps for specimens immersed for an initial 12 h: (a) (c) unSMGTed and (b) (d) SMGTed surface morphologies; (e) unSMGTed and (f) SMGTed topographic maps; (g) typical height fluctuations of the surface of the topographic maps.

Fig. 7. Antibacterial results of Mg-0.2Cu against S. aureus after co-cultured with bacteria at 37 °C.
The dissolution of the intermetallic Mg2Cu particles accompanied the nano-crystallization of at the topmost surface layer, as shown in Fig. 3, which was attributed to the physically crushing and redissolution of these coarse precipitates into the matrix by the severe plastic deformation caused by the SMGT. The SMGT introduced a mass of dislocations.The Mg2Cu particles strengthened the stress field of the dislocations because the unmovable Mg2Cu particles bent and twisted the surrounding dislocations. The strengthened stress fields of the dislocations raised the free energy of the elemental atoms and led to a high energy storage in the crystal structure. When the stored deformation energy is higher than the formation free energy required for the supersaturated solid solution, the solid solubility of the second phase in the matrix is extended. The solubility enhancementx/x0is given by the following [31]:
wherex0is the equilibrium solubility,μis the chemical potential of the solute atom,Ris the gas constant,Tis the temperature,σis the stress acting on the solute atom,Vmis the molar volume of the solute atom,Gis the shear modulus,bis the Burgers vector,νis the Poisson ratio of the host metal,Θis the angle about the glide plane, andris the distance from a dislocation core to the solute atom.
Furthermore, the dislocation strain energy is reduced when solute atoms are segregated at dislocations. The second phase concentration at dislocations was several tens of times higher than the average solid solubility [32]. Fig. 8 illustrates that a high density of dislocations formed during deformation provides a large number of dislocation pipelines to promote solute diffusion. The mechanism of pipe diffusion indicates that dislocations were forced to move during plastic deformation,and solute atoms were retained in situ to form a solute rich zone. At the new dislocations, a new rich zone was formed due to the diffusion of solute. Therefore, the solute atoms were continuously dissolved into the matrix by the continuous slip of dislocations. The increase of solid solubility of the second phase in Mg made the Mg matrix to have a more positive corrosion potential. This was verified by the dynamic potential polarization curve as presented in Fig. 4b, in which the corrosion potential of the SMGTed surface was a little more positive than the un-SMGTed surface.
4.2. Lower initial biodegradation rate
The above indeed verified the expectation: the gradient nanostructured surface layer produced by SMGT acted as a self-coating and greatly decreased the initial biodegradation rate. The severe plastic deformation by SMGT caused a major decrease of grain size, an increase of crystallographic defects and dissolution of the second phase precipitates; all of which determined the biodegradation rate. The mechanism by which the surface nanocrystallization, the increase of surface crystallographic defects and the dissolution of second phase precipiates affected the biodegradation of the SMGTed Mg-0.2 Cu alloy is described subsequently.

Fig. 9. Schematic illustration showing the influence of grain refinement on limiting propagation of the preferred crystallographic pits in Mg alloy. (a) coarse grained Mg alloy; (b) fine grained Mg alloy.
Usually, Mg alloys suffer localized corrosion, particularly micro-galvanic corrosion at second phase particles. An obvious enhancement in degradation resistance of Mg alloys was achieved by decreasing their susceptibility to micro-galvanic corrosion. In the present work, the grain refinement caused by SMGTed had a significant influence in promoting homogeneous corrosion, which were supported by the corrosion surface morphologies and topographic maps as shown in Fig. 6. That is, the corrosion surface of the SMGTed specimens was relatively smooth, while that of the unSMGTed specimens was obviously rough and had large deep pits. The Mg substrate behaved as an anode in the electrochemical corrosion process due to its strongly negative electrode potential.The corrosion occurred first in the Mg substrate, next to the intermetallic particles. The number of grain boundaries in the surface nanocrystalline layer was largely increased, and accordingly the number of obstacles to corrosion propagation was largely increased. The degradation behavior as stated above was schematically illustrated in Fig. 9. The surface nanocrystallization layer was a major feature for the SMGTed Mg-0.2Cu alloy and had a distinctly modified degradation behavior. This was substantiated by Zhang et al. [15], who demonstrated that the corrosion propagation was reduced by refining the grain size of Mg alloys. Furthermore, the geometrical mismatch between the Mg substrate and the oxide layer was high because the Piling-Bedworth ratio was only 0.8 [33]. The volume was approximately doubled when the cubic MgO transforms into hexagonal Mg(OH)2in an aqueous solution [34]. The compressive rupture caused by this process led to the continual corrosion. Nano-crystallization released this stress and accordingly decreased the risk of oxide cracking because the fraction of grain boundaries was high. As a consequence, there was a better surface coverage provided by the initial MgO layer that was formed on the surface nanocrystalline layer, and accordingly the cracking of the exterior Mg(OH)2layer was inhibited. To a certain extent,this also accounted for the enhanced initial biodegradation resistance of the SMGTed Mg-0.2Cu alloy.
On the other hand, surface nano-crystallization induced strain-induced crystalline defects with an extremely high density in near-surface region [30,35], which mainly included grain boundaries, subgrain boundaries and dislocations. In order to understand the effect of these strain-induced crystalline defects, pure Mg was used as a comparative study,which might exclude the effect of the second phase precipitations on the corrosion. The polarization curve of pure Mg before and after SMGT is presented in Fig. 10 and showed that the corrosion current density of SMGTed samples(23 μA/cm-2) was a little higher than that of unSMGTed samples (15 μA/cm-2). The higher corrosion current density corresponds to a higher biodegradation rate. This indicated that for pure Mg, although the grain refinement produced by SMGT reduced the biodegradation rate, the surface crystallographic defects induced by the severe plastic deformation during SMGT increased the biodegradation rate. Similar results for an aluminum alloy was found by Ma et al. [36],that is, the high energy crystallographic defects were considered to be prioritized corrosion areas and increased the biodegradation rate. Consequently, for SMGTed Mg-0.2Cu,the increased crystalline defects increased the biodegradation rate, and counteracted the influence of grain refinement and the absence of intermetallic particles on the decrease of the biodegradation rate.
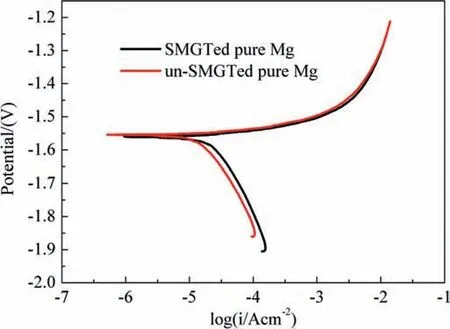
Fig. 10. Dynamic potential polarization curve of pure Mg before and after SMGT.
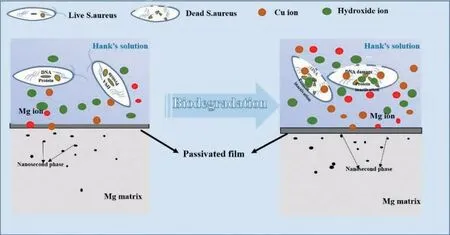
Fig. 11. Schematic illustration on antibacterial mechanism of the SMGTed Mg-0.2Cu.
The presence of Cu in the Mg-Cu alloys caused the biodegradation behavior to be sensitive to the micro-galvanic corrosion between the Mg2Cu particles and the matrix.Mg2Cu acts as a cathode phase,which formed a corrosion battery with the magnesium matrix and accelerated the biodegradation. This is also the main reason for the poor biodegradation resistance of Mg-Cu alloys [8,19]. The number of intermetallic Mg2Cu particles was significantly decreased and they even disappeared after SMGT,and accordingly micro-galvanic corrosion was reduced. This is another important reason for improving the initial biodegradation resistance of the SMGTed Mg-Cu alloy.
4.3. Antibacterial efficacy and safe intake of Cu
The results of antibacterial experiments showed that the SMGTed Mg-0.2Cu alloy had a strong antibacterial ability,which indicated that the releasing of Cu2+ions was effective for antimicrobial use. The Cu2+ions were inferred to be released from the SMGTed Mg-0.2Cu alloy by the following considerations during corrosion. Cu in the alloy in contact with Hanks’ solution had the following reaction: Cu released an electron to form Cu+ions, the Cu2O was formed under an action of Cl-ions and OH-ions, and finally Cu2+ions were formed under an action ofH+ions and O2-irons due to the Cu2O instability in aqueous solution [37]. Cl-ions in Hank’s solution destroyed the continuity of the corrosion film on the surface of the alloy and promoted the Cu2+ion release. The released Cu2+ions came into contact with the bacteria and killed the bacteria by destroying their cell walls and membranes.The antibacterial mechanism of the SGTMed Mg-0.2Cu alloy in simulated body fluid environment is illustrated in Fig. 11. Therefore, Cu had good antibacterial function in either its chemical compound form or metallic form under the action of the released Cu2+ions. In this work,the SMGT treatment of the Mg-0.2Cu alloy fragmented the coarse Mg2Cu second phase in the surface and caused redissolution into the matrix, but the good antibacterial function was still retained although Cu existed in a slightly different form before and after SMGT. The concentrations of the released Cu2+ions immersed in Hank’s solution for 24 h are depicted in Fig.12.The unSMGTed Mg-0.2Cu had the higher concentration of ∼50 μg/L, and the SMGTed Mg-0.2Cu had the lower released Cu2+ion concentration of ∼20 μg/L.Nevertheless, the antibacterial efficacy was good as shown in Fig. 7. In contrast, Yan et al. [19] reported that the Mg-0.1Cu alloy showed good antibacterial activity had a much lower maximum released Cu2+ion concentration of 1.2 μg/L(ppb) after immersion for 24 h in Hank’s solution. In addition, a slow Cu2+ion release rate from the Ti-Cu alloy at ∼0.16 μg/(Lday) also provided an efficient antimicrobial activity [38], although this Cu release rate was much lower than that of the SMGTed Mg-0.2Cu alloy. Therefore, the SMGTed Mg-0.2Cu still had a good antibacterial effect by the release of Cu2+ions.
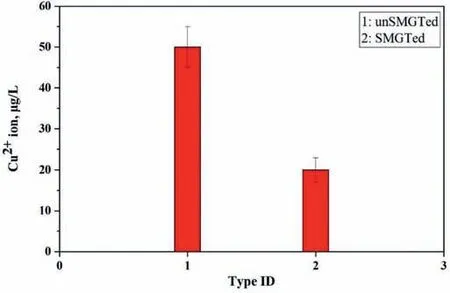
Fig.12. Cu2+ release from the Mg-0.2Cu alloy after 24 h in Hanks’solution.
The maximum amount of the released Cu2+ion concentration from the SGMTed Mg-0.2Cu immersed in Hank’s solution for 24 h was 20 μg/L (ppb) (Fig. 12), which meant 0.016 μg/cm2for a sample with an immersion ratio of 1.25 cm2/ml. If a SMGTed Mg-0.2Cu implant of size 10 mm x 10 mm x 4 mm was implanted into the human body, the released Cu2+ion was approximately 0.05 μg per day, which was much lower than the tolerable Cu upper intake level of 10 mg per day for adults [39]. A previous study found that an as-cast Mg-0.25Cu alloy with faster corrosion rate and higher released Cu2+ion concentration showed favorable biocompatibility in a rabbit model [40]. Thus, the safe Cu release of the SMGTed Mg-0.2Cu was acceptable.
5. Conclusions
1. A gradient nanostructured surface layer with ∼500 μm in thickness was successfully produced on a Mg-0.2 Cu alloy by a surface mechanical grinding treatment (SMGT).The surface layer had a decreasing micro-hardness with increasing distance from the topmost surface into the substrate.
2. The gradient nanostructured surface layer acted as a selfcoating and markedly decreased the initial biodegradation rate in Hank’s solution, which was attributed to the surface nanocrystallization, and the fragmentation and redissolution of Mg2Cu particles in the surface.
3. The SMGTed Mg-0.2 Cu alloy had good antibacterial efficacy and the amount of released Cu ions was safe.
4. A lower initial biodegradation rate by SMGT overcomes a main obstacle to clinical application of Mg alloys. This work creatively used SMGT technology to produce a highperformance Mg alloy implant material.
Data availability statement
All data included in this study are available upon request by contact with the corresponding author.
Declaration of competing interest
All authors agree this submission and declare no interest conflict.
Acknowledgments
Financially supported by Natural Science Foundation of China (No. 51874368).
杂志排行
Journal of Magnesium and Alloys的其它文章
- NaH doped TiO2 as a high-performance catalyst for Mg/MgH2 cycling stability and room temperature absorption
- Microstructure, mechanical properties and fracture behaviors of large-scale sand-cast Mg-3Y-2Gd-1Nd-0.4Zr alloy
- Elucidating the evolution of long-period stacking ordered phase and its effect on deformation behavior in the as-cast Mg-6Gd-1Zn-0.6Zr alloy
- Effect of long-period stacking ordered structure on very high cycle fatigue properties of Mg-Gd-Y-Zn-Zr alloys
- The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition
- The role of melt cooling rate on the interface between 18R and Mg matrix in Mg97Zn1Y2 alloys
