NaH doped TiO2 as a high-performance catalyst for Mg/MgH2 cycling stability and room temperature absorption
2023-11-18JoshuAdedejiBolrinZhoZhngHujunCoZhiLiTengHePingChen
Joshu Adedeji Bolrin, Zho Zhng, Hujun Co,∗, Zhi Li, Teng He, Ping Chen,∗
a Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, P. R. China
b University of Chinese Academy of Sciences, Beijing 100049, P. R. China
c Shangdong Energy Group Co., LTD., No.10777, Jingshi Road, Jinan City, Shandong Province, 250014, P. R. China
d School of Material Science and Engineering, Xi’an Jiaotong University, Xi’an, Shaanxi Province, 710049, P.R. China
Received 20 August 2021; received in revised form 9 October 2021; accepted 5 November 2021
Available online 6 December 2021
Abstract This paper presents the catalytic effect of NaH doped nanocrystalline TiO2 (designated as NaTiOxH) in the improvement of MgH2 hydrogen storage properties. The catalyst preparation involves ball milling NaH with TiO2 for 3 hr. The addition of 5 wt% NaTiOxH powder into MgH2 reduces its operating temperature to ∼185 °C, which is ∼110 °C lower than the additive-free as-milled MgH2. The composite remarkably desorbs ∼7.2 wt% H2 within 15 min at ∼290 °C and reabsorbs ∼4.5 wt% H2 in 45 min at room temperature under 50 bar H2. MgH2 dehydrogenation is activated at 57 kJ/mol by the catalyst. More importantly, the addition of 2.5 wt% NaTiOxH catalyst aids MgH2 to reversibly produce ∼6.1 wt% H2 upon 100 cycles within 475 hr at 300 °C. Microstructural investigation into the catalyzed MgH2 composite reveals a firm contact existing between NaTiOxH and MgH2 particles. Meanwhile, the NaTiOxH catalyst consists of catalytically active Ti3O5, and “rod-like” Na2Ti3O7 species liberated in-situ during preparation; these active species could provide multiple hydrogen diffusion pathways for an improved MgH2 sorption process. Furthermore, the elemental characterization identifies the reduced valence states of titanium (Ti<4+) which show some sort of reversibility consistent with H2 insertion and removal. This phenomenon is believed to enhance the mobility of Mg/MgH2 electrons by the creation and elimination of oxygen vacancies in the defective (TiO2-x) catalyst. Our findings have therefore moved MgH2 closer to practical applications.
Keywords: Magnesium hydride; NaH doped nanocrystalline TiO2; Kinetics; Room temperature absorption; Reversibility.
1. Introduction
The increasing environmental pollution and the depletion of nonrenewable energy have raised awareness towards the development of hydrogen storage systems as a viable source of clean energy.However,the suitability for on-board application has been a challenge to realizing the much-anticipated hydrogen economy [1]. MgH2is one of the most promising candidates for hydrogen storage and has been extensively investigated over the last few decades. This is because of its large gravimetric and volumetric hydrogen capacities of about 7.6 wt% and 110 g/L, respectively. MgH2also has the benefits of natural abundance, low cost, and non-toxicity [2,3]. However,the sluggish sorption rates and high operating temperature of MgH2ranging between 300 and 400 °C which result from high kinetic barriers and stable thermodynamics (dehydrogenation enthalpyΔHf≥75 kJ/mol),prevent its mobile application [4,5]. To overcome these barriers, tremendous efforts have been given to nano-structuring [6–8], alloying [9–14],and catalysis [15–19] in the past few years with significant progress made on the sorption properties. However, hydrogen capacity loss by the formation of a multicomponent alloy and the introduction of confined porous materials that result from nano-structuring and alloying [6,12] has drawn attention towards catalytic doping as one of the most effective ways of improving the hydrogen storage properties of MgH2. The use of catalysts such as transition metals [20,21], transition metal oxides [19,22,23], carbides [18,24], hydrides [25,26],etc., have been shown to improve the kinetics of MgH2.
Among the various catalysts, TiO2has recently attracted great interest due to its particle size and tunable Ti valence state (via oxygen vacancy creation) which can enhance the hydrogen storage properties of MgH2[27,28]. For instance,MgH2doped with 5 mol% of rutile, anatase, and P25 TiO2synthesized by high-energy ball milling were investigated to enhance the hydrogenation properties of Mg [27]. From the results, the rutile TiO2doped composite showed the fastest absorption kinetics and highest capacity; this was attributed to the formation of an ultrafine nanocomposite MgH2-TiO2.Pandey et al.[29]also observed that MgH2catalyzed by 7 and 50 nm TiO2exhibited the optimum catalytic effect for hydrogen desorption and absorption respectively among the particle sizes of 7, 25, 50, 100, and 250 nm. It was stressed that the TiO2could partially be reduced at temperatures below 340°C to form defective TiO2-xwith oxygen vacancies during the sorption process of MgH2. Furthermore, nanocrystalline TiO2supported on carbon (TiO2@C) has also shown good catalytic performance in the hydrogen storage reaction of MgH2[30]. The addition of 10 wt% of the catalyst into MgH2reduced the onset dehydrogenation temperature to 205 °C with the release of 6.5 wt% H2within 7 min at 300 °C and reabsorption of 6.6 wt% H2within 10 min at 140 °C. The mechanism behind the improvement was based on the weakening/breaking of the Mg-H bond by TiO2as obtained from DFT calculations which agreed with the experimental results.Some recent investigations have revealed that hydrothermally synthesized TiO2-based catalysts such as the 2D graphene-like TiO2nanosheet, and graphene-supported TiO2nanoparticles(TiO2@rGO), could improve the sorption properties of MgH2[31,32]. The perovskite oxides of titanium also demonstrated some level of efficiency towards improving the sorption properties of MgH2. For example, Zhang et.al reported the catalytic performance of Na2Ti3O7nanotubes, with a diameter of 10 nm, which could facilitate the hydrogen de/absorption kinetics of MgH2by providing a lot of hydrogen diffusion channels [33]. With 5 wt% of the catalyst, MgH2could desorb 6.5 wt% H2at 300 °C in 6 min and absorb 4.1 wt% H2at 150 °C in 10 min. Some other examples include MgH2catalyzed with Li2TiO3[17], SrTiO3[34], BaTiO3[35], and NiTiO3[36]. On a general note, it was resolved that fairly stable metal oxides with a large number of possible structural defects and high valence states possess high catalytic efficiency on the sorption reaction of MgH2[22,37,38].
Given the above investigations, it is reasonable to examine the catalytic effect of light metal hydride (NaH) pre-activated TiO2in the improvement of MgH2hydrogen storage performance. The choice of NaH as a reducing agent for TiO2is based on its strong reducing ability which can help boost vacancy concentration, and defect sites in TiO2under a mild preparatory condition; this was confirmed in our previous works [39,40] by using Na/NaCl induced-oxygen vacancy in TiO2for hydrogenation and water-gas shift reactions. Highenergy milling of TiO2with NaH under room temperature yields black TiO2powder (TiO2-x) which is reportedly characterized by surface disorders, surface defects, and oxygen vacancies [41,42]. Introducing 5 wt% of this powder reduces the operating temperature of MgH2to ∼185 °C, with room temperature absorption of 4.5 wt% H2in 45 min. 2.5 wt%of the catalyst also enables MgH2to undergo 100 cycles of de/absorption at 300 °C. To the best of our knowledge, no investigation has been conducted before now on this branch of knowledge.
2. Methods
2.1. Samples preparation
Pure MgH2(98%), pure NaH (95%), and anatase TiO2(99.8% metal basis 25 nm particle size) powders were commercially purchased from Alfa-Aesar, Sigma Aldrich, and Macklin chemical respectively. The powders were used without further treatment. All samples were prepared under an argon atmosphere in the glovebox with a circulative purification system (O2< 10 ppm, H2O< 0.1 ppm) to avoid the influence of oxygen and moisture. NaH doped TiO2catalyst was prepared by ball milling NaH with TiO2separately in mole ratios of 0, 0.5, 1, and 2 to 1 for 3 hr. After that, a preselected amount of each catalyst obtained was added into fresh MgH2powder and ball-milled. Pristine MgH2was also ball-milled separately with and without TiO2. Ball milling of all the MgH2composites lasted for 16 hr with batch weight kept at 2 g.All of the milling processes were performed using Retsch PM 400 at room temperature with a rotation speed of 200 RPM. The mixtures were sealed in 150 ml stainless steel vials in a glovebox with a ball-to-powder weight ratio (BPR)of about 80:1.The milling process was interrupted for 2.5 min after every 10 min of rotation to dissipate accumulated heat.
2.2. Samples characterization
Powder X-ray diffraction (XRD) measurements were conducted using an X’Pert3Materials Research Diffractometer(Malvern Panalytical) with Cu Kαradiation (λ= 0.154 nm)at 40 kV and 40 mA. Samples were measured into the steel sample holders and covered with Kapton to avoid contamination during the measurement. Each measurement was done at a scan speed of 2°/min over diffraction angles of 10°to 90°. The microstructure and morphology of the samples were investigated using Hitachi S-4800 scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDS) analysis unit and an FEI Tecnai G2 F20 S-TWIN transmission electron microscopy (TEM). For TEM analysis,the samples were dispersed in hexane,sonicated,and drop cast on a copper grid. Image processing was performed using Digital Micrograph (Gatan) software. X-ray photoelectron spectroscopy(XPS;Thermo Fisher Scientific,ESCALAB 250Xi, Al-Kα= 1486.6 eV) technique was used to analyze the surface state of the catalyst. The binding energy was calibrated using C-C binding energy at 284.4 eV as a reference.
The thermal decomposition properties of the samples were first investigated by using a homemade temperatureprogrammed desorption system equipped with a mass spectrometer (HPR20, Hiden) (TPD-MS). About 15–20 mg of the samples were loaded into an air-tight sample holder and sealed to the reactor in the glovebox. The analysis was carried out between room temperature and 400 °C at a heating rate of 2 °C/min under 20 mL/min argon flow.
The volumetric desorption of the samples was carried out using Gas Reaction Controller (Advanced Material Corporation, USA). 120–150 mg of each sample was tested. Samples were heated up from room temperature to 400°C with a heating rate of 2 °C/min under 0.001 bar of H2. However, room temperature absorption was conducted at 10, 30, and 50 bars of H2backpressure.
Thermodynamic and kinetic de/re-hydrogenation behaviors of samples were evaluated by using a conventional Hy-Energy PCT pro-2000 pressure-composition-isotherm (PCI) analyzer.200–250 mg of each sample was loaded into a standard autoclave steel reactor. Isothermal desorption was investigated at 260, and 290 °C under 0.001 bar of H2pressure,while isothermal absorption was investigated at 50, 100,200, 230, and 260 °C under 30 bars of H2pressure. The composite’s reversibility was evaluated at 300 °C under 0.001 and 30–50 bars of H2pressure for de/absorption. PCI desorption measurement was performed at 300, 320, and 340 °C. The thermodynamic property was determined by using the Van’t Hoff equation [43], which is expressed as a function of the equilibrium pressures recorded during PCI measurements.
WherePH2,ΔH, andΔSare the hydrogen equilibrium pressure, enthalpy, and entropy change, respectively.
The apparent activation energy of each sample under investigation was determined using the Kissinger method[44] through the mass spectra data obtained from TPD-MS.Samples were heated from room temperature to 400 °C with heating rates of 2, 6, 8, and 10 °C/min under 20 mL/min of argon flow. The method is as described in equation 2.
Whereβis the heating rate, Tp2is the peak temperature of desorption given by the result of TPD-MS, R is the gas constant,A is a linear constant andEais the activation energy calculated from the slope value of the Kissinger plot.
3. Results and discussion
3.1. Hydrogen storage properties
The optimum ratio of NaH to TiO2with the best catalytic performance was determined by TPD-MS(Fig.S1),which indicates that NaH doped TiO2in a 1:1 mole ratio (designated as NaTiOxH)has the best catalytic effects.Following that,the dehydrogenation properties of as-milled MgH2, MgH2–5 wt%TiO2, and MgH2-y wt% NaTiOxH (y= 2.5, 5, and 10) composites were measured by TPD-MS and the results are summarized in Fig. 1a. The results show that 10 wt% NaTiOxH catalyzed MgH2starts to desorb hydrogen from ∼174 °C and reaches its peak at 237 °C; which is ∼100 °C lower than the as-milled MgH2. However, reducing the amount of NaTiOxH from 10 to 5 and 2.5 wt% only influences the dehydrogenation peak slightly.
To clearly show the catalytic effects of the different catalysts and doping amounts, temperature-programmed volumetric desorption of the prepared samples was measured and plotted in Fig. 1(b). It shows that 2.5, 5, and 10 wt% NaTiOxH catalyzed MgH2begin to release H2from temperatures below 200 °C, while they desorb ∼6.9, 7.2, and 6.2 wt% H2at∼260 °C; finally, the composites release off ∼7.5, 7.4, and 6.5 wt% H2at ∼320 °C. However, as-milled MgH2starts to dehydrogenate at ∼295 °C which is ∼100 °C higher than these catalyzed samples, and it liberates ∼7.5 wt% H2at∼375 °C. Taking the kinetics and H2capacity into consideration, the 5 wt% NaTiOxH catalyzed MgH2was selected for further investigations.
The hydrogenation properties of MgH2–5 wt% NaTiOxH were measured under varying conditions as shown in Fig. 2.At room temperature (Fig. 2a), the dehydrogenated MgH2–5 wt% NaTiOxH absorbs ∼4.5 and more than 5.0 wt% H2under 50 bars of H2pressure within the first 45 and 120 min,respectively. Meanwhile, under 10 and 30 bars of H2pressure, the composite respectively absorbs ∼4.1 and 5.5 wt%H2within the first 180 min; ∼5.5 and 6.0 wt% H2in 6 hr;∼6.0 and 6.4 wt% H2(∼87.7% of the total) in 12 hr (shown in Fig. S2). The observed fluctuation of absorption curves between 30 and 50 bars at the apex could be ascribed to an uncontrollable variation in environmental temperature. Furthermore, a moderate temperature absorption measurement of the composite at 30 bars of H2pressure (Fig. 2b) shows an absorption capacity of about ∼4.4 and 4.5 wt% H2within the first 30 min at 50 °C and 100 °C, respectively; while asmilled MgH2could barely absorb at these temperatures. This enhanced absorption kinetics further confirms the catalytic efficiency of NaTiOxH catalyst on MgH2.
Subsequently, a comparative measurement of isothermal de/re-hydrogenation at high temperatures of both the noncatalyzed and catalyzed MgH2was conducted at four (4) different constant temperatures of 200, 230, 260, and 290 °C under 0.001 bar and 30 bars of H2, respectively (Fig. 3).Figure 3(a) shows that the composite releases ∼7.2 wt% H2within the first 15 min at 290 °C, and ∼6.9 wt% in 60 min at 260 °C. However, as-milled MgH2releases only ∼3.1 wt%H2after 120 min at 290 °C, with no significant release at 260 °C. Furthermore, as shown in Fig. 3(b), the composite absorbs ∼6.6 wt% H2within the first 120 s at 260 °C and∼6.9 wt% after 20 min at 230 °C, while as-milled MgH2only absorbs ∼6.0 and 6.1 wt% H2within the same time range at 230 °C and 260 °C, respectively. Lastly, the composite charges ∼6.0 wt% H2after 30 min at 200 °C while the as-milled sample absorbs less.
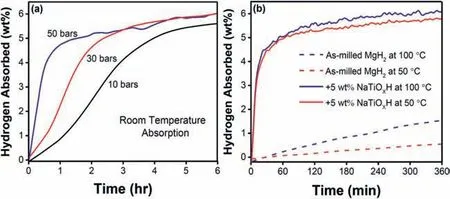
Fig. 2. (a) Room-temperature absorption curves of MgH2–5 wt% NaTiOxH at 10, 30, and 50 bars of hydrogen pressures; (b) Isothermal hydrogenation curves of as-milled MgH2 and MgH2–5 wt% NaTiOxH under 30 bars H2 at 50 and 100 °C.

Fig. 3. Isothermal dehydrogenation (a) at 260 and 290 °C, and isothermal hydrogenation (b) at 200, 230, and 260 °C for as-milled MgH2 and MgH2–5 wt%NaTiOxH.
A reversibility test was carried out to investigate the stability of the de/re-hydrogenation kinetics. In pursuit of a high hydrogen capacity with reasonable de/re-hydrogenation kinetics, MgH2–2.5 wt% NaTiOxH was chosen for cycling measurement across 100 cycles at 300 °C with a total of 475 hr(Fig. 4). Dehydrogenations were measured under 0.001 bar of H2while hydrogenations were measured under 30, 40,and 50 bars of H2, respectively. The data profiles of the 1st, 50th, and 100th cycles of de/absorption (Fig. S3 and S4) show that the composite remains fairly intact with only a slight variation in the hydrogen capacity. Aside from the drop in kinetics, after 100 cycles, the hydrogen desorption capacity remained at ∼6.1 wt%, equivalent to ∼84% capacity retention and 0.012 wt% hydrogen loss per cycle. The kinetic relaxation observed could be attributed to the agglomeration of Mg/MgH2particles and their separation from the catalyst during cycling. The zero-point slight variation noticeable between the 50th and 100th cycle absorptions could be attributed to the H2backpressure increase from 40 to 50 bars.

Fig. 4. Reversibility measurement of MgH2–2.5 wt% NaTiOxH at 300 °C under 0.001 and 30–50 bars of H2 pressures.
3.2. Kinetic and thermodynamic properties
The dehydrogenation kinetic improvement of MgH2–5 wt% NaTiOxH was investigated by applying the Kissinger model to calculate the apparent activation energy (Ea). First,the mass spectra data of as-milled and catalyzed MgH2were collected at heating rates of 2, 6, 8, and 10 °C/min as shown in Fig. 5 (a and b). The as-milled MgH2shows clearly two-step desorption behavior similar to reported studies [36,45,46]. This was attributed to either the formation of metastable high-pressureγ-MgH2or the non-uniformity of its grain/particle sizes. As shown in Fig. 5(c), the Kissinger plots of as-milled and 5 wt% NaTiOxH catalyzed MgH2indicate that ball milling pristine MgH2could reduce itsEafrom the reported value of ∼180 kJ/mol [34,45,47,48]to ∼101 (±4) kJ/mol, and further reduction to ∼57 (±1)kJ/mol by adding NaTiOxH catalyst. The PCI desorption measurement of the composite at 300, 320, and 340 °C exhibits a distinct plateau region at each isotherm as shown in Fig. 5(d). The Van’t Hoff plot of equilibrium pressure against temperature (inset) provides the dehydrogenation enthalpy change of ∼77 (±1.5) kJ/mol-H2. This indicates that Na-TiOxH does not have any thermodynamic improving effect on MgH2.
A comparative tabulation of the room temperature absorption capacity,activation energy,and reversibility of MgH2catalyzed by a few representative TiO2-based catalysts is shown in Table 1. This confirms the improved H2storage performance of MgH2by adding NaTiOxH catalyst.
3.3. Characterization of NaTiOxH catalyst
X-ray diffraction (XRD), transmission electron microscopy(TEM), selected area electron diffraction (SAED), and scanning electron microscopy (SEM) equipped with energydispersive X-ray spectroscopy (EDS) analysis unit were used to evaluate the phase composition, morphology, and crystallography of the synthesized NaTiOxH catalyst. X-ray photoelectron spectroscopic(XPS)investigation was also conducted to examine the chemical status of the component species consisted in the catalyst as shown in Fig. 6. The XRD profile of NaTiOxH (Fig. 6a) displays the diffraction peaks corresponding to TiO2. The reduced intensity and broadness of some of the peaks have previously been attributed to an amorphous layer of defective TiO2-xnanoparticles formed near the surface [52]. In addition, a careful resolution of the peaks (Table S1) shows the emergence of a few crystalline phases of Ti3O5and Na2Ti3O7near the TiO2. The presence of these crystalline phases and the formation of black TiO2-xpowder suggest that a reaction occurred between NaH and TiO2during ball milling [42,53,54]. The SAED pattern of the catalyst(Fig. 6b) exhibits a typical point-ring diffraction characteristic of polycrystalline materials. The agreement in the calculated d-spacing values (Table S1) of indexed Ti3O5, and Na2Ti3O7between the SAED and XRD patterns of the catalyst (shown in Fig. 6a and b), confirms theirin-situformation. In addition, the analysis of selected areas on the HRTEM image of NaTiOxH (Fig. 6c) shows the lattice fringes corresponding to Ti3O5(110), and Na2Ti3O7(101 and 103). The Fast Fourier Transformed(FFT)image(inset)reveals a rod-like diffraction pattern of the crystalline Na2Ti3O7on the (101) plane. More TEM images of the catalyst that show the distribution and lattice fringes of Na/TiOx species on different sides could be seen in Fig. S5 and S6. For the morphology, the SEM micrograph of pristine TiO2anatase (Fig. 6d) shows spherical nanoparticles (∼23 nm) with reduced size and increased surface area that is noticeable after milling with NaH (Fig. 6e).Bright patches on the particles’ surface could be attributed to contaminations on exposure to air before/during measurement. Furthermore, sodium 1s XPS spectrum of the catalyst(Fig. 6f) reveals its existence as Na-O-Ti, and Na-O/OH positioned at 1070.5 and 1072.1 eV, respectively [55]. The energy peak at 1069.2 eV is attributed to the Ti-Auger effect (Ti LMM) [56,57]. Titanium 2p XPS of the catalyst(Fig. 6g) shows that it exists in four (4) different valence states of +4, +3, +2, and 0; as distinct from the spectra of pure TiO2(Fig. S7). This is due to the formation of defects and oxygen vacancies after milling with NaH; a phenomenon that could generate trapped-in electron densities around vacant 3d orbitals of the corresponding adjacent Ti atoms [49,52,58,59]. The appearance of the peak corresponding to Ti3+at 457.5 eV could be attributed to the Ti3O5and/or its related species with other possible oxyhydrides [60,61].Ti2+peak at 455.1 eV could be due to TiO species probably covered up in the amorphous layer of the defective TiO2-x[28,61,62], while Ti0peak at ∼452.5 eV is considered to be the characteristic peak of Ti metal [28,60,63]. The 1 s XPS spectrum of oxygen (Fig. 6h) identifies its existence as OTi/Na (530.0 eV), H-O-Ti/Na, and/or peroxides (531.9 eV)with the binding energy slightly shifting to the higher region as compared to oxygen 1s spectra of pure TiO2sample (Fig.S8). An adsorbed contaminant on exposure of the sample to air before measurement accounts for the peak at 532.4 eV,while the peak at 536.8 eV is attributed to the Na-Auger effect (Na KLL) [64–66]. Summarily, ball milling NaH with TiO2liberates defective TiO2-xspecies which is characterized by reduced valences of titanium, and oxygen vacancies. A few crystalline phases of Ti3O5and Na2Ti3O7also emergein situduring the reaction.

Fig. 5. TPD-MS curves of as-milled MgH2 (a) and MgH2–5 wt% NaTiOxH (b) at heating rates of 2, 6, 8, and 10 °C/min; (c) Kissinger plot of as-milled MgH2 and MgH2–5 wt% NaTiOxH; (d) PCI desorption curves at 300, 320 and 340 °C and Van’t Hoff plot of MgH2–5 wt% NaTiOxH.

Table 1 Room temperature absorption capacity, activation energy, and reversibility of MgH2 catalyzed by some TiO2-based catalysts.
3.4. Phase transition and mechanism analysis of MgH2–5 wt% NaTiOxH composite

Fig. 6. (a) XRD pattern of pure TiO2 and NaTiOxH catalyst; (b) SAED pattern of NaTiOxH; (c) TEM image of NaTiOxH; (d) SEM image of pure TiO2;(e) SEM image of NaTiOxH; (f) Na 1 s spectra of NaTiOxH; (g) Ti 2p spectra of NaTiOxH; (h) O 1 s spectra of NaTiOxH.
As revealed in Sections 3.1 and 3.2, NaH doped TiO2in a 1:1 mole ratio (NaTiOxH) exhibits excellent catalytic performance on the hydrogen storage properties of MgH2. To unravel the mechanism behind this performance, XRD patterns of the as-milled and de/re-hydrogenated samples before and after cycling were collected as shown in Fig. 7(a).The patterns show characteristic peaks of MgH2, Mg, and MgO. The MgO peak could arise due to oxygen contamination before/during the XRD measurement[67]or the presence of MgO characteristic of the oxide additives loaded MgH2[28]. Aside from these regular phases of MgH2/Mg/MgO, the phase stability of Ti3O5, Na2Ti3O7, and defective TiO2-xafter 100 cycles show a probable reason behind the improved de/absorption behaviors of MgH2. Meanwhile, the SAED image of the as-milled composite (Fig. 7b) shows the plane corresponding to Ti3O5and Na2Ti3O7species. The TEM image of the as-milled composite reveals the contacting catalyst nanoparticles around the MgH2particles (Fig. 7c), while the HRTEM images of the as-milled and hydrogenated composites reveal the corresponding lattice fringes of MgH2, MgO,Na2Ti3O7,and TiO2-xspecies (Fig. 7d and e). The calculatedd-spacing values of the resolved lattice fringes as detected by SAED and TEM agree with both the XRD pattern and the standard values ofd-spacing as shown in Table S2. More TEM images of the composites with some lattice fringes on different sides are shown in Fig. S9 and S10.Moreover, from the morphological perspective, the SEM micrograph of pristine MgH2(Fig. 7f) shows micro-particles with a size distribution>30 μm. High energy milling reduces the size below 10 μm (Fig. 7g) with the size distribution profile shown in Fig. S11. The addition of NaTiOxH catalyst (Fig. 7h) further reduces the size of MgH2coupled with an increased contact which could help promote its kinetics of de/re-hydrogenation [34]. The SEM micrograph of the dehydrogenated sample (Fig. S12) shows fine sponge-like Mg particles with bright patches on the surface attributed to MgO. A slight expansion of the particles could be observed on hydrogenation (Fig. S13). This expansion is believed to promote contact between the particles of Mg/MgH2and Na-TiOxH which could facilitate the reversibility process as observed in Fig. 4. Likewise, EDS mapping of the composite(Fig. 7i) also reveals the bright spots of Na, Ti, and O species well dispersed around the network of MgH2; a phenomenon that could positively influence the electron dynamics around MgH2for an improved hydrogen storage performance. Furthermore, titanium 2p XPS spectral of the as-milled sample(Fig. 7j) shows its existence in four (4) different states similar to Fig. 6(g) above. However, the addition of MgH2causes a significant electronic effect around Ti3+at 457.0 eV, and Ti2+at 454.8 eV as compared to the Ti spectra of NaTiOxH catalyst in Fig. 6(g). This kind of shift in binding energy(∼1 eV difference) was previously attributed to the reduction in ionic contribution in the respective titanium chemical bond formation; the unstable ionic character could arise due to factors such as lattice distortion, hybridization, and crystal field stabilization effects[68–71].On dehydrogenation, the compositional changes around the valences due to H2release result in a slight shift to the higher binding energy region. Interestingly, the Ti0state at 452.5 eV in the as-milled sample which corresponds to titanium metal disappears. Considering the high chemical reactivity between titanium and oxygen,the reason for this disappearance could be attributed to the oxidation of the titanium metal into higher state sub-oxides even as more oxygen atoms are likely to be present after dehydrogenation [41,54,72–74]. After re-hydrogenation, a similar pattern to the as-milled sample in Fig. 7(j) above re-emerges (Fig.S14). This indicates probably the occurrence of a redox reaction via H2insertion and removal; a process that causes some sort of vacancy creation and elimination between the valence and conduction bands of Ti-O bonds as previously reported by the following studies [17,75]. The Oxygen 1s data plot of the as-milled sample (Fig. S15) shows the bond states corresponding to phases of Mg-O, Na/Ti-O, hydroxides, and/or peroxides [65]. The binding energy shift of the corresponding O-based species(while Mg-O binding energy peak remains)is also consistent with the redox process as shown in Fig.S16.It should be noted at this point that the Na2Ti3O7species formedin-situremains after 100 cycles of de/re-hydrogenation from XRD analysis (Fig. 7a); hence, a logical conclusion could be reached that a ‘topotactic’ reaction probably occurs between Mg-H and this catalytic species similar to the reported investigations on TiO2surface topotactic reactions [73,76]. This reaction probably generates nonstoichiometric layers around the Na-doped TiO6octahedrons which provide multiple diffusion channels that enhance the Mg-H bond de/association[17,33]. In addition, the disappearance and re-appearance of Ti3O5species in the respective XRD patterns of the dehydrogenated and re-hydrogenated composites, and the unstable valences of Ti3+/Ti2+/Ti0species in the composites (consistent with H2insertion and removal),evidently confirm that the redox process around these defects facilitates hydrogen diffusion in Mg/MgH2[17,32,75]. This is schematically illustrated in Fig. 8. However, apart from the multivalent titanium states,and the Ti3O5/Na2Ti3O7species formedin situ, some other components of the defective TiO2-xcould have played their respective roles towards improving the electronic conductivity around the MgH2bond; this creates the gap for further investigations.
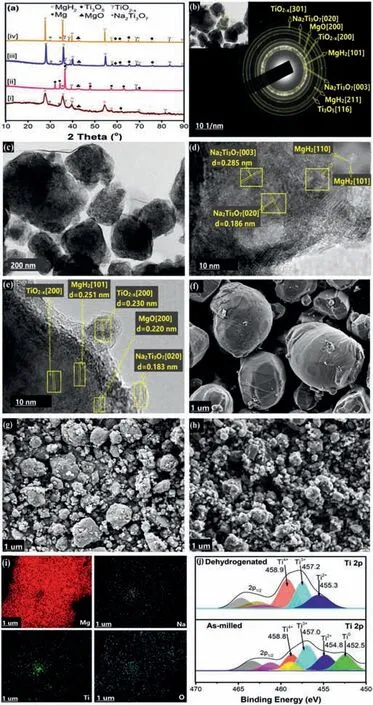
Fig. 7. (a) XRD pattern of MgH2–5wt% NaTiOxH as-milled (i), dehydrogenated (ii), 1st hydrogenation (iii), and 100th hydrogenation (iv); (b) SAED pattern of as-milled MgH2–5wt% NaTiOxH; (c) TEM image of as-milled MgH2–5wt% NaTiOxH; (d, e) HRTEM images of as-milled and hydrogenated MgH2–5wt% NaTiOxH; (f, g) SEM images of pristine and as-milled MgH2; (h) SEM image of as-milled MgH2–5wt% NaTiOxH; (i) EDS mapping of MgH2–5wt% NaTiOxH; (j) Ti 2p spectra of MgH2–5wt% NaTiOxH.

Fig. 8. The schematic diagram of the catalytic mechanism in MgH2-NaTiOxH composites.
4. Conclusion
This work presents NaH doped nanocrystalline TiO2Na-TiOxH as an effective catalyst for the hydrogen storage properties of MgH2. The introduction of 2.5 wt% of the catalyst stabilized the reversibility of Mg/MgH2up to 100 cycles with ∼6.1 wt% H2retained afterward. Interestingly, 5 wt% of the catalyst could influence a remarkable absorption of ∼4.5 wt% H2in 45 min at room temperature under 50 bars of H2pressure. This composite also absorbed ∼5.0 wt% H2in 60 min at 50 °C under 30 bars of H2pressure. In addition to these improvements, the desorption analysis revealed that 5 wt% NaTiOxH catalyzed MgH2could start to release H2from ∼185 °C and desorb ∼7.2 wt% H2within 15 min at∼290 °C; thanks to the apparent activation energy of desorption calculated to be ∼57 kJ/mol which is 44 kJ/mol below as-milled MgH2, and 123 kJ/mol below pristine MgH2. However, the observed dehydrogenation enthalpy of ∼77 (±1.5)kJ/mol-H2indicates that NaH only acted as a reducing agent for TiO2without any positive influence on the thermodynamic property of MgH2. The results obtained from XPS measurement revealed that NaTiOxH is an effective catalyst for MgH2possibly due to the existence of reduced titanium valences(Ti<4+) which showed partial/full reversibility via hydrogen insertion and removal, due to vacancy creation and elimination. Additional information from XRD, TEM, SEM, and EDS complementarily revealed an intimate contact between the homogeneously dispersed NaTiOxH and MgH2particles.It was also observed that the presence of catalytically active Ti3O5and “rod-like” Na2Ti3O7formedin-situwith some other possible multivalent titanium sub-oxides in the defective black TiO2-xpowder could enhance the hydrogen storage performance of MgH2by providing multiple diffusion channels during the de/absorption.
Conflicts of interest
The authors declare no competing interest.
Supplementary information
Figure S1. Temperature programmed desorption curves of MgH2catalyzed with 10 wt% NaH @ TiO2at ratio 0.5, 1,and 2:1.
Figure S2.Room-temperature absorption curves of MgH2–5 wt% NaTiOxH in 12 hr at 10, and 30 bars of hydrogen pressure.
Figure S3. 1st, 50th, and 100th desorption cycles of MgH2–2.5 wt% NaTiOxH at 300 °C and 0.001 bar of H2pressure.
Figure S4. 1st, 50th, and 100th absorption cycles of MgH2–2.5 wt% NaTiOxH at 300 °C and 30–50 bars of H2pressure.
Table S1. Comparisons between calculatedd-spacing of phases in the catalyst and standards.
Figure S5. TEM image of NaTiOxH catalyst.
Figure S6. HRTEM images of NaTiOxH catalyst.
Figure S7. XPS profile of Titanium 2p in pure TiO2sample.
Figure S8.XPS profile of Oxygen 1s in pure TiO2sample.
Table S2. Comparisons between calculatedd-spacing of phases in the composite and standards.
Figure S9. HRTEM images of as-milled MgH2–5 wt%NaTiOxH.
Figure S10. HRTEM images of hydrogenated MgH2–5 wt% NaTiOxH.
Figure S11. Size distribution profile of as-milled MgH2.
Figure S12. SEM image of dehydrogenated MgH2–5 wt%NaTiOxH.
Figure S13. SEM image of hydrogenated MgH2–5 wt%NaTiOxH.
Figure S14. XPS profile of Ti 2p in MgH2–5 wt% Na-TiOxH at different phases.
Figure S15. XPS profile of Oxygen 1s spectra in MgH2–5 wt% NaTiOxH.
Figure S16. XPS profile of O 1s in MgH2–5 wt%NaTiOxH at different phases.
Acknowledgments
The authors acknowledge the Project supported by the National Key R&D Program of China (2019YFE0103600,2018YFB1502101), the Key R&D Program of Shandong Province, China (2020CXGC010402), the National Natural Science Foundation of China (51801197), the Liaoning Revitalization Talents Program (XLYC2002076), the Dalian Highlevel Talents Program(2019RD09),the Youth Innovation Promotion Association CAS (2019189) and K.C. Wong Education Foundation (GJTD-2018–06).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Microstructure, mechanical properties and fracture behaviors of large-scale sand-cast Mg-3Y-2Gd-1Nd-0.4Zr alloy
- Enhanced initial biodegradation resistance of the biomedical Mg-Cu alloy by surface nanomodification
- Elucidating the evolution of long-period stacking ordered phase and its effect on deformation behavior in the as-cast Mg-6Gd-1Zn-0.6Zr alloy
- Effect of long-period stacking ordered structure on very high cycle fatigue properties of Mg-Gd-Y-Zn-Zr alloys
- The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition
- The role of melt cooling rate on the interface between 18R and Mg matrix in Mg97Zn1Y2 alloys
