Research advances of magnesium and magnesium alloys worldwide in 2022
2023-11-18YnYngXiomingXiongJingChenXiodongPengDolunChenFushengPn
Yn Yng, Xioming Xiong, Jing Chen, Xiodong Peng, Dolun Chen, Fusheng Pn
aNational Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing 400044, China
b Department of Mechanical and Industrial Engineering, Toronto Metropolitan University (formerly Ryerson University), Toronto, ON M5B 2K3, Canada
Received 4 July 2023; accepted 31 July 2023
Available online 9 September 2023
Abstract More than 4600 papers in the field of Mg and Mg alloys were published and indexed in the Web of Science (WoS) Core Collection database in 2022. The bibliometric analyses indicate that the microstructure, mechanical properties, and corrosion of Mg alloys are still the main research focus. Bio-Mg materials, Mg ion batteries and hydrogen storage Mg materials have attracted much attention. Notable contributions to the research and development of magnesium alloys were made by Chongqing University (>200 papers), Chinese Academy of Sciences, Shanghai Jiao Tong University, and Northeastern University (>100 papers) in China, Helmholtz Zentrum Hereon in Germany,Ohio State University in the USA, the University of Queensland in Australia, Kumanto University in Japan, and Seoul National University in Korea, University of Tehran in Iran, and National University of Singapore in Singapore, etc. This review is aimed to summarize the progress in the development of structural and functional Mg and Mg alloys in 2022. Based on the issues and challenges identified here, some future research directions are suggested.
Keywords: Magnesium alloys; Cast magnesium alloys; Wrought magnesium alloys; Bio-magnesium alloys; Mg-based energy storage materials; Processing technologies; Corrosion and protection.
1. Introduction
Magnesium (Mg) is the lightest commercial structural metal and a promising energy storage material. It has broad prospects in achieving the strategic goals of “carbon neutrality” and “emission peak” and alleviating the energy crisis[1–5]. Mg alloys have high specific strength and stiffness, superior damping performance, good biocompatibility, large hydrogen storage capacity, and high theoretical specific capacity for batteries, etc. Hence, magnesium and its alloys have application potential in aerospace, automotive, 3C (computers,communications, and consumer electronics), biomedical and energy sectors, etc. in the world [6–11]. However, a lot of difficulties still need to be overcome to expand the further applications of magnesium alloys [12–15]. The relatively low strength, poor plasticity, and inferior corrosion resistance of magnesium alloys impede the structural applications, while the problems on the fast degradation rate of Mg alloys and narrow hydrogen charging and discharging window need to be solved in functional materials to broaden the future application of Mg alloys [16–18].
In the past year of 2022, more than 4600 papers in the field of Mg and Mg alloys were published and indexed in the authoritative database of “Web of Science Core Collection”.The research trends and hotspots of magnesium alloys were analyzed based on such a literature search. The present work aims to review the important advances of magnesium and its alloys worldwide in 2022 to boost the multifaceted scientific research of magnesium alloys and promote the global development and application of magnesium alloys.
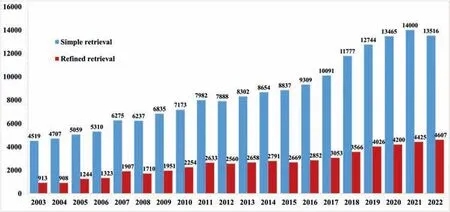
Fig. 1. Published Mg-related papers in the past 20 years in the Web of Science (WoS) Core Collection database (searched on February 10, 2023).
2. Overview of Mg research in 2022
2.1. Overall status of Mg research
The published Mg-related papers in 2022 were searched in the Web of Science (WoS) Core Collection database on February 10, 2023. Fig. 1 presents simple search results in the past 20 years using ‘Magnesium or Mg alloy’ as the topic(blue columns). To reveal more precisely the publications on Mg and Mg alloys, a more sophisticated retrieval strategy is applied. Briefly, ‘Mg alloy’, ‘Magnesium hydrogen’, ‘Magnesium battery,’ ‘Magnesium biodegradable’, ‘Magnesium corrosion’, and ‘Magnesium mechanical’ were used as topics with a certain rule in the WoS Core Collection database. After the duplicates are automatically eliminated, the results are shown in the red columns in Fig. 1. It is seen from the simple searches that the number of publications gradually increases from 2003 to 2021, and the number of publications in 2022 is slightly lower than that of 2021. The slight decrease in 2022 would mainly be due to the fact that some publications in 2022 have not yet been indexed in WoS by February 10,2023. It is expected that the total number of publications in 2022 would be higher than that of 2021 in the WoS database.It is seen from the retrieval searches that the number of publications gradually increased from 2003 to 2022. The gradual increase in publications illustrates that the research on Mg and Mg alloys continues to be a hot spot in the field of materials science and engineering in the past 20 years and has attracted more and more attention.
With the refined or more precise retrieval, 4607 papers on Mg and Mg alloys in total were collected by February 10,2023, indeed being more than those in 2021 and previous years. Statistical analysis was conducted via the VOS viewer software. The distributions of countries and regions and organizations that published Mg papers were analyzed on the basis of the above-mentioned literature. A total of 79 countries and regions published Mg and Mg alloys in 2022. Fig. 2 shows a statistical analysis of the distribution of countries and regions with at least 5 Mg publications in 2022. As seen from Fig. 2(a), China remains the country that publishes the most Mg papers with a contribution of 45.49%, which is an increase from 40.38% in 2021, followed successively by India,USA, Germany, and Japan, etc. Fig. 2(b) shows the network visualization among different countries and regions. The circle size represents the number of published papers while the width of the link lines among different countries and regions indicates the collaboration intensity. This analysis reveals that about 20.85% of Mg papers were published based on international collaborations in 2022, with a slight decrease from 23.81% in 2021. At the same time, there are many Mg papers on international collaborations among China, USA, Germany,Australia, Japan, South Korea, India, etc.
Fig. 3 shows the statistical analysis of organizations that published at least 15 Mg papers in 2022. The top 20 organizations are shown in Fig. 3(a). Chongqing University published 228 Mg papers, a significant increase from 163 papers in 2021, and remained the top spot in recent years, followed by the Chinese Academy of Sciences, Shanghai Jiao Tong University, Northeastern University, and Harbin Institute of Technology. Helmholtz Zentrum Hereon from Germany, the University of Tehran from Iran, and the National University of Singapore from Singapore are also positioned in the top 20 spots in 2022. Fig. 3(b) shows the network visualization among different organizations. Similarly, the circle area or size represents the number of published papers, while the width of the link lines among different organizations indicates the collaboration activities.The proportion of Mg papers based on collaboration is 65.82%, which is similar to the proportion of collaborative papers published in 2021, suggesting that collaborations among different organizations can significantly accelerate Mg research and development.
2.2. Statistics and analysis of journals publishing Mg papers
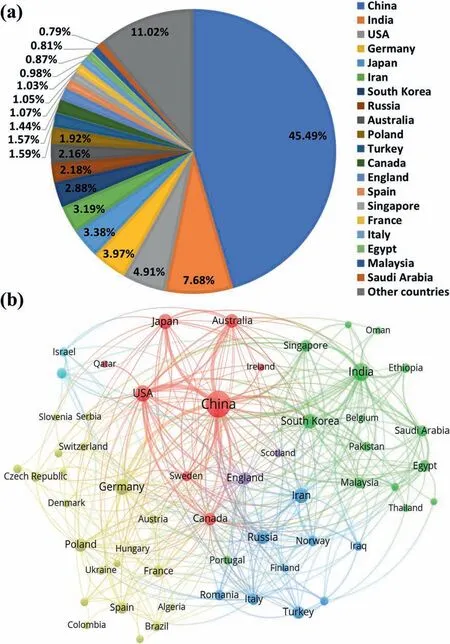
Fig. 2. Statistical analysis of the distribution of countries with at least 5 Mg papers published in 2022: (a) Paper percentage in different countries and regions;(b) network visualization among different countries.

Fig. 3. Statistical analysis of organizations publishing at least 15 Mg papers in 2022: (a) Top 20 organizations; (b) network visualization among different organizations.
According to the number of magnesium papers published in 2022, the top 20 journals are listed in Table 1.Journal of Magnesium and Alloyspublishes the most papers, followed byMaterialsandMaterials Science and Engineering A. The network visualization among different journals that published at least 10 Mg papers in 2022 is shown in Fig. 4. Similarly,the wider links between the two journals reveal more citations between them. The results indicate that theJournal of Magnesium and Alloys, Materials Science and Engineering A, and Journal of Alloys and Compoundshave a wider link,indicating that their correlations are pretty close.
Journal of Magnesium and Alloys(JMA)published 230 papers in 2022,representing an increase of 42%from 2021.The impact factor (IF) of JMA increases rapidly from 111.862 in 2021 to 17.6 in 2022, ranking No. 1 among the 78 journals in Metallurgy & Metallurgical Engineering category (JCR Q1).Fig. 5 shows the statistical analysis result of country (region)distribution of Mg papers published in the JMA in 2022. The number of collaborations among different institutions is 161,accounting for 70%, up from 67% in 2021. More than half of the articles in the JMA involved institutional collaborations, which is also similar to the whole trend. The statistical results confirm that the JMA enjoys collaborative academic achievements.
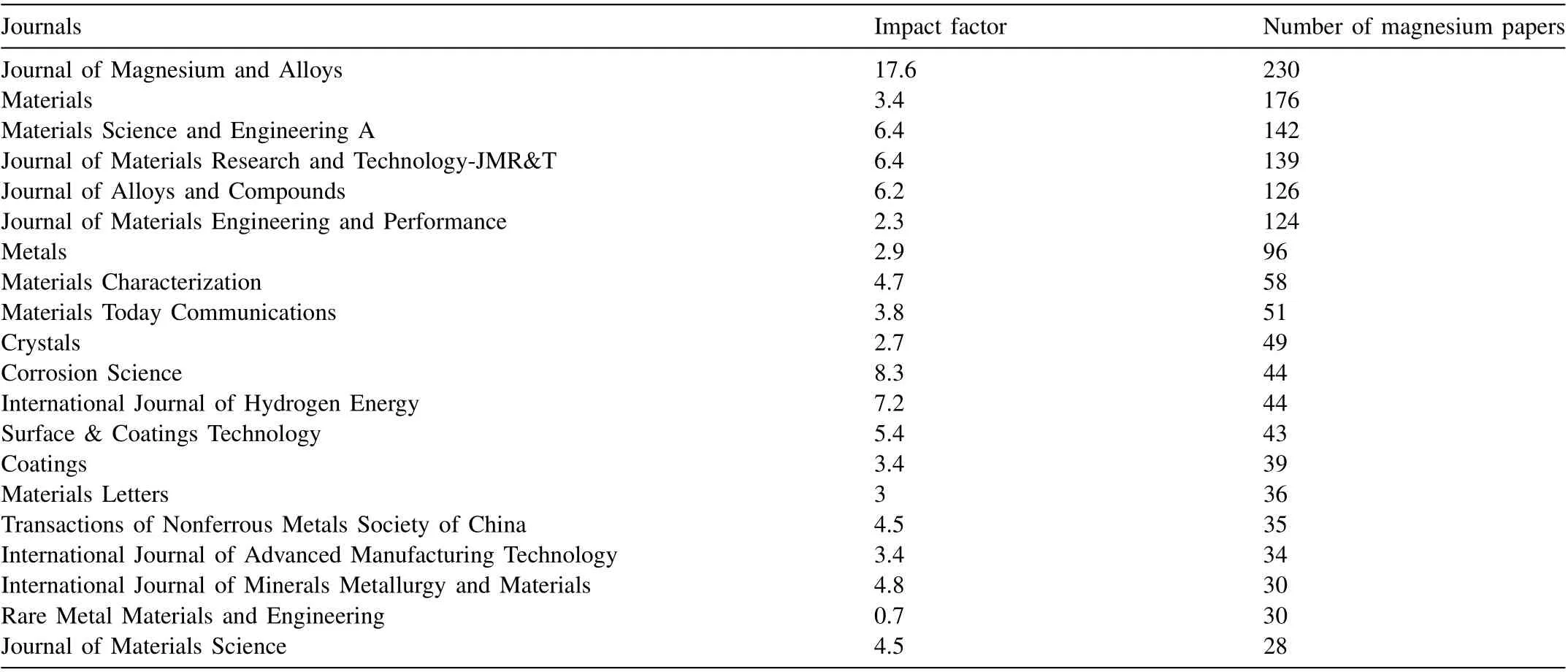
Table 1 Top 20 journals with Mg papers worldwide in 2022.
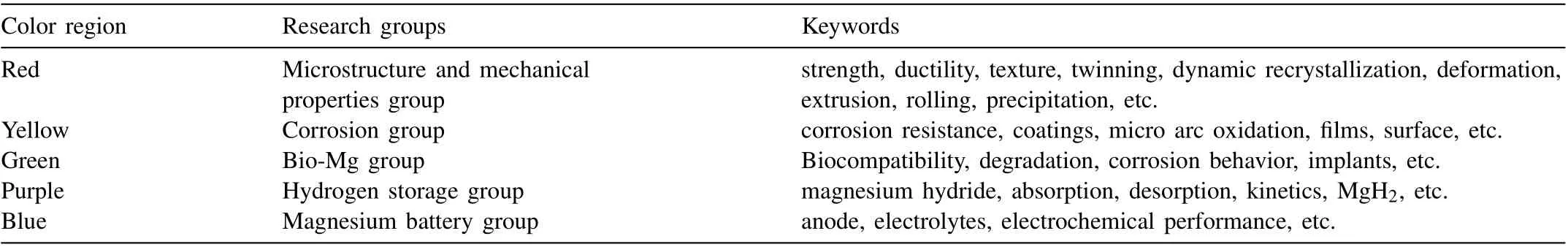
Table 2 Keywords in each research group.
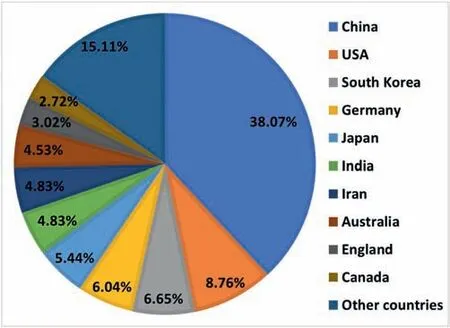
Fig. 5. Statistical analysis of county (region) distribution of Mg papers published in the Journal of Magnesium and Alloys in 2022.
2.3. Research hotspots in 2022 based on bibliometric analysis
The top 150 keywords by relevance, based on the Mg and Mg alloy articles published in 2022 are shown in Fig. 6. The larger size of the circle reflects the more times of keywords used. For instance, ‘Microstructure’, ‘Mechanical-Properties’,‘Corrosion Resistance’, and “biocompatibility” are the top four keywords, which implies that the microstructure, mechanical properties, corrosion and bio-Mg of magnesium alloys continue to be the research hot areas.
In addition, the similar color of circles indicates a high relevance of the keywords. There are mainly four colors in Fig. 6, i.e., red for ‘microstructure’ and ‘mechanical properties’ group, yellow for ‘corrosion’ group, green for ‘bio-Mg’,purple for ‘hydrogen storage’ group, and blue for ‘magnesium battery’. The largest group is the red ‘microstructure’and ‘mechanical properties’, suggesting that the mechanical properties and microstructures of structural Mg alloys as well as their processing technologies in 2022 still attracted much attention in the R&D of Mg and Mg alloys. Research in the second largest yellow group is very near green and ‘bio-Mg’group, indicating that bio-Mg alloys have attracted increasing attention and are closely related to the corrosion properties. Interestingly, in each color group, the interconnected keywords are identified as the sub-hot spots in each hot field,as listed in Table 2.
In short, the bibliometric analysis of keywords is capable of showing both the hot fields and the hot spots in each hot field, which could be used to guide the research directions and topics. Based on the bibliometric analysis results, the research fields on Mg and Mg alloys could be generally classified into four main categories: (1) traditional structural cast and wrought Mg alloys that focused mainly on microstructure and mechanical properties, (2) functional materials including Mg battery, hydrogen storage Mg materials, and bio-Mg materials, (3) processing technologies of Mg and Mg alloys, and(4) corrosion and protection of Mg and Mg alloys.
3. Structural Mg alloys
3.1. Cast Mg alloys
3.1.1. Cast Mg alloys containing rare-earth elements
In 2022, many studies on rare-earth cast magnesium alloys were reported, mainly focusing on Mg-Gd and Mg-Y alloys.Ultra-light and high-strength magnesium alloys are widely used in aviation, aerospace, missile, automobile, and other fields.It is known that rare-earth elements can effectively control the microstructure of magnesium alloys,providing a feasible method of attaining high-strength and high-plasticity magnesium alloys [19–21]. However, rare-earth elements are expensive. Therefore, developing ultra-light, high-strength, and high-plasticity magnesium alloy with a low rare-earth content is worthy of in-depth study. Moreover, this paper lists some research reports on cast magnesium alloys containing rare-earth elements in 2022, as shown in Table 3.
The mechanical properties of different cast magnesium alloy systems vary greatly. The ultimate tensile strength (UTS)of the Mg-La series is lower than 200 MPa [22,23], while Mg-Gd series alloys have a much higher UTS [25,27, 29,30].Other series alloys also have a UTS of more than 200 MPa[26,28]. Taking Mg-4Al-1Si-1.5Ce-1.5La alloy [26] as an example, its UTS reaches 263 MPa after die casting and has high plastic properties. It can be seen that the mechanical properties of cast magnesium alloys containing rare-earth elements in different systems are significantly different.
Compared with previous studies, the Mg-7.8Gd-2.7Y-2.0Ag-0.4Zr alloy developed by Huisheng Cai et al. [25] of Shanghai Jiao Tong University through gravity casting and subsequent solution treatment (510 °C × 6 h) + aging treatment(200°C×32 h)has a UTS of ∼411 MPa,yield strength(YS) of ∼273 MPa, and elongation (EL) of ∼4.9%. On the one hand, the strength is improved due to the presence ofβ’andγ’ precipitates; the alloy shows prominent double-peak aging characteristics during isothermal aging due to the difference in the precipitation process. A higher aging temperature is beneficial to shorten the time to reach the hardness peak.On the other hand, due to the precipitation-strengthening effect of Ag, its contribution to the YS of the alloy is about 51%.
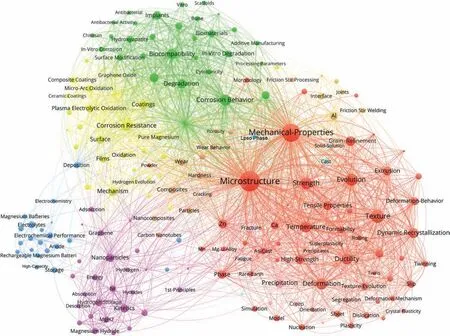
Fig. 6. Network visualization among different keywords in Mg-related papers.
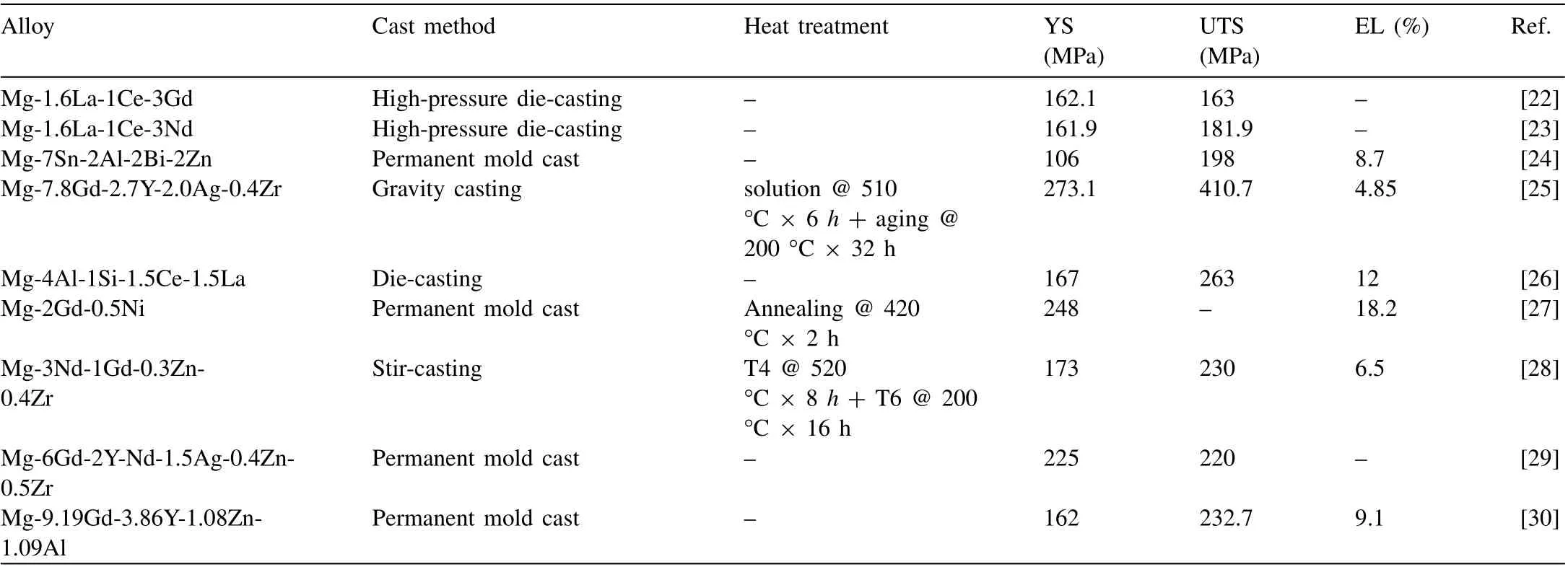
Table 3 Mechanical properties of cast magnesium alloys containing rare-earth elements.

Table 4 Mechanical properties of cast magnesium alloys without containing rare-earth elements.
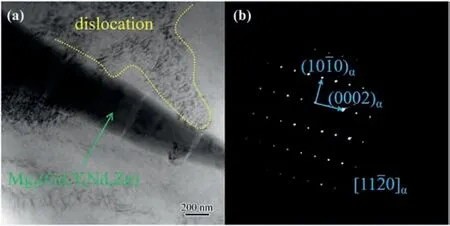
Fig. 7. (a) The TEM image of near elevated tensile fracture of as-cast Mg-6Gd-2Y-Nd-0.4Zn-0.5Zr, (b) SAED patterns corresponding to the (a) [29].
To explore the high-temperature strengthening mechanism of Mg-6Gd-2Y-Nd-0.4Zn-0.5Zr alloy, Tao Ma’s group[29] studied the microstructure and mechanical properties of Mg-6Gd-2Y-Nd-0.4Zn-0.5Zr alloy by adding Ag. It is believed that adding Ag not only plays a role in grain refinement but also promotes the formation of the second phase. Moreover, the content of Ag is more than 1.5 wt.%,and a small amount of Mg17Ag2(Gd, Y, Nd, Zn) phase is formed at the grain boundary, as shown in Fig. 7. At the same time, Mg-6Gd-2Y-Nd-1.5Ag-0.4Zn-0.4Zr alloy exhibited higher mechanical properties, with a UTS 220 MPa at room temperature, which is 11.3% higher than that of Agfree alloy.
3.1.2. Cast Mg alloys without rare-earth elements
In 2022, research scholars designed some new rare-earthfree magnesium alloys,whose mechanical properties are summarized in Table 4. Jian Rong et al. [31] prepared highstrength Mg-7Al-3Ca alloy via high pressure die casting,with a UST of 238 MPa, a YS of 196 MPa, and an EL of 3.9%. The high YS was attributed to the combinations of fine-grain strengthening, second phase strengthening from numerous Al2Ca eutectic phases, and solid solution strengthening from Al solutes. Especially after aging treatment, the Al2Ca phase further precipitates, and the room temperature YS of Mg-7Al-3Ca alloy increases from 184 MPa to 196 MPa.
3.1.3. Effect of reinforcing particles on the properties of cast magnesium alloys
This article lists some research reports on the effect of reinforced particles on the properties of cast magnesium alloys in 2022, as shown in Table 5. M.S. Santhosh et al. [34] added fly ash to the vortex formation process when casting magnesium matrix composites using the stir casting method. The tensile strength of the composite material was obtained up to∼252 MPa, while the tensile strength of pure magnesium was only ∼177 MPa,with a 42%increase in the tensile strength of magnesium alloy after adding fly ash. In addition, the Vickers hardness value of pure magnesium is ∼65 HV. After adding fly ash during casting, the hardness of magnesium alloy increases by up to 21%, reaching ∼79 HV.
In the production of composite materials, some ceramic reinforcing particles are usually used to strengthen the material, such as SiC, Al2O3, TiB2, carbon nanotubes (CNT), etc.Murugan Subramani et al. [35] obtained AZ31 nanocomposites with better properties by adding SiC nanoparticles produced by the plasma arc vaporization method during gravity stir casting of AZ31 magnesium alloy. The hardness of the sample was ∼51 HV, the UTS was ∼157 MPa, the YS was ∼109 MPa, and elongation was ∼4.6%. Emadi et al.[36] added Al2O3(wt.%) particles with different contents to the samples during the preparation of AZ91E cast samples. The UTS of the samples reached a maximum value of 159 MPa and elongation (%EL) reached a maximum value of 2.5% with the addition of 0.5% Al2O3. However,the YS of the samples only reached a maximum value of 106 MPa with the addition of 1.0% Al2O3. Wuxiao Wang et al. [37] added 50 nm TiB2particles to molten Mg-4Al-1.5Si and finally obtained cast samples with substantially increased mechanical properties. Compared with the original cast alloy, the addition of TiB2nano-sized particles increased the hardness of the sample by ∼102% to 113 HV; the UTS by 69.8% to 142.4 MPa ± 11.4 MPa, the YS by 10.6% to 76.4 MPa ± 10.2 MPa; and the elongation by 187.5% to 9.2% ± 1.1%.
3.1.4. Defect control of cast Mg alloys
Cast defects, e.g., porosities, inclusions, and hot tearing,are inevitable in cast Mg alloys due to complex thermalsolute-convection interaction during solidification[38,39].For instance, in Mg-RE alloys RE elements show a greater oxidation tendency than Mg (e.g., Y>Gd>Nd>Mg), and it is much easier to generate oxidation slags in Mg-RE alloys. The cause of cast defects is complicated, and it is not only related to casting processes but also associated with a series of factors such as alloy properties, melting processes, and mold-ing materials. Therefore, when analyzing the causes of cast defects, it is necessary to perform a comprehensive analysis according to characteristics, locations, processes, and material properties, and then take corresponding technical measures to reduce and/or eliminate cast defects.
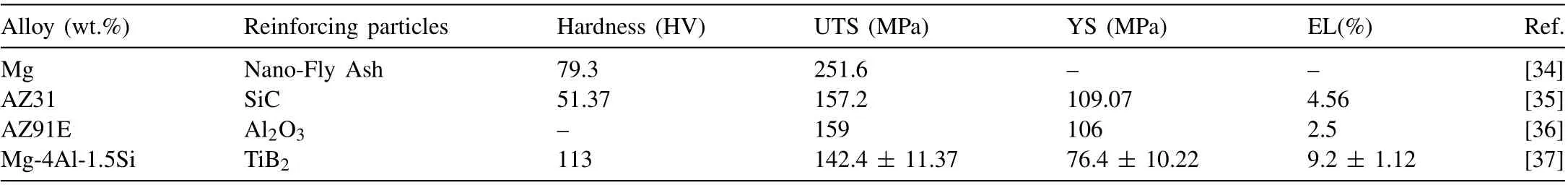
Table 5 Mechanical properties of some reinforced particulate cast magnesium alloys samples.

Fig. 8. Evolution of five dendrites and bubble in a 3D view. The phase interfaces of the dendrites and bubble are extracted by setting the phase-field values to 0.5. The arrows denote the flow velocity vectors [40].
For the defects related to the gas phase, Ang Zhang et al.[40,41] developed a solid-liquid-gas multiphase-field lattice-Boltzmann model to investigate the interaction between the gas phase and the solidifying microstructures during solidification. Fig. 8 shows the evolution of multi-dendrites and gas phase in a 3D view. The gas bubble rises under buoyancy,and it is hindered and entrapped by the growing dendrites.Restricted by the dendrite skeleton, the bubble exhibits a near-spherical shape due to the pinching effect. The proposed model is regarded as a significant progress in solving the formation of the gas porosity, and it is suitable for addressing the problems involving the solid-liquid-gas multiphase and multiphysical characteristics.
Dejiang Li et al.[42]investigated the formation of porosity in die cast AZ91D and EA42 alloys. The volumetric porosity at the near-gate location was higher than that far from the gate location, and the latent heat released by a large amount of the second phase could inhibit porosity formation in the defect band. Shoumei Xiong et al. [43] discussed the effects of runner design and pressurization on the porosity defect of the die cast Mg-3.0Nd-0.3Zn-0.6Zr alloy, and they found that net-shrinkage porosity was transformed into isolated islandshrinkage porosity when ESCs were reduced, and the casting pressurization could greatly reduce porosity morphology, volume, and size.
For the hot tearing defect, Xiaoqin Zeng et al. [44] proposed a new criterion based on solidification microstructures.This criterion focused on the events occurring at the grain boundary, and it was validated according to the hot tearing behavior of Mg-Ce alloys. Hiroshi Noguchi et al. [45,46]investigated the notch sensibility of a non-combustible cast AZX912 (X = Ca) alloy, and they found that the stable crack propagation always occurred in a fracture process from notches smaller than 1 mm. Yi Meng et al. [47] studied the effects of Al content on the solidification behavior and analyzed the change of the hot tearing tendency with Al content.
However, cast defects are inevitable even in precision casting processes with upgraded casting parameters and modified cooling systems. To ensure the quality and usability of as-cast components, economic and maneuverable repair techniques are required. Yuling Xu et al. [48] studied the effects of welding wire composition on the microstructures and mechanical properties of Mg-Gd-Y repair welds, and they found that the Zr added alloys had better thermal stability because Zr promoted the grain refinement in the fusion zone of the repair welds. Guohua Wu et al. [49] developed an economical repair welding technique for Mg-Y-RE-Zr castings based on tungsten inert gas welding, and defect-free repaired joints with good appearance were obtained with welding currents at 170 A and 190 A. Nevertheless, there is a lot of work to be done to reduce and eliminate the defects in cast magnesium alloys.
In a word, the research of casting magnesium alloys reported in 2022 has made some progress. In particular, substantial research has been made in developing alloy components, enhancing particle strengthening, and controlling alloy defects. However, the increase in strength in casting magnesium alloy is often accompanied by a decrease in plasticity,which seriously hinders the practical applications. Therefore,how to synergize the strength and plasticity is an essential direction of future research on cast magnesium alloys.
3.2. Wrought Mg alloys
3.2.1. Traditional commercial wrought Mg alloys
In 2022, many papers focused on controlling the microstructure through deformation processes to improve themechanical properties of commercial wrought magnesium alloys [50–52]. The mechanical properties of traditional commercial wrought Mg alloys are summarized in Table 6.

Table 6 Mechanical properties of traditional commercial wrought Mg alloys at room temperature reported in 2022.
Ruizhi Peng et al. [53] prepared AZ31B alloy by high differential temperature rolling (HDTR). A large shear strain(>0.4) in the HDTR sample led to the activation of multiple twinning systems, especially the formation of contraction twins (CTWs) and double twins (DTWs). After annealing, the HDTR-annealing sample achieved excellent ductility(∼33.6%). Bin Jiang’s group proposed two types of novel extension approaches. One adopted slope extrusion (SE) to prepare AZ31 alloy sheets. Compared to the conventional extrusion, SE brings more asymmetric deformation and stronger accumulated strain along the ND,which benefits the enhancement of strength and ductility. The SE sheets exhibited a higher yield strength (219 MPa) and ultimate tensile strength(410 MPa) than the counterparts of the conventional extrusion sheet[56].The other new process is to use transverse gradient extrusion (TGE) to fabricate (AZ31) alloy sheets, thereby tailoring the strong basal texture of conventional extruded (CE)sheets. The TGE sheet exhibited a large Erichsen value (IE)of 6.7 mm at room temperature, which was approximately 258% of the CE sheet [63].
In terms of texture design, Lingyu Zhao et al. [64] revealed that the yield asymmetry in Mg-3Al-1 Zn alloy is directly related to bimodal texture components and clarified the relationship between pre-deformation parameters and texture component distribution by quantitative research. R. Roumina et al. [65] investigated the effect of texture on the flow stress during hot deformation of the RE-containing Mg alloys. The results show that lower flow curves and peak stresses observed for the AZ31–1RE alloy at higher temperatures were associated with lower Taylor factor values, which confirms texture softening by the addition of RE elements. Hang Li et al.[66] developed a plasticity model of single crystal and polycrystals, which can effectively describe the elasto-viscoplastic deformation and texture evolution and reasonably predict the multiaxial ratcheting of AZ31 Mg alloy. Wenke Wang et al.[67] investigated the asymmetry evolution in the microstructure and the plastic deformation behavior between the tension and compression for AZ31 magnesium alloy by combining the experiment and the visco-plastic self-consistent (VPSC)model. The VPSC model clarified the texture evolution asymmetry between the tension and compression by the contribution of each deformation mechanism to the macroscopic strain.
3.2.2. High-strength wrought Mg alloys
Some high-performance wrought Mg alloys were developed in the past year. Table 7 summarizes the mechanical properties of the high-strength wrought Mg alloy developed in 2022.
R.G. Li et al. [68] prepared an Mg-15Gd alloy plate with a yield strength of 504 MPa, ultimate tensile strength of 518 MPa, and elongation of 4.5% by the conventional extrusion, warm-rolling and aging. The high strength is mainly attributed to the nano substructure with boundary segregation of Gd atoms, the high-density nano-clusters induced by dislocations in the interior of grains, the high-density dynamical precipitates with submicron size, and the strong basal texture. Adil Mansoor et al. [69] studied the effects of rare-earth elements (RE = Gd and Er) contents on the microstructural evolution and mechanical performance of Mg-Gd-Er-Zr alloys. After peak-aging treatment, the double-pass extruded Mg-14Gd-2Er-0.4Zr alloy exhibited a high yield strength of 481 MPa ± 3.7 MPa and an adequate elongation of 3.2% ± 0.6%. Hucheng Pan et al. [71] fabricated a low-RE-alloyed Mg-4Sm-0.6Zn-0.4Zr alloy by a simple lowtemperature and low-speed extrusion process. The alloy exhibits extraordinarily high strength and acceptable ductility: a TYS of 458 MPa and an elongation of 4.8%. The ultrahigh yield strength of the alloy is closely related to the combined effect of fine recrystallized grains, highly textural hot-worked grains, and numerous nanoscale particles.
Weili Cheng et al. [70] developed a novel extruded Mg-5Bi-5Sn-1Mn alloy exhibiting high yield strength (YS) of 446 MPa and ductility of 13.2%, resulting from the bimodal microstructure composed of coarse unDRXed grains with strong extrusion texture and dislocation storage capacity as well as ultra-fine DRXed grains (0.48 μm). Kaibo Nie et al. [74] developed the as-extruded Mg-2.0Al-2.8Ca-0.6Mn alloy with tensile yield strength and ultimate tensile strength of ∼402 and ∼426 MPa, respectively. The dynamic precipitation of nanoprecipitates effectively prevents the movement of dislocations during plastic deformation, thus significantly improving the strength. Abdul Malik et al. [84] fab-ricated an extruded low alloyed Mg-0.5Zn-0.5Y-0.15Si alloy with the TYS, UTS and EL% being 248 MPa ± 1.1 MPa,348 MPa ± 2.3 MPa and 19% ± 0.12%, respectively. The high UTS, EL% and high strain hardenability is the synergistic effect of the interaction of basal and profuse

Table 7 Mechanical properties of high strength wrought Mg alloys at room temperature in 2022.
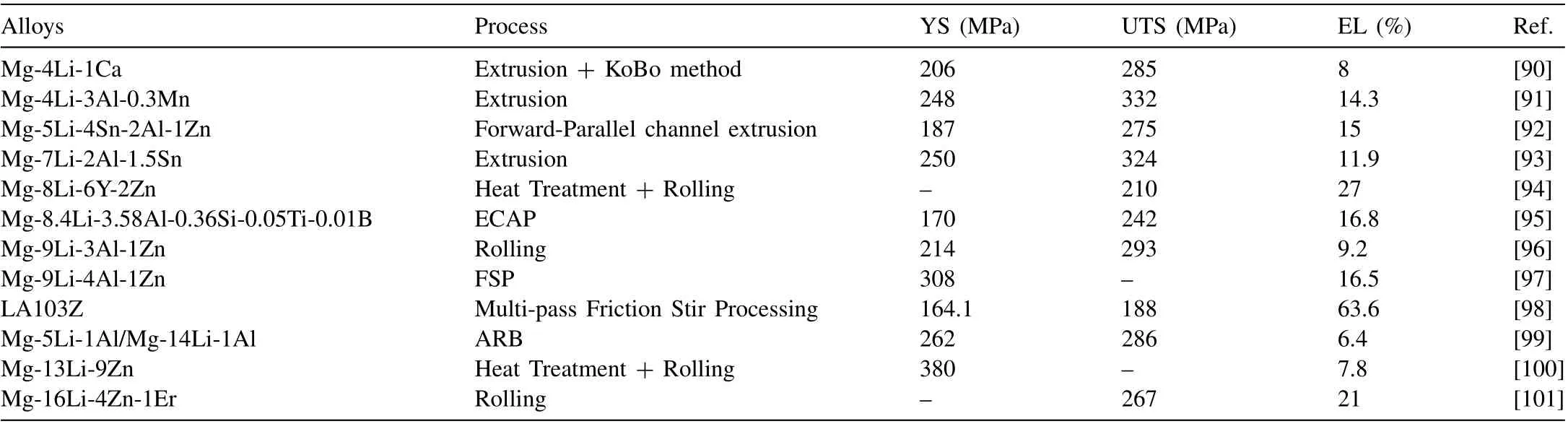
Table 8 Mechanical properties of the superlight wrought Mg-Li alloys developed in 2022.
3.2.3. Superlight wrought Mg alloys
The use of lightweight materials in the transportation field is one of the most practical methods to reduce the energy consumption of vehicles [89]. Superlight wrought alloys are mainly Mg-Li alloys with a density of 1.4 g/cm3–1.65 g/cm3,and the relevant mechanical properties of wrought Mg-Li alloys developed in 2022 are summarized in Table 8.

Fig. 9. (a) BF-STEM image showing dense nano-precipitates and (b, c) atomic-scale HAADF-STEM image showing the structure of nano-precipitates in the β-phase matrix, as indicated by red arrows. d–g HAADF-STEM images and corresponding EDS-STEM Al mapping of FSP sample after natural aging for (d,e) 3 months and (f, g) 2 years. Yellow and red frames in the inset FFT pattern in (a) indicated the diffractions from the α and β phase, and arrows in inset FFT in (a, c) and diffraction pattern in (f) indicated the diffraction from the θ phase. The incident beam was parallel to [001]β. [97].
I. Bednarczyk [90] prepared Mg-4Li-1Ca alloys by extrusion and KoBo method. Compared with the conventional extrusion, a banded structure was formed and the microhardness increased from 56 HV0.2 to 76 HV0.2 via the KoBo method, due to the given plastic deformation and the impact of defects. G. Zhou et al. [93] reported that a high strength dual-phase Mg-7Li-2Al-1.5Sn alloy was prepared by hot extrusion. A large amount of nano-precipitates were introduced into as-extruded alloy and massive residual dislocations were retained, which is beneficial for the improvement of the mechanical property. Moreover, Sn-rich precipitates (Mg2Sn or Li2MgSn) with good thermal stability can effectively prevent grain growth, which is suitable for the improvement of the high-temperature performance of the alloy.The as-extruded alloy shows excellent mechanical properties with a YS of 250 MPa, a UTS of 324 MPa and an EL of 11.9%. Zhuoran Zeng et al. [97] improved the corrosion resistance and mechanical properties of a dual-phase Mg-Li-Al-Zn alloy by FSP. The coarse AlLi phase was suppressed by FSP followed via liquid CO2quenching, which is beneficial to decrease the degradation rate of LAZ941 alloy. Fig. 9 shows the microstructure of nano-precipitates in the as-FSP samples, demonstrating the suppression effect on the AlLi phase. The alloy has a low electrochemical degradation rate of 0.72 mg·cm-2·day-1, and a high specific strength of 209 kN·m·kg-1, with minor softening from natural aging within 2 years. Kai Hu et al. [98] studied the effect of multi-pass friction stir processing on LA103Z alloy plate. They found that multi-pass friction stir processing effectively refined the microstructure of LA103Z alloy and weakened the rolled texture, enhancing the mechanical properties. Huajie Wu et al.[99]adopted accumulative roll bonding(ARB)to prepare Mg-Li alloy composite sheets. They indicated that the grain size of both Mg-5Li-1Al (α-Mg) and Mg-14Li-1Al (β-Li) alloys gradually decreases with the increase of ARB passes and the initial thickness ratio ofα-Mg/β-Li. Whenα-Mg/β-Li is 1:1, the composite sheet possesses good mechanical properties with a YS of 262 MPa and UTS of 286 MPa after 5 passes of ARB. Ruizhi Wu’s group [100] reported that a high-strength Mg-13Li-9 Zn alloy was prepared by quenching, annealing, and rolling. The rolling process introduced ordered B2 nanoparticles into the alloy and activated the transformation ofα-Mg grains. Qing Ji et al. [101] achieved a high strength Mg-16Li-4Zn-1Er alloy by cryogenic rolling.The UTS of 267 MPa and EL of 21% is mainly because of the delay of the DRV and DRX stages and massive residual dislocations caused by cryogenic rolling. Shun Zhang et al.[102] reported that the homogenization treatment causes the phase transformation of as-cast Mg-14Li-0.5Ni alloy, which benefits the synergistic enhancement of strength and plasticity. C. Ravikanth Reddy et al. [103] found the addition of Li to refine the microstructure of Mg-0.5Ni-2Gd alloys and prepared a high yield strength Mg-0.5Ni-2Gd-5Li (at.%) alloy.
In general, the research of Mg-Li alloys is increasingly concerned by researchers, but over-aging and corrosion resistance of Mg-Li alloys are still a considerable challenge.Moreover, the low content of Li tends to show higher strength and lower plasticity in Mg-Li alloys, while the addition of high lithium content is the opposite. Therefore, finding an effective way to balance the relationship between the strength and plasticity of Mg-Li alloys is necessary.
3.2.4. High-plasticity wrought Mg alloys
Magnesium and magnesium alloys have poor intrinsic plasticity because of their hexagonal close-packed (HCP) crystal structure, which needs to be improved to maintain good plasticity due to the limited independent slip systems at room temperature.Recently,many reports have involved alloying[104–106] and deformation [107–109] to acquire high-plasticity magnesium alloys. Table 9 summarizes the mechanical properties of typical high-plasticity magnesium alloys developed in 2022.
On the one hand, multi-element synergistic strengthening is an effective way to enhance the performance of magnesium alloy. Qichi Le’s group [104] fabricated multi-element synergistic strengthened high-performance Mg alloys (AXMZT-5,Mg-1.4Sn-0.93Zn-0.83Ca-0.67Mn-0.39Al) by extrusion. The bimodal microstructure along with a large number of refined recrystallized grains promotes the formation of a ductiledominated fracture mode in the alloy, which is beneficial to the improvement of plasticity. Massoud Emamy et al.[111] investigated the effect of Al addition on Mg-5Ni-xAl(x=2.5,5,and 7.5 wt.%)alloys.The Mg-5Ni-7.5Al alloy exhibits a high UTS of 355 MPa with an excellent EL of 23.9%.The dissolution of unfavorable intergranular Mg17Al12intermetallic phase and solid solution hardening during homogenization effectively improve the mechanical properties of the alloy. Mg-1Zn-0.4Gd-0.2Ca-0.5Zr developed by Jianfeng Nie et al. [112] exhibited a good UTS of 224 MPa with an excellent EL of 38%. Junxiu Chen et al. [119] studied the effects of rare-earth elements on ECAPed Mg-2Zn-0.5Y-0.5Zr alloy.Y and Nd are beneficial to the enhancement of strength and ductility of the alloy due to the dispersive second phase, grain refinement and activation of the non-basal slips. Jihua Chen’s group [120] studied the high plasticity mechanism of Mg-Ga alloy sheets prepared by high strain rate rolling(HSRR).They indicated that the Ga addition promoted the activation of the non-basal slip, which is beneficial to the work-hardening of the alloy to achieve better plasticity. The schematic diagram of microstructure evolution during HSRR is shown in Fig. 10.Peng et al. [121] developed a high-ductility Mg-0.5Mn-0.1Al alloy with an EL of 56.3%, which is caused by the dislocation annihilation at the grain boundary and the occurrence of DRX during the deformation process at room temperature.The low-cost and low-alloyed Mg-1.2Zn-0.1Ca alloy prepared by Datong Zhang’s group [122] exhibited a good elongation of 35.2% and a TYS of 159 MPa. Wenxue Fan et al.[123] prepared Mg-2Zn-0.2Mn-0.5Ca-0.5Sm alloy with high formability and good mechanical properties by Ca and Sm alloying. The synergistic addition of Sm and Ca adjusts the distribution of Ca elements in the alloy,while introducing a large number of fine Ca-containing particles into the alloy, which is helpful to the improvement in the mechanical property.

Table 9 The mechanical properties of typical high-plasticity wrought Mg alloys at room temperature in 2022.

Fig. 10. The schematic diagram of microstructure evolution of Mg-Ca alloy during high strain rate rolling [120].
On the other hand, the deformation method is a crucial aspect to enhance the plasticity of the alloy. Zhenzhen Gui et al. [110] investigated the precipitation behavior and mechanical properties of Mg-2.9Gd-1.5Nd-0.3Zn-0.3Zr alloy after solution-treatment (ST) and ECAP. They observed spherical precipitates(∼200 nm)and fine phases(∼100 nm)precipitated along the stripe-like Zn2Zr3phase and new dislocations existing in refined grains after solution-treatment and ECAP,which contributes to the improvement in the mechanical property. The YS, UTS, and elongation of the ST-ECAPed alloy reached ∼211 MPa, ∼264 MPa, and 27.9%, respectively. M.Kavyani et al. [107] reported that half equal channel angular pressing (HECAP) improves the mechanical and biocorrosion properties of Mg-Zn-Ca-Mn alloy and Mg-3.95Zn-0.5Ca-0.75Mn alloy. Compared to the homogenized alloy, the microstructure is significantly refined after two passes of the HECAP process from 345 μm to 2 μm. Most grains were reoriented parallel to (0001) basal plane after HECAP process. The HECAPed Mg-Zn-Ca-Mn alloy has great mechanical properties with a UTS of 342 MPa and an EL of 23.2%.Hao Lv et al. [124] investigated the effects of extrusion ratio and temperature on Mg-Zn-Yb-Zr alloys. Prismatic and basal slips dominate the deformation of the extruded alloy at 320 °C, which is beneficial for the formation of intensified basal texture. Moreover, the low extrusion temperature and high extrusion ratio can achieve a good strength-ductility synergy with a TYS of 259 MPa, a UTS of 370 MPa and an EL of 25.9%. Zihong Wang et al. [125] used wire and arc additive manufacturing (WAAM) to fabricate AZ31 alloy with an equiaxed-grain-dominated microstructure, achieving a synergistic improvement in the damping and mechanical properties of the alloy, and the elongation of the alloy at room temperature reached 28.6%.
In the case of commercial wrought Mg alloys, while the high content of rare-earth elements is related to the high strength, low-alloyed Mg alloys were also developed to achieve good strength-ductility synergy. In the case of highstrength wrought Mg alloys, the ultimate tensile strength and elongation of Mg-15Gd alloy via extrusion, warm-rolling and aging have exceeded 518 MPa and 4.5%, respectively. In the superlight wrought Mg alloys, the development of superlight wrought Mg-Li alloys is fruitful. Mg-7Li-2Al-1.5Sn alloys prepared by conventional extrusion exhibit an excellent tensile strength of 324 MPa and a relatively good plasticity of 11.9%.In the plasticity wrought Mg alloys,substantial reports mainly focused on low alloying to improve the plasticity due to the low cost and high efficiency.
4. Functional Mg materials
In addition to structural magnesium alloys’ high specific strength and stiffness, magnesium alloys exhibit good biocompatibility, large hydrogen storage capacity, high theoretical battery specific capacity, and damping properties. Therefore, magnesium and magnesium alloys have enormous application potential in energy and biomedical science. In the past year, a large number of papers have focused on the research of magnesium-based functional materials.
4.1. Bio-magnesium alloys
Biodegradable Mg alloys are expected to be promising candidates for implantation in the clinic due to their excellent biocompatibility and non-secondary surgery [126–129]. However, the clinical application of Mg alloys is limited by poor mechanical formability and a high degradation rate [130,131].Alloying design and surface modification are the key to improving the corrosion resistance of Mg alloys, but the in-vivo biological properties should be considered, including healing rate, inflammatory responses, and side effects [132,128].
The development of Mg alloys has attracted great attention from both the commercial sector and research community all over the world. When determining the alloying design, it is important to adjust the acceptable dosage range of alloying elements and consider the issues of material degradation products [133]. For different clinical applications, alloying elements (Zn, Ca, andSc, etc.) are contributed to the mechanical performance and biocompatibility of Mg alloys and currently represent the dominant alloying additions.Zn as an inflammatory agent can inhibit the expression of inflammation-related genes[134]and the degradation products of Mg-Zn alloys can significantly reduce the growth of gallbladder cancer cells and promote their apoptosis [135]. Therefore, Mg-Zn alloys are generally used in the preparation of cardiovascular stents and bile duct stents.The degradation rate of Mg-xZn(x≤4 wt.%)is 4.85 mm/y∼4.62 mm/y,while its application is very limited because of its low yield strength (<170 MPa) [136–138]. The corrosion resistance of Mg-Zn alloys increases with increasing Zn content up to 4 wt.%, but the mechanical performance cannot meet the requirements of implantation. Thus, Mg-Zn alloys are further alloyed by adding other elements, including Ca, Gd, and Y. The as-extruded Mg-2Zn-1Gd alloy exhibited a low in-vivo degradation rate (0.31 mm/y), low cytotoxicity (grade 0–1) to MC3T3-E1 cells and excellent mechanical performance (YTS: 284 MPa, UTS: 338 MPa and EL: 24%)[139]. Mg-Ca alloy focuses on the bone repair, and adding elements Zn into Mg-Ca alloys can effectively improve cytocompatibility, osseointegration, and osteogenesis [140]. It is noted thatScexhibits no chronic effects in organs compared with all other rare-earth elements. In addition, the addition ofSccan improve effectively mechanical performance and corrosion rate. Mg-30Sc alloy exhibits an acceptable in-vivo corrosion rate (0.06 mm/y), no cytotoxicity on the MC3T3 cell model, and good mechanical integrity (maintain up to 24 weeks in the rat femur bone) [141].
Surface modification, including chemical conversion,micro-arc oxidation, and electrophoretic deposition, is used to control the degradation of Mg alloys. For example,MAO/poly(l-lactide)/paclitaxel have been generated on the surface of Mg-Zn-Y-Nd alloy stents to improve their corrosion resistance and biocompatibility in physiological environments[14]. Antibacterial Cu is also applied to the surface of Mg via a chemical conversion technique to improve corrosion resistance and biocompatibility.Adding 0.24 wt.%Cu content into PA coating can promote the proliferation, and differentiation of MC3T3-E1 cells as well as exhibit excellent anti-bacterial rates in 24 h (E. coli: 94.6%,S. aureus: 81.3%) [142].
In the aspect of bio-magnesium alloys, alloying design and surface modification is the key to improving the properties,the developed Mg-30Sc alloy exhibits excellent properties.The surface treatment as an effective method needs to be further developed to achieve a better control of degradation of Mg alloys.

Table 10 Average discharge voltage and anode efficiency of Mg alloys for Mg-air battery.
4.2. Mg batteries
4.2.1. Mg-air battery
As one of the most important directions of future energy development, metal air batteries are a safe and efficient way.The metal-air battery is a special kind of primary battery.It uses metal as a negative electrode for discharge reaction,oxygen in the air as a positive electrode for reaction, and water electrolyte solution (mainly NaCl solution). Current metal air batteries include aluminum air batteries, zinc air batteries,lithium air batteries and magnesium air batteries. Compared with other metal air batteries, magnesium air battery has the advantages of low cost, good safety, high energy density and high theoretical discharge voltage. Magnesium air battery is one of the most promising energy storage materials [143]. As a type of energy storage material, magnesium is characterized by low density, high energy storage capacity, low price and abundant energy storage [144].
(1) Anode of Mg-air batteries
Due to the side effect of Mg anode self-corrosion reaction(Mg + 2H2O →Mg(OH)2+ H2), Mg metal anode in the magnesium air battery has low anode efficiency and low discharge voltage. In addition, the discharge product Mg(OH)2accumulates on the anode surface, causing the anode to polarize and destroying the anode properties. Thus magnesium air battery has low anode efficiency and high polarization [145].Anode efficiency, also known as Faraday efficiency, Coulomb efficiency or anodic corrosion efficiency, is defined as the ratio of energy conversion to energy consumption. The anode efficiency of a magnesium air battery is about 30%–60%compared with the nearly 100% anode efficiency of a lithium battery, depending on the discharge current density of the battery. The corrosion behavior of magnesium is also affected by hydrogen evolution reaction (HER), negative difference effect(NDE), block effect and impurity. More hydrogen is released from the Mg electrode during discharge, which does not occur in conventional metals. Since the development of air batteries, especially magnesium air batteries, many studies have been carried out to understand the corrosion mechanism and discharge mechanism of Mg and Mg alloy.
The metal anode is the crucial factor affecting the discharge performance of magnesium air batteries. To solve the above problems, many investigations have been done on alloying, heat treatment and deformation of magnesium [146–149]. Among them, the alloying of magnesium is the most widely used method. Since the beginning of the last century,Mg-Zn alloys (typically ZC63), Mg-Li alloys, Mg-Al alloys(such as AZ31, AM60, etc.) and Mg-RE alloys have been used as anode of magnesium air batteries. At the same time,adding a proper amount of rare-earth elements to a magnesium alloy can obviously improve the discharge performance and anode efficiency of a magnesium air battery. Moreover,after heat treatment and deformation treatment, the properties of the anode can be further enhanced.
The addition of rare-earth elements can also improve the discharge performance of the magnesium alloy anode when magnesium alloy is used as an air battery. Bingjie Ma et al.[150] prepared four kinds of Mg-xLa containing La as an anode of magnesium air battery. The results showed that adding five wt.% La could improve the discharge performance and corrosion resistance of air batteries.Adding La can reduce the self-corrosion ofα-Mg phase and accelerate discharge product spalling. Jianchun Sha et al. [151] investigated the effects of microalloying on the microstructure, electrochemical behavior and discharge properties of as-rolled Mg-4Li alloys.The addition of Al and Y elements results in the regulation of grain size and a second phase, and a significant increase in corrosion resistance. Table 10 summarizes the discharge properties of Mg alloys.
(2) Electrolyte for Mg-air batteries
Over the past decades, electrochemical reaction mechanisms of Mg in aqueous electrolyte have been extensively studied. The addition of corrosion inhibitors such as stannates, quaternary ammonium salts, dithiobiuret and their mixtures has been demonstrated as an effective method to suppress the HER, improving the performance of Mg-air batteries [157,158]. Linqian Wang et al. [159] have added Mg2+complexing agents, such as citric acid (CIT), salicylic acid(SAL),2,6-dihydroxybenzoic acid(2,6-DHB),5-sulfosalicylic acid (5-sulfoSAL) and 3,4-dihydroxybenzoic acid (3,4-DHB),into 3.5 wt.% NaCl electrolyte to enhance the discharge performance of (Mg-Ca)-air battery. They have found that these additives can efficiently increase the discharge voltage and specific energy of respective Mg-air batteries. Min Liu et al.[160]reported NaBF4-dimethyl sulfoxide(DMSO)/NaCl-H2O biphasic electrolytes to alleviate the anode HER corrosion by allowing an anodic reaction to take place in organic solution DMSO while ensuring high cathode activity with applying cathode reaction to occur in NaCl-H2O solution. The discharge results show that the utilization rate of AZ61 alloy in NaBF4-DMSO/NaCl-H2O biphasic electrolytes is increased to 67% at 1 mA·cm-2, which is 17% higher than that in NaCl-H2O single electrolyte, and the anode energy density is up to 2020 Wh·kg-1.
In summary, NaCl solution as a neutral electrolyte has the lowest discharge potential with pure Mg, and Cl-has lower Faradaic efficiency than NO3-, NO2-and HPO4-. Therefore,NaCl solution has been widely used as the aqueous electrolyte in Mg-air batteries for scientific research and commercial applications. Developing suitable inhibitors for aqueous electrolytes could significantly enhance the discharge performance of Mg-air batteries. The introduction of an organic phase with an aqueous phase as a double liquid electrolyte could also significantly improve the anodic efficiency. However, further studies on the ionic conductivity and diffusion coefficient of Mg in the organic phase are needed to improve the discharge performance of Mg-air batteries [161].
(3) Cathode of Mg-air batteries
The discharge performance of Mg-air battery also relies on an air cathode, which consists of a waterproof breathable layer, a gas diffusion layer, a catalyst layer, and a current collector [162]. The waterproof breathable layer is used to separate electrolyte and air, such as paraffin or Teflon. The gas diffusion layer comprises carbon material and hydrophobic binder (e.g., polytetrafluoroethylene (PTFE)) to allow air penetration and seepage water [163]. The oxygen reduction reaction (ORR) takes place on the catalyst layer. The current collector is typically made of a Ni metal mesh with good electron conductivity.
Prussian blue analogues (PBA) were employed as precursors to prepare bimetallic M-N-C electrocatalysts. The primary Mg-air batteries assembled with the CuCo@N/C-800 as the cathode catalyst display better discharge performances than that of Co@N/C-800 [164].
The common catalysts of air cathode are noble metals,carbon materials, and transitional metal oxides. Noble metal catalysts (e.g., Pt, Pd, Au, and Ag) have high catalytic activity, low overpotential, and large limited current density, but high cost [165]. In the last decades, numerous investigations have been working on developing high-performance and lowcost non-noble metal catalysts for air cathodes. Recently several research groups made good progress on catalysts and air cathodes [166,167].
4.2.2. Rechargeable magnesium battery
In light of the escalating energy crisis and the scarcity of lithium resources, it is imperative to develop novel batteries for the next generation that can supplant lithium batteries. Among various alternatives, magnesium batteries exhibit a remarkable theoretical specific capacity (3833 mAh·cm-3and 2205 mAh·g-1) and abundant resource (23,399 ppm in the earth’s crust) [168,169]. Furthermore, due to the lower diffusion energy of magnesium ions on the magnesium surface compared with that of lithium ions on the lithium surface, magnesium batteries are less prone to dendrite formation, which enhances their safety [170,171]. These advantages render magnesium batteries as promising candidates for the next generation of secondary batteries. However, magnesium batteries still face challenges such as the incompatibility of electrolytes and the scarcity of the high-performance cathode and anode materials, which require further research.
(1) Anode of rechargeable Mg batteries
A common issue is that the magnesium anode reacts with the electrolyte and forms a passivation layer that impedes magnesium ion transport. This severely deteriorates the electrochemical performance of magnesium ion batteries[172].To address this issue, Ye Yeong Hwang et al. [173] grafted TFSI anions onto the backbone of poly (vinylidene fluoride co hexafluoropropylene) (PVDF-HFP) polymer, which substantially increased its amorphousness and enhanced the conductivity of Mg2+ions. They thus prepared an Mg2+ion-permeable polymer layer that protects the magnesium metal anode, as shown in Fig. 11. It achieved highly reversible magnesium deposition for 300 cycles in a symmetrical cell using a carbonate electrolyte that reacts severely with magnesium metal. Furthermore, Clément Pechberty et al. [174] coated the surface of the magnesium electrode with liquid gallium to form an alloy interface, which reduced the anode polarization, stabilized the plating/stripping process of the anode, and prolonged the battery cycle life.
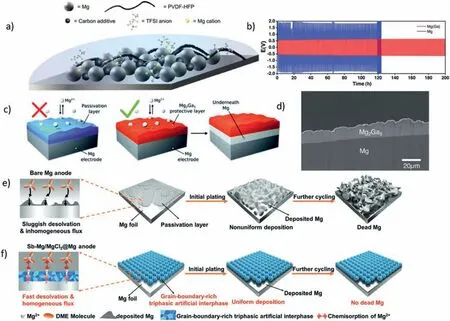
Fig. 11. (a) Schematic of Mg powder electrode coated with TFSI-anion-grafted PVDF-HFP [173]; (b) Evolution of the overpotential during subsequent magnesium plating/stripping processes in symmetrical cells with bare and Ga-protected magnesium electrodes (blue and red lines, respectively) in 0.8 mol·L-1 Mg(TFSI)2/DME electrolyte and at 40 °C, with sweeps of 30 min at a current density of 0.1 mA cm-2, (c) SEM cross-sectional image of a Ga-treated magnesium disk (d) schematic representation of plating underneath [174]; (e) Schematic of Uneven deposition on bare magnesium [175]; (f) Schematic of uniform Mg deposition on triphasic artificial hybrid interphase [175].
Uneven deposition of magnesium anode poses a serious challenge to the cycling stability and safety of magnesium batteries. It induces the formation of a porous “dead” magnesium layer that consumes electrolytes rapidly and shortens battery life as shown in Fig. 11(e). To address this issue,Yuanjian Li et al. [175] devised a grain-boundary-rich triphasic artificial hybrid interphase, as illustrated in Fig. 11(f). The interface consists of Sb metal, Mg3Sb2alloy, and MgCl2. It is formed on the surface of the magnesium anode by a simple solution treatment that enables more uniform deposition of magnesium ions. The treated anode exhibits a cycling life of up to 350 h at a current density of up to 5 mA·cm-2.The full cell composed of the anode and the Mo6cathode achieves a long lifespan of 8000 cycles at a high rate of 5C. Besides uneven deposition, G. Cui et al. [176] also observed uneven stripping in the magnesium anode.At moderate current densities (0.1 mA·cm-2–1 mA·cm-2), they witnessed abnormal self-accelerated pit growth on the magnesium stripping side. This phenomenon could result in magnesium anode failure or a battery short circuit. By in-situ spectroscopy,they revealed that this phenomenon was induced by chlorinecontaining complex ions near the interface. This finding has implications for the development of long-life magnesium anode. P. Li et al. [177] investigated the homogenization and stripping processes and discovered that the constant current cycling of thin magnesium metal and APC electrolyte was essentially asymmetric during stripping and plating. That is,Mg stripping occurred through the formation of round holes,but reverse plating did not fill these holes; instead, it deposited on the top surface. This resulted in shape instability and low coulombic efficiency (CE) and anode utilization rate(AUR). By using gas chromatography (GC), they determined that magnesium loss was due to the formation of a solidelectrolyte interphase (SEI) and “dead” magnesium. Moreover, they found that applying a -0.5 V overpotential beforehand could make the electrochemical behavior more symmetric and improve the AUR. This finding has great significance for magnesium anode research.
Besides using pure magnesium as an anode, researchers also attempted to use alloy anodes, which could avoid some problems caused by pure magnesium anodes. For instance, Dachong Gu et al. [178] explored and synthesized a Bi10Sb10Sn80anode. After alleviating the passivation-induced kinetic and cycle life issues, it still retained a high capacity of 417 mAh·g-1and 517 mAh·g-1at 500 mAh·g-1and 20 mA·g-1, respectively. The new challenges arising from using alloy anodes require further solutions.To address the poor wettability of liquid GaSn anodes at room temperature, Meijia Song et al. [179] constructed a bifunctional intermetallic compound (Ag3Ga) layer on the current collector. Furthermore, Ag3Ga can provide additional capacity. This enhanced the battery capacity. In the half-cell configuration, it cycled stably up to 600 times, and in the full-cell configuration, it cycled stably 100 times.Re-optimizing alloy anodes is an extension of magnesium battery anode development.

Fig. 12. Reactions of (a) intercalation materials (b) The rate performance of VSO, VSO-1, MVSO and MVSO-1 from 50, 100, 200, 500, 1000 mA g-1 and back to 50 mA g-1 (c) The cycling performance of MVSO under the current density of 1000 mA g-1 [163] (d) conversion materials (e) Electrochemistry performance of the developed Mg||Te batteries: GCD curves from 1 to 100 cycles at 0.1 A g-1; (f) the rate performance at different current densities [164] (g)organic materials. (h) charge/discharge profiles at 50 mA g-1 of N26 (i) charge/discharge profiles at 50 mA g-1 of P26 [166].
To advance the commercialization of magnesium-ion batteries, Toshihiko Mandai et al. [180] fabricated ultrathin magnesium anodes. They fabricated ultrathin magnesium foil with a thickness less than 45 μm by controlling the initial microstructure of the extruded magnesium and then hot-rolling it. The laminate-type cell prepared with a MnO2cathode had an initial discharge capacity of 220 mAh·g-1. Rolling the magnesium anode to be thinner could greatly improve the energy density of magnesium batteries, which is important for their commercialization.
(2) Cathode of rechargeable Mg batteries
The cathode will severely limit the development of magnesium batteries. Based on the reaction mechanism with Mg2+ions,the cathode materials can be classified into three groups:(a)intercalation-type,(b)conversion-type and(c)organic-type(Fig. 12.).
For the intercalated electrode, Shiqi Ding et al. [181] modified the VS4cathode with Mo and O co-doping (MVSO),which enhanced the conductivity, produced abundant sulfur and oxygen vacancies, induced the coexistence of V3+/V4+,and shaped it into hollow flowers. It had a high specific capacity (140.5 mAh·g-1at 50 mA·g-1), ultra-long cycle life(1000 cycles), high capacity retention rate (95.6%), excellent rate performance (when the current density increased to 1000 mA·g-1, capacity reached 75.2 mAh·g-1), and low selfdischarge ratio (Fig. 12b, c).
For conversion-type cathodes, Z. Chen et al. [182] utilized the high-conductivity and increased surface conversion sites of Te encapsulated in carbon spheres (CSs) to prepare a conversion-type Mg||Te battery. It has a discharge specific capacity of up to 387 mAh·g-1and a rated capacity of 165 mAh·g-1at 5 A·g-1(Fig. 12e, f). Changliang Du et al.[183]used selenium ion substitution strategy and crystal engineering to adjust the electrochemical reaction kinetics and enhance the magnesium storage performance of a CuS nanotube cathode. It exhibited excellent magnesium storage capacity(372.9 mAh·g-1at 100 mA·g-1), remarkable cycle stability(1600 cycles at 2.0 A·g-1) and good rate capability (112.4 mAh·g-1at 2.0 A·g-2).
For organic electrodes, Yiyuan Ding et al. [184] prepared three kinds of organic polyanthraquinonimide (PAQI),namely N14,N26 and P26,and compared them as RMB cathodes. N26 (122 mAh·g-1) and P26 (164 mAh·g-1) showed higher capacity and better cycle stability than N14 (111 mAh g-1) (after 500 cycles at 500 mA·g-1, N26 maintained 115 mAh·g-1and P26 maintained 111 mAh·g-1) (Fig. 12h, i). In addition, there are many other studies on cathode materials.We will list some of them in Table 11.
(3) Electrolyte for rechargeable Mg batteries
In the development of magnesium-ion batteries, the nature of the electrolyte will greatly affect the battery performance,so that it is essential to develop electrolytes. MingxiangCheng et al. [190] synthesized an amino-magnesium halide TMPLA electrolyte by using a one-step reaction of LiCl congenital-containing Knochel-Hauser base TMPL (2,2,6,6-tetramethylpiperidyl magnesium chloride lithium chloride complex) and AlCl3Lewis acid. It had excellent anode stability (>2.65 V vs. SS), high ionic conductivity (6.05 mS cm-1), low overpotential (<0.1 V) and excellent coulombic efficiency (97.3%). When it was used as the electrolyte of a CuS || Mg cell, it displayed a very high discharge capacity of 458.8 mAh·g-1in the first cycle and stabilized at 170.2 mAh·g-1after 50 cycles at 0.05C with high coulombic efficiency (99.1%). The synthesis method of this electrolyte was simple and low-cost, which was significant for the development of magnesium battery electrolytes.
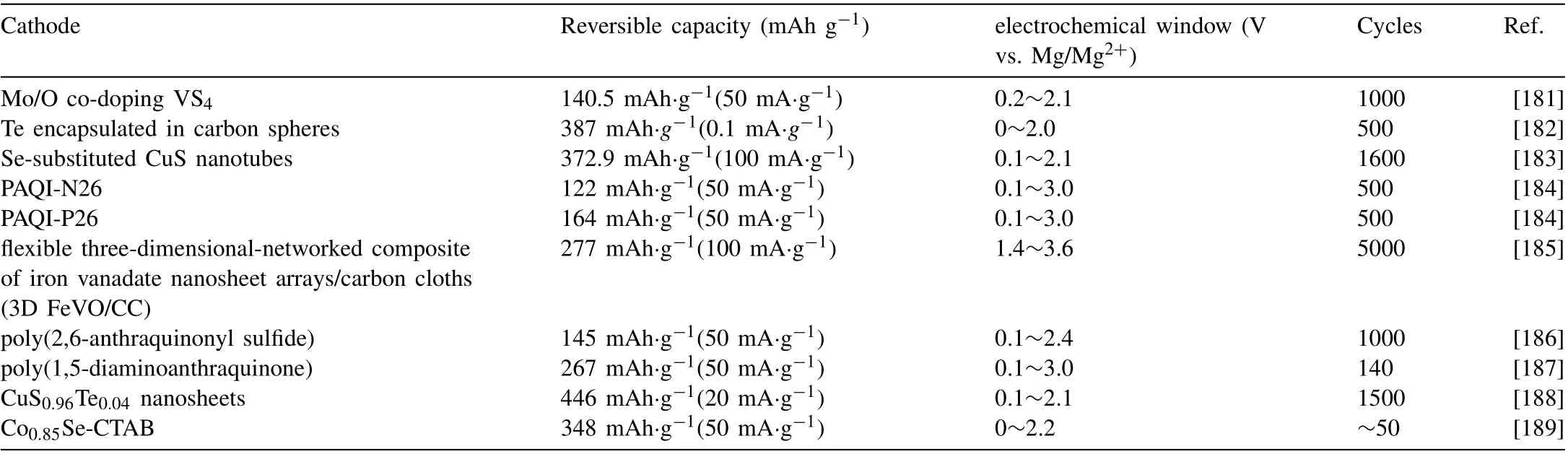
Table 11 Summary of the electrochemical performance of cathode materials for Mg-ion batteries.
Boron-based electrolytes have attracted much attention due to their high coulombic efficiency, low polarization and remarkable anode stability. However, the application of boronbased electrolytes is limited by their complex preparation process, high cost and sensitivity to impurities and water.To solve this problem, X. Huang et al. [191] prepared a novel boron-centered non-nucleophilic electrolyte (BMCM)by using the in-situ reaction of B(TFE)3, MgCl2, CrCl3and Mg powder in DME. This electrolyte has low overpotential(∼139 mV), high coulombic efficiency (∼97%), high anode stability (∼3.5 V vs Mg/Mg2+) and long-term cycling stability (more than 500 h) (Fig. 13a, b). In addition, the CuS |BMCM|Mg full cell prepared with this electrolyte has a specific discharge capacity of 231 mAh·g-1at a current density of 56 mA·g-1, which can retain ∼88% even after 100 cycles.Most importantly, this electrolyte can tolerate trace water and impurities, which is beneficial for developing practical magnesium battery electrolytes.
In studying magnesium battery electrolytes, new electrolytes need to be continuously developed. Vadthya Raju et al. [192] designed a deep eutectic solvent (DES) synthesized by 1-ethyl-3-methylimidazolium chloride (EMIC) and Mg(ClO4)2or Mg(CF3SO3)2. EMIC-Mg(ClO4)2and EMICMg(CF3SO3)2have relatively high conductivities, which are 2.8 and 2.4 mS·cm-1at 25 °C. The anode stability measured with graphite as the working electrode is∼3.0 V vs Mg/Mg2+, and the capacity of the assembled graphite|DES|Mg battery is ∼40 mAh·g-1in 50 cycles,which shows significant stability. Their research has a pioneering role in eutectic electrolytes for magnesium batteries.
The use of additives for electrolytes is also a way to improve the performance of electrolytes. Ahiud Morag et al. [193] adjusted the MgxCly2x-ycluster in the electrolyte of magnesium batteries (Mg bis(hexamethyldisilazide)(Mg(HMDS)2)/MgCl2dissolved in tetrahydrofuran (THF))by adding 1–butyl–1-methylpiperidinium bis(trifluorome thylsulfonyl)imide (PP14TFSI) ionic liquid additive. In their study, they observed that PP14TFSI can decompose large MgxCly2x-yclusters into small Mg species (such as MgCl+and Mg2+), where 1–butyl–1-methylpiperidinium (PP14+)and bis(trifluoromethylsulfonyl)imide (TFSI-) can stabilize the generated MgCl+and accelerate the decomposition of Mg-Cl, which can enable the TiS2cathode to have a twoplateau insertion/extraction behavior. This is in contrast to the non-insertion property of TiS2cathode in the electrolyte without PP14TFSI. Finally, the battery prepared with PP14TFSI added electrolyte and TiS2cathode has good specific capacity (81 mAh·g-1at 10 mA·g-1), high-rate capability (63 mAh·g-1at 200 mA·g-1), and excellent cycling stability(86.3% capacity retention after 500 cycles).
The corrosion of chlorine-containing electrolytes to battery components cannot be ignored. However, the use of non-chlorine electrolyte is easy to cause negative electrode passivation. To prevent the commercially accessible chlorine-free Mg(TFSI)2/DME electrolyte [magnesium bis(trifluoromethanesulfonyl)imide dissolved in 1,2-dimethoxyethane (DME) passivation, W. Zhao et al.[194] modified the solvation coordination by adding an additive solvent with high electron abundance. They found that the oxygen atom in the phosphoroxy group of DME competed with the that in the carboxy group for Mg2+coordination, resulting in a softer solvation sheath deformation. Moreover, they observed that the organic phosphorus molecules in the rearranged solvated sheath decomposed on the Mg surface, enhancing Mg2+transport and reducing resistance by three and one order of magnitude, respectively. Using this additive, they fabricated a symmetrical battery that exhibited over 600 cycles with low polarization. Based on the nature of the coordination bond, they hypothesize that the phosphorus-oxygen bond with extreme electron richness can compete with carbon-oxygen groups of DME for the coordination with Mg2+. To test this hypothesis, they select a series of organophosphorus molecules with different degrees of steric effect and electron enrichment and evaluate their capability for solvent sheath rearrangement. They focus on trimethyl phosphate (TMP) as the representative organophosphorus molecule, and find that it can replace one of the three bound DME molecules to coordinate with Mg2+, which facilitates the decomposition of bound TMP into ionic conductive interphase on the Mg surface (Fig. 13c, d, e). The use of noncorrosive electrolytes can improve the lifespan and safety of magnesium batteries, which is a very promising development direction in the context of environmental protection.
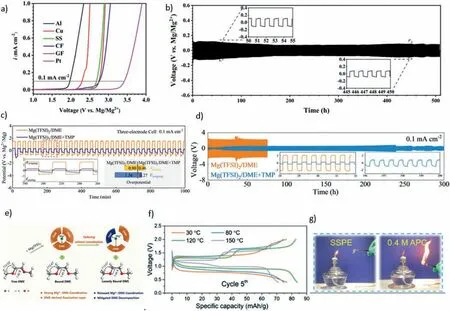
Fig. 13. (a) LSV curves of different working electrodes (Al, Cu, SS, CF, GF, and Pt) in the as-synthesized BMCM electrolyte (scan rate: 1 mA·s-1) [191];(b) Galvanostatic voltage profiles of a Mg|BMCM|Mg symmetric cell cycled at a current density of 0.1 mA·cm-2 for 500 h [191]; (c) The voltage profiles of three-electrode system with Mg foil, Mg belt, and Mg foil serve as the working, reference and counter electrodes, respectively. Inset: the enlarged profiles and the comparison of overpotentials for the plating and stripping processes in Mg(TFSI)2/DME and Mg(TFSI)2/DME+TMP electrolytes; (d) rate performance of Mg2+ plating/stripping with a fixed capacity of 0.125 mAh·cm-2 in Mg(TFSI)2/DME and Mg(TFSI)2/DME+TMP electrolytes; (e) Scheme of the tailoring solvation coordination and the corresponding characteristics of the free DME, bound DME and loosely bound DME; (f) The voltage profiles of the fifth cycle for the Mo6S8//SSPE//Mg batteries at different temperatures [195]; (g) Flammability test results of PECH-OMgCl@G3 SSPE and conventional 0.4 m APC electrolyte [195].
In order to expand the application of magnesium batteries,it is also important to develop electrolytes that can work at elevated temperatures.Xuesong Ge et al.[195]proposed a solid self-supporting single-ion polymer electrolyte (SSPE), which is a polyepichlorohydrin-based self-standing polymer electrolyte plasticized by triglyme (PECH–OMgCl@G3 SSPE).This electrolyte has a wide electrochemical stability window(∼4.8 V vs Mg2+/Mg), high Mg2+ion transference number(∼0.79), and highly reversible magnesium plating/stripping performance. Most importantly, the Mo6S8/Mg battery assembled with this electrolyte has excellent high-temperature (up to 150 °C) performance (Fig. 13f). It has also excellent dimensional thermal stability and non-flammability characteristics, and high safety under abuse conditions (Fig. 13g). Its proposal has improved the usage scenario and safety of magnesium batteries and contributed to the development of functional electrolytes.
(4) Magnesium-sulfur (Mg-S) battery
Magnesium-sulfur (Mg-S) batteries have attracted wide attention due to their high theoretical capacity and low cost of active materials. However, they face major challenges such as low utilization of active materials and short battery life caused by sulfur dissolution and polysulfide shuttle effects in the liquid electrolyte during charge/discharge cycles [196].
To address these issues, some researchers have focused on improving electrolyte. Liping Wang et al. [197] synthesized a novel non-corrosive gel polymer electrolyte based on tetra(hexafluoroisopropoxy) Mg[B(hfip)4]2through in-situ polymerization. This electrolyte has high ionic conductivity(10-3S·cm-1), reversible magnesium plating/stripping ability(coulombic efficiency ∼99%, 1000 cycles) and low voltage polarization. It also prevents the dissolution and diffusion of soluble electrode materials. This is the first demonstration of using gel polymer electrolytes to inhibit polysulfide shuttle in Mg-S batteries.
Another strategy to mitigate this problem is to modify the separator. Y. Yang et al. [198] coated a dual-functional layer of Cu3P confined in a carbon matrix on a commercial polypropylene film to form a porous film with high electrolyte wettability and good thermal stability. This film can adsorb magnesium polysulfides and catalyze the conversion reaction of S and Mg2+, thus enabling the stable cycling of Mg-S batteries. The Mg-S battery prepared with this separator can achieve high specific capacity, fast rate capability(449 mAh-1at 0.1C, 249 mAh·g-1at 1.0 C), long cycle life(500 cycles at 0.5C), and even work at elevated temperatures.L. Wang et al. [199] modified the separator with a Mo6S8functional layer to reduce the polysulfide shuttle and enhance the polysulfide conversion, thus significantly improving the Mg-S battery performance in terms of reversible discharge capacity and cycle life (≈200 cycles). The Mg-S battery assembled with this separator has a high specific energy density(942.9 mAh·g-1in the first cycle), can cycle 200 times at 0.2 C, and has a coulombic efficiency of 96%.
Besides the shuttle effect, the passivation of the magnesium anode will also affect the Mg-S battery. Ruinan Li et al.[200] used Mg-Li alloy to address the passivation problem caused by a pure magnesium anode. The Mg-Li/S battery showed an enhanced discharge voltage plateau of 1.5 V and an energy density of 1829 Wh·kg-1, which was superior to the Mg-S battery samples(0.3 V,287 Wh·kg-1)of the control group and the reported Mg-S batteries. Moreover, the surface film impedance of the Mg-Li/S battery is five orders of magnitude lower than that of the Mg/S battery.
4.3. Magnesium-based hydrogen storage
Magnesium-based hydrogen storage materials have become solid hydrogen storage materials of great concern in the 21st century due to their rich resources, high hydrogen storage capacity (7.6 wt.%), and its function of purifying hydrogen.However, their large-scale use is hindered because of the high thermodynamic stability and the slow reactions kinetics of magnesium-based hydrogen storage materials. To overcome these shortcomings, researchers continue to study from the aspects of alloying, nanostructuring, adding catalysts, changing preparation methods, etc. [201–205].
Reducing the hydrogen release temperature and enthalpy of Mg-based hydrogen storage materials to improve the thermodynamic properties are two key issues in the practical applications of Mg-based hydrogen storage materials.Xiong Lu et al. [206] prepared different Mg2Ni samples and studied their hydrogen storage thermodynamics properties. Carbon-coated nanocrystalline Mg2Ni began to absorb hydrogen at room temperature and began to release hydrogen at 180 °C. The enthalpy of hydrogen evolution of carbon-coated nanocrystalline Mg2NiH4samples also decreased from 89.9 kJ/mol ± 4.0 kJ/mol of MgH2to 67.0 kJ/mol ± 0.5 kJ/mol. The remarkable improvement of hydrogen storage properties of Mg2NiH4is a result of the joint action of alloying, carbon coating and nanocrystalline.Rashmi Kesarwani et al. [207] studied the effect of MgF2and CsH double catalysts formed by the dehydrogenation reaction of MgH2and CsF on the thermodynamics of Mg-based hydrogen storage materials. Moreover, the catalyst reduces the decomposition temperature of MgH2to 249 °C, which is 106°C lower than that of pure ball milling MgH2under the same conditions. The decomposition enthalpy and formation enthalpy of MgH2using catalyst are 66.6 kJ/mol ± 1.1 kJ/mol and 63.1 kJ/mol ± 1.2 kJ/mol, respectively. Luxiang Wang et al. [208] studied the effect of MoS2catalyst on the thermodynamic properties of MgH2, and found that Mo weakened the Mg-H bond, promoted the dissociation of MgH2,and greatly reduced the hydrogen absorption and desorption temperatures. The sample added with MoS2catalyst absorbed 4.5 wt.% hydrogen within 20 min at 280 °C and released hydrogen within 5 min at 200 °C. Heng Lu et al. [209] studied the synergistic catalytic effect of AlH3-TiF3composite material. The results showed that the in-situ generated Al∗of the catalyst reacted with TiF3and Mg during the heating process to form Al3Ti and AlF3, which can act as the actual catalytic active center in the hydrogen desorption and absorption process, accelerate the reversible conversion between Mg and MgH2, and help reduce its hydrogen desorption enthalpy and temperature. The hydrogen desorption enthalpy of MgH2decreased from 75.1 kJ/mol H2to 68.51 kJ/mol ± 4.05 kJ/mol H2.The dehydrogenation peak temperature of the sample with catalyst decreased to 291.1 °C, which was 89.6 °C lower than that of ball milled MgH2(380.7 °C).
Improving the kinetic properties of hydrogen storage materials is mainly to improve their activation energy and hydrogen absorption and desorption rate by adding catalysts[210,211] or changing alloy composition [212]. Table 12 lists the research on using catalysts to improve the kinetic properties in recent years. The research shows that using catalysts can reduce the activation energy of hydrogen release and accelerate the rate of hydrogen desorption under isothermal conditions. Cong Peng et al. [213] proposed a new method for preparing highly dispersed nickel nanoparticles to catalyze MgH2by using the in-situ hydrogenolysis reaction of NiCp2during ball milling. After ball-milling for 15 h in 4 MPa hydrogen atmosphere, the MgH2–16.1 wt.% NiCp2sample showed uniform morphology, and the in-situ Ni nanoparticles were highly dispersed in the MgH2matrix, with a sizeof about 8 nm. In the initial dehydrogenation process, Ni reacts with MgH2to form Mg2Ni that inherits its highly dispersed structure. In the subsequent hydrogen absorption and desorption cycle, the fine and highly dispersed nickelbased catalyst phase helps improve the hydrogen release kinetics of MgH2. The activation energy of hydrogen release is 88.2 kJ/mol, which is far lower than the activation energy of 160.7 kJ/mol when no catalyst is added. In addition to single-phase catalysts, some researchers also explored the effect of heterogeneous catalysts on kinetic properties. Zhen Jia et al. [214] used boron nitride (BN) as a catalyst carrier,5 wt.% Nix@BN (x= 40, 50, 60, 70, 80) were doped into MgH2fabricated via hydriding combustion synthesis by ball milling to improve the kinetics of MgH2. The Ni70@BN has the best kinetic properties, and its nickel particle size is only 10–30 nm, which is uniformly dispersed. Due to the synergy between BN and Mg2Ni(H4),Ni70@BN shows excellent catalytic activity, and the sample rapidly releases 6.21 wt.% H2within 15 min at 300 °C. The activation energy of dehydrogenation is 59.77 kJ/mol ± 3.96 kJ/mol, which is mainly due to the synergistic effect between BN and Mg2Ni(H4)promoting the “hydrogen pump” effect. In addition to using BN as the catalyst carrier,Zhiqiang Lan et al.[215]used nanoporous carbon as the carrier to support Ni and V2O3nanoparticles((Ni-V2O3)@C) as the catalyst and found that samples containing 10 wt.% (Ni-V2O3)@C showed excellent hydrogen storage properties and could absorb hydrogen at room temperature, and their initial hydrogenation temperature was 100 °C lower than the initial hydrogenation temperature of the original MgH2. The sample can absorb 5.50 wt.% H2at 25 °C in 10 min, and release 6.05 wt.% H2at 275 °C in 10 min.The activation energies of hydrogen absorption and desorption are 42.1 kJ/mol and 84.6 kJ/mol, respectively, which are 46.8 kJ/mol and 51.6 kJ/mol lower than the pure MgH2sample. The working principle of the catalyst is that V2O3is partially converted into VO during ball milling, and Ni reacts with Mg after the first dehydrogenation to form in-situ Mg2Ni. The Mg2Ni/Mg2NiH4coating formed in-situ around Mg/MgH2particles acts as a“hydrogen pump”,promoting the diffusion and decomposition of hydrogen, and the presence of C inhibits the agglomeration of Mg/MgH2particles.
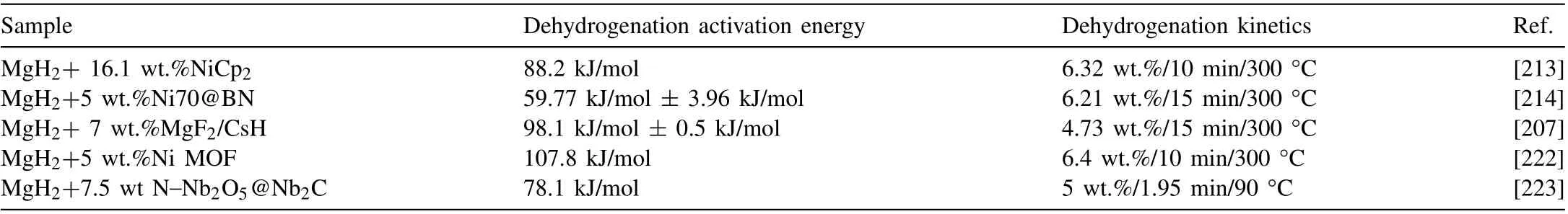
Table 12 Information on catalyst doping systems.
In addition to adding catalyst, the kinetic properties of materials depend largely on alloy elements. The addition of alloy elements forms new phases [216,217] and multiphase structure and increases the H atom diffusion path to improve the kinetic properties. In the study of alloying modification,the long-period-stacking-ordered (LPSO) phase has been a research hotspot in recent years. R.S. Jin et al. [218] prepared Mg-9.1Y-1.0 Zn, Mg-9.1Y-1.8 Zn and Mg-9.2Y-3.1 Zn alloys with different Zn contents by semi-continuous casting. Their results show that with increasing Zn content, the LPSO phase increases and mainly distributes on the grain boundary of the alloy. The 12R-type LPSO phase in Mg-Ni-Y system shows good kinetic properties and cycle stability,but the accurate composition and phase relations with different LPSOs are not clear. Cheng Liu et al. [219] provided a thermodynamic description of Mg-Ni-Y system focusing on LPSO phase in the Mg-rich corner based on many experimental observations, which is helpful for the composition design and preparation of high-performance Mg-based hydrogen storage alloys. The LPSO phase decomposes and in-situ forms YH2/YH3hydride during hydrogenation. With increasing Zn content, these YH2/YH3hydrides tend to concentrate at the grain boundary, which leads to the uneven distribution of YH2/YH3hydrides in these alloys. The more uneven the distribution of these YH2/YH3hydrides on the Mg matrix, the worse the hydrogen desorption kinetics of the alloy.At 320 °C, the time for Mg-9.1Y-1.0 Zn, Mg-9.1Y-1.8 Zn,and Mg-9.2Y-3.1 Zn alloys to reach the maximum dehydrogenation value is 2.7 h, 4.4 h and 4.7 h. The first-principles calculation results show that the presence of YH2reduces the H transfer energy and H2recombination energy of the MgH2(110) surface, which shows that YH2does have a catalytic effect in the alloy.
Reducing the size of magnesium-based hydrides by means of nanostructuring is another way to improve its kinetic properties. Nanostructuring can increase the specific surface area of magnesium-based hydrogen storage materials, enrich grain boundaries/defects, and shorten the transmission path of hydrogen atoms. Most of the current research uses the methods of a combination of nanostructuring and alloying. Bing Han et al.[220]prepared Mg85Ni14Ce1amorphous alloy films with a thickness of 500, 300, 250, and 50 nm by DC magnetron sputtering. The effect of nanometer size on kinetic properties and cycle properties was studied. With the decrease in size,the hydrogen storage kinetics increases significantly. In addition, the nanoscale amorphous alloy can absorb and desorb hydrogen reversibly, and the desorption kinetics is almost unchanged at 120 °C. The structure of amorphous alloy can be completely restored after the hydrogen absorption and desorption cycle.
Other researchers have developed new preparation methods in addition to traditional melting, ball milling, and other methods, which can directly generate nanopowders and improve the kinetic properties of hydrogen storage alloys at the same time. Raffaello Mazzaro et al. [221] prepared Mg-Ni nanoparticles (20 at.% Ni) by vapor condensation using two hot steam sources. The prepared Mg and Ni are mixed to form nanoparticles, while the second phase Mg2Ni is not completely formed. The formation of Mg-Mg2Ni or MgH2-Mg2NiH0.3nanocomposites was observed after the nanoparticles were kept at 150 °C for 2 h under a high vacuum or at a mild hydrogen pressure of 0.15 bar.At 150 °C, its activation energy is 80 kJ/mol±8 kJ/mol(hydrogen absorption) and 60 kJ/mol±6 kJ/mol (hydrogen desorption), respectively. However, the maximum hydrogen storage capacity is only 2.5 wt.%.
In addition to its potential hydrogen storage properties,magnesium-based materials can react with water, which can be used to produce hydrogen by hydrolysis. Jingru Liu et al.[224] prepared a kind of Mg: Li 17:3 nanoporous Mg-Li material by a physical vapor deposition method. The size of nanopores is in the range of 100 nm to 600 nm, the average pore size is 280 nm, and the porosity is 42.4%. This nanoporous Mg-Li alloy shows excellent hydrogen production characteristics. It can efficiently and rapidly use brine to produce hydrogen. At 0 °C, 25 °C, 35 °C, and 50 °C, the hydrogen yield can reach 962 mL-1·g-1, and the hydrogen production rate is 60 mL-1·g-1·min-1, 109 mL·g-1·min-1,256 mL·g-1·min-1and 367 mL·g-1·min-1,respectively.The improvement of its hydrogen production properties is mainly attributed to the high specific surface area of the nanoporous structure and the addition of lithium, which acts as an active center in the process of hydrogen production. This porous magnesium-based material provides a new idea for improving the speed of hydrogen production by hydrolysis.
In the past year, researchers have achieved some improvements in the poor thermodynamic and kinetic properties of magnesium-based hydrogen storage materials through alloying, nanostructuring, adding catalysts, and changing preparation methods.However,there is still a certain distance to fully solve these two problems.
4.4. High damping Mg alloys
Magnesium and its alloys are renowned for their superior damping properties. This excellent performance contributes to their widespread use in vibration and noise reduction in the industrial sector [225,226]. However, magnesium alloys cannot always maintain outstanding damping properties due to the addition of alloying elements. Thus, it is crucial to develop high-performance magnesium alloys that combine these exceptional properties to extend magnesium alloys in other applications.
Jiahao Wang et al. [227] reported that heat treatment and hot rolling processes could produce Mg-8Li-6Y-2 Zn alloys with high damping properties and sufficient strength. The tensile strength of this alloy increased from 140 MPa to 210 MPa(as shown in Table 13). Meanwhile, due to the formation and fragmentation of the 18R-LPSO phase, the damping properties increased from 0.006 to 0.018. Furthermore, Gang Zhou et al. [228] concurrently improved the strength and plasticity of the Mg-4Li-3Al-0.3Mn alloys through a hot extrusion pro-cess. As a result, the tensile strength and elongation of the alloy reached 332 MPa and 14.3% due to a large number of residual dislocations and nano-sized AlMn particles formed by extrusion, and the damping capacity increased from 0.022 to 0.028 at a strain amplitude of 1 × 10-3after extrusion.
Table 13 Damping capacity and tensile properties of different magnesium alloys.
Solid solution atoms also influence the damping properties of magnesium alloys. Elements with a robust solid solution strengthening effect lead to a sharp decrease in damping properties, so many high-damping magnesium alloys contain only a few alloying elements with the low solid solubility in the magnesium matrix, such as Zr, Si, Ni, Mn, Ca, Ce [229].Wensen Huang et al. [230] found that the difference between the radii of Mg and Gd atoms is significant in Mg-Gd binary alloys.At the same time,both the lattice distortion and modulus mismatch due to the addition of Ga increase the resistance to dislocation motion, resulting in better-damping properties.In addition, Jiale He et al. [231] found that by studying rolltreated Mg-xZn-yGa (x+y= 1 at.%), the elongation and damping properties of this alloy increased progressively with decreasing Zn/Ga ratios. Among them, Mg-1Ga (at.%) alloy had the highest elongation (31.1%) and damping capacity,with a Q21 of 0.019 at 0.1% strain.
In brief, functional magnesium materials have made some new progress. In the case of bio-magnesium alloys for different clinical applications, some studies mainly focus on the contribution of alloying elements (Zn, Ca, Sc, etc.) and surface modification to enhance the mechanical properties and biocompatibility of magnesium alloys. The developed Mg-30Sc alloy exhibits an acceptable in-vivo degradation rate(0.06 mm/y), no cytotoxicity on the MC3T3 cell model, and excellent mechanical properties. In the aspect of Mg batteries,significant advances have been made in the cathode/anode materials and electrolytes for magnesium batteries. However,the highly efficient and low-cost preparation method for largescale artificial-SEI-coated Mg anode is still lacking. For the cathode,the high-capacity and high-voltage oxide and polyanionic salt materials still need to be developed extensively. For electrolytes, researchers are gradually leaning toward developing low-cost and chlorine-free liquid electrolytes and hightemperature-resistant solid electrolytes. In the case of hydrogen storage materials, some new catalysts have been used to reduce the activation energy of hydrogen release and accelerate the rate of hydrogen desorption under isothermal conditions, such as YH2, NiCp2, Ni/BN, Ni/V2O3, and so on.Additionally, some new preparation methods which can directly generate nanopowders and improve the kinetic properties of hydrogen storage alloys at the same time have been developed. In the aspect of damping Mg alloys, researchers mainly focus on the effects of heat treatment, deformation process and solid solution atoms on the damping properties of magnesium alloys. The developed Mg-4Li-3Al-0.3Mn alloy processed by hot extrusion shows excellent damping performance (0.028) and a good combination of strength and plasticity.
In summary, the damping performance can be improved through some metal plastic processes and solid solutionenhanced elements to the alloy. In the actual research work,the appropriate process should be selected according to the actual situation and experimental conditions to improve the damping performance of magnesium alloys.
5. Processing technologies
5.1. Recovery and reuse of waste magnesium and magnesium alloys
With the development of science and technology, magnesium alloy products are becoming increasingly popular. There will inevitably be scrap in the production and application process of magnesium alloy. The accumulation of magnesium alloy waste has caused environmental and safety problems.Therefore, how to recycle the scrap of magnesium alloys and utilize the recycled magnesium alloys is of great importance to the environmental protection field.
The waste dust from Mg alloy grinding not only has the danger of explosion, but also reduces the performance of Mg due to the oxidation and hydrolysis of Mg, which seriously limits the reuse of Mg alloy waste dust. Yuyuan Zhang et al.[232] selected four inert agents such as sodium citrate and sodium alginate for the inert treatment of waste magnesium alloy dust. The results show that the combination of organic sodium citrate and inorganic sodium silicate can make the inerting efficiency of Mg alloy dust reach 99.4%, which is more than 20% higher than that of any inerting agent used alone. XRD and FTIR results show that a composite conversion film composed of[SC-Mg]and hydrated magnesium silicate is formed on the surface of waste Mg alloy dust particles,which blocks the contact between external water molecules and Mg2+ions.
Magnesium alloy hydrolysis is considered an environmentally friendly and efficient method for hydrogen production.Some studies have applied industrial magnesium alloy waste to the hydrolysis process to promote hydrogen production. To achieve rapid initial kinetic and high hydrogen capacity, Xiaojiang Hou et al. [233] analyzed the role of (Mg10Ni90)Ce10(MNC) in hydrolysis process. AZ91D and ZK60 attle after activated by MNC exhibits 0.81 and 0.56 H2yield in initial 2 min, being higher than the samples without MNC(0.56 and 0.04). Meanwhile, the maximum hydrogen generation rates can reach 340.9 and 262.8 mL•g-1•min-1.Kaiming Hou et al.[234]creatively used carbon nanotubes(CNTs)and(Mg10Ni)85Ce15(MNC15) as additives to activate Mg-Al alloy (MA) waste synergistically by the ball milling process.The results show that the simultaneous addition of MNC15 and CNTs can significantly improve the hydrolysis kinetics and hydrogen yield of MA. MA-5CNTs-10MNC15can generate 806.6 mL•g-1H2with 0.92 yield at 328 K with an initial 10 min. J. L. Bobet’s group [235] discussed the experimental and theoretical model of hydrolysis of pelleted magnesium alloy scraps. The results show that the industrial magnesium alloy scrap containing 3% of Al and 3% of Zn (and about 20%of MgO and Mg(OH)2)exhibits the best hydrolysis yield and kinetics (for example, almost 90% yield in 1 min) after adding 5 wt.% C and 5 wt.% Ni, Cu, Co or SiO2ball milling for 3 h.
5.2. Cast technology for high-strength Mg alloys
An annual rise of 10%–20% in the casting production of these alloys in the last few decades has been reported, and it is projected to maintain at a similar rate [236]. Therefore,optimizing and developing the casting process is one of the crucial measures to shorten the production process, improve production efficiency, reduce energy consumption and obtain high-strength magnesium alloy products [237–241].
The twin-roll casting process helps to refine the grain structure and secondary phase particles, thus improving the mechanical properties of magnesium alloy sheets.T.Nakata et al.[242]have successfully enhanced the strength and plastic synergism of Mg-3 wt.% Al-Mn (AM30) alloy sheets through twin-roll casting and low-temperature annealing. The AM30 alloy sheet produced by twin-roll casting, homogenization,hot-rolling, and subsequent annealing at 170 °C for 64 h exhibits a good 0.2% proof stress of 170 MPa and a large elongation to failure of 33.1% along the rolling direction.Matthew S. Dargusch et al. [33] prepared Mg-0.5Zn-0.5Ca alloy using a new twin-roll casting process (TRC). The TRC Mg-0.5Zn-0.5Ca alloy showed excellent refinement with grain sizes less than 150 μm and exhibited a UTS and EL of 221.9 MPa ± 1.8 MPa and 9.3% ± 2.1%, respectively.
It is well known that theβ′phase,β2phase,βphase,andβ′′phase precipitates are important strengthening phases in Mg-RE-Zn alloys. To take advantage of the strengthening effect of the second phase, W. Luo et al. [243] developed a method based on the spark plasma sintering rapid solidification strip process to successfully prepare a multiple microstructure with fine Mg grains, LPSO phase,β1phase,andβ′phase. Different sintering parameters can control the microstructure and mechanical properties of the as-cast Mg-15Gd-1 Zn (wt.%) alloy. The microstructure investigation showed that the sintered alloys consisted of fine grains, theβ1phase, and long–perioded stacking ordered (LPSO) phase.The sintering temperature and time have a significant effect on the microstructural evolution. The mechanical tests indicated that the YS and UTS were in the range of 170 MPa–320 MPa,320 MPa–410 MPa, respectively, and the corresponding EL was in the range of 10%–22% with varying process parameters.
Feng Wang et al.[244]fabricated the vacuum die-cast Mg-7Al-2Sn alloy and studied its microstructure and mechanical properties. After solution treatment, the mechanical properties of Mg-7Al-2Sn alloy are improved significantly. The values of ultimate tensile strength, yield strength and elongation are∼276 MPa, ∼203 MPa and ∼10.6%, which is attributed to the compact microstructure of the alloy after heat treatment.
Fig. 14. Pictures of surface and cross sections, dimensional error ΔP and relative density ρS of porous scaffolds: (a–d) S300D, (e–h) S400D, (i–l) S500D[249].
5.3. Additive manufacturing technology of Mg alloys
Due to the great potential of magnesium alloys in structural and biomedical applications, research on additive manufacturing (AM) of magnesium alloys has been gradually carried out to break through the limitations of traditional processes for producing commercial magnesium alloys with complex structures [245–248].
5.3.1. Additive manufacturing technology of biomedical Mg alloys
Porous biodegradable Mg and its alloys have great potential to serve as ideal bone substitutes. Jinge Liu et al.[249] fabricated the biodegradable WE43 alloy porous scaffolds by laser powder bed fusion and optimized the processing parameters, and carried out in-vitro and in-vivo investigations. The surface and cross sections of the porous scaffolds are shown in Fig. 14. With the customized energy input and scanning strategy, the relative density of struts reached over 99.5%, and the geometrical error between the designed and the fabricated porosity declined to below 10%. The compressive strength of this porous scaffold is 4.4 MPa–23.5 MPa and the elastic modulus is 154 MPa–873 MPa, which is equivalent to that of cancellous bone. Good biocompatibility was also observed by in-vitro cell viability and in-vivo implantation. J. Dong et al. [250] used pre-alloyed Mg-Zn (4 wt.%Zn) powders as raw material to prepare Mg-Zn binary alloy scaffolds with a geometrically ordered and fully interconnected porous structure through an extrusion-based AM technique and deboning and sintering, which can avoid some crucial issues encountered when applying powder bed fusion AM techniques. The shape of the porous scaffold is cylindrical with a diameter of 12.38 mm and a height of 12.64 mm.The porosity and strut density of the Mg-Zn scaffolds were 50.3% and 93.1%, respectively. The yield strength and elastic modulus of the Mg-Zn scaffolds (14.5 MPa ± 3.0 MPa and 448.8 MPa ± 42.4 MPa, respectively) were much higher than those of the pure Mg scaffolds (4.7 MPa ± 0.7 MPa and 184.4 MPa ± 37.3 MPa, respectively). The biodegraded specimens showed a notable decline in their strain to failure from 30% ± 8% before the in-vitro immersion to 16% ± 1% after 14 days. In addition, a Mg-Zn/bioceramic composite scaffold ex-situ based on extrusion additive manufacturing technology was developed. The yield strength (24.4 MPa ± 13.4 MPa),elastic modulus(431.6 MPa±102.9 MPa)and in-vitro rate of biodegradation (0.5 mm/y) of Mg-Zn/5TCP composite scaffolds were superior to those of the monolithic Mg-Zn scaffold[251]. Warren L. Grayson’s group [252] present an exciting alternative to conventional additive manufacturing techniques: 3D weaving with Mg wires that have controlled chemistries and microstructures. The weaving process offers high throughput manufacturing as well as porous architectures that can be optimized for stiffness and porosity with topology optimization. 3D woven Mg scaffolds with precisely controlled porosity and pore size were fabricated using ZXM100 wires and were coated with PLA using a modified dip-coating approach. The PLA coating dramatically improved the bending strength of the as-woven STD (the ’standard’ architecture,each available position in fill and warp layers was inserted with a pair or a single wire, respectively) and HPFW (The architecture has higher porosity in both the fill and warp direction) to 19.2 MPa ± 4.1 MPa, 19.1 MPa ± 2.2 MPa,and their Young’s moduli to 1259.1 MPa ± 153.8 MPa,1021.8 MPa ± 202.4 MPa, corresponding to improvements of 11 ×, 14 × in bending strength, and 43 × and 43 × in Young’s moduli. Xuezheng Yue et al. [253] prepared AZ91 Mg alloy scaffolds with two parametric frames (a newly hybrid three period minimal surface (TPMS) cellular structure and a Voronoi structure) by the SLM method. Experimental results showed that the average deviations between designed models and printed specimens are within 5%. The scaffolds with TPMS and Voronoi structures are designed with a high porosity ranging from 83%to 95%,and they have achieved low density in the range of 0.09 g/cm3to 0.31 g/cm3.Meanwhile,hybrid TPMS structures display superior mechanical properties compared to Voronoi structures with the same porosity, which is mainly ascribed to the unique structure revealed from the simulation results.

Fig. 15. (a) Evaporative fumes during the LPBF process and (b) side view of the as-built GWZ1031K samples where red ovals indicate macro cracks[77].
5.3.2. Additive manufacturing technology of structural Mg alloys
Wenjiang Ding’s group [77] investigated the microstructural evolution and mechanical properties of a high-strength Mg-10Gd-3Y-1Zn-0.4Zr alloy fabricated by laser powder bed fusion (LPBF). The size of the printed sample is 10 mm × 10 mm × 10 mm cube (as shown in Fig. 15).The as-built alloy is composed of fine equiaxed ɑ-Mg grains with an average grain size of 4.1 μm ± 0.5 μm, reticularβ-(Mg, Zn)3(Gd, Y) eutectic phase and flaky Y2O3oxide phase, and exhibits a YS of 310 MPa ± 8 MPa, UTS of 347 MPa ± 6 MPa and EL of 4.1% ± 0.8%. Yangyang Guo et al. [254] studied the effect of heat treatment on the microstructure and mechanical properties of AZ80M magnesium alloy fabricated by wire arc additive manufacturing. Each sample was deposited with 50 layers, the dimensions were 8 mm in thickness, 80 mm in width and 300 mm in length.The T6 samples (410 °C for 1.5 h, water quenched then 180 °C for 25 h and air cooled) effectively improved the microstructure uniformity. The average microhardness was 66.9 HV, and the UTS and EL of the T6 samples were 292 MPa and 16%in the horizontal direction,and 283 MPa and 14%in the vertical direction, respectively. The anisotropy was somewhat reduced compared with the AD state, and strength and plasticity were improved. In addition, a high-strength ZK60 Mg alloy fabricated by laser powder bed fusion (LPBF) is subjected to in-situ aging treatment by preheating the substrate. The sample size is 10 mm × 10 mm × 8 mm. The specimens treated at a preheating temperature of 180 °C exhibit a YS of 201 MPa ± 5 MPa, UTS of 291 MPa ± 7 MPa,and EL of 14.7%±0.8%[255].Qianhui Cao et al.[256]used a novel ultrasonic frequency pulsed arc(UFP)as a heat source to improve the anisotropy of mechanical properties caused by wire arc additive manufacturing (WAAM). As a result, the UFP-WAAM deposit exhibits a good forming quality with no defects and fewer micropores, full equiaxed-grain microstructure and isotropic ultimate tensile strength along the building direction (UTS = 203 MPa) and traveling direction(UTS = 211 MPa).
It is well known that additive manufacturing of magnesium alloys is associated with high Mg loss due to vaporization and the high incidence of many defects in the manufactured parts/samples. Faridreza Attarzadeh et al. [257] presents a combined modeling and experimental approach applied for a widely WE43 alloy by laser-based powder-bed fusion (LPBF) to address this difficulty in element loss, densification,and defects. The optimized L-PBF processing parameters are determined by considering the Mg loss, densification, and the characteristics of defects. Their study yields 0.23 wt.%Mg loss compared to 2 wt.% Mg loss reported in the previous studies. Furthermore, more than 99.5% densification is achieved, while only ∼2% and ∼0.5% of the total defects are characterized as keyhole and lack of fusion defects, respectively. Xiaochi Yuan et al. [258] studied the defect of the Mg-Gd alloy additively manufactured by selective laser melting(SLM). The as-built Mg-3Gd, Mg-6Gd, and Mg-10Gd samples are relatively dense with relative densities of 98.4%—98.8%. By contrast, macro-cracks occasionally occur within the Mg-15Gd sample. Jingwei Liang et al. [259] eliminated the hot cracks of laser powder bed fusion (LPBF) processed ZK60 components by adding rare-earth element Y.The cracks in ZK60 LPBF samples were mainly caused by the lowmelting eutectic phase Mg7Zn3at the grain boundaries. The elimination of cracks was mainly attributed to the I-phase at the grain boundary and grain refinement.
5.4. Plastic processing technologies of Mg alloys
5.4.1. Progress in extrusion technology
In 2022, with environmental protection, energy saving, and high-efficiency requirements, the extrusion process was continuously improved to further refine the structure and improve the mechanical properties [260–263].
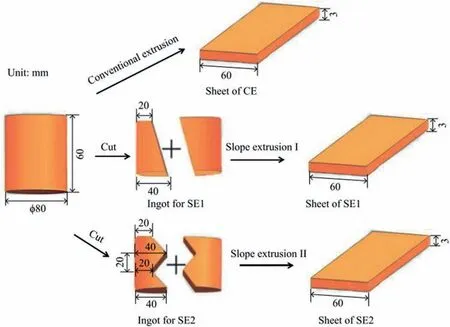
Fig. 16. Schematic diagrams for the fabrication of the CE and SE sheets [264].
Fig. 17. ESE process: (a) Schematic illustration of the extrusion die, and (b) mold and a sample of extrusion [265].
Yan Xu et al. [60] designed a new accumulative backward extrusion (ABE) process for AZ91D magnesium alloy. The initial as-cast grains were significantly refined, and a lowstrength matrix texture was formed. As a result, excellent ultimate tensile strength and elongation to failure were obtained,which were 296 MPa and 21.4%, respectively. Huabao Yang et al. [264] designed a new extrusion process of slope extrusion (SE) for the manufacture of AZ31 alloy plates, which can significantly refine the grains (from 9.1 μm to 7.7 μm and 5.6 μm) and strengthen the matrix texture to improve the comprehensive properties of the alloy, as shown in Fig. 16.The inclined interface can produce more extensive asymmetric deformation and stronger cumulative strain along the ND direction. The YS of SE1 and SE2 sheets (191 MPa and 220 MPa, respectively) is much higher than that of the CE sheet (178 MPa). The UTS shows an upward trend from CE to SE1 and SE2 sheets (from 393 MPa to 394 MPa and 411 MPa). Bo Che et al. [265] designed a new expansion shear extrusion (ESE) process to prepare AZ31 magnesium alloy sheets with uniform microstructure and fine grain size(the average grain size is (2.4±0.3) μm, as shown in Fig. 17.When the diameter of the expansion ball is 30 mm, the strain inhomogeneity and flow velocity inhomogeneity parameters are as low as 0.262 and 0.049, respectively, resulting in a better sheet-forming effect.
5.4.2. Progress in rolling technology
Conventional rolling usually leads to high anisotropy and strong substrate texture. Therefore, some methods have been proposed to control the microstructure and texture to improve materials’ mechanical properties and formability.

Fig. 18. Working diagram of ultrasonic surface rolling processing (USRP)treatment [266].
Fig. 19. Schematic diagram of closed forging extrusion [270].
Linbo Chen et al. [266] developed an ultrasonic surface rolling processing (USRP) after equal channel angular extrusion, as shown in Fig. 18. The surface roughness, yield strength, ultimate tensile strength, and microhardness of the prepared Mg-Y-Nd-Zr alloy (216.06 MPa, 330.61 MPa, and 105.6 HV, respectively) were significantly improved. This is mainly due to grain refinement and synergistic deformation between gradient nanostructure(GNS)and coarse-grained(CG) matrix. Zhike Deng et al. [267] designed a simple processing scheme of electric pulse treatment (EPT) after cold rolling. Compared with conventional heat treatment (HT), the ultimate tensile strength (198.7 MPa ± 4.4 MPa) and elongation (20.2% ± 0.6%) at the break of the prepared Mg-1Gd(G1) alloy achieve a good balance. Due to the uniform grain structure, weakened texture, and selective heating effect after EPT, the microcracks generated during cold rolling can be cured.
Biquan Xiao et al. [268] designed an asymmetric rolling(AR) process. The AZ31 sheet prepared on the online heating mill has a more uniform structure, a higher degree of recrystallization, and a symmetrical distribution of the substrate texture. Compared with the yield strength(270.1 MPa ± 6.5 MPa) and elongation (3.9% ± 1.4%) of the SR sheet. The yield strength (230.6 MPa ± 6.7 MPa and 213.3 MPa ± 3.9 MPa, respectively) and elongation(14.3% ± 0.6% and 14.9% ± 0.8%, respectively) of AR1 and AR2 reached a good balance. The AR steel plate has fewer shear bands and a layered bimodal structure. Kamil Majchrowicz et al. [269] designed a differential speed rolling(DSR) process to prepare TZ61 (Mg-6Sn-1 Zn) alloy with fine grain size and weakened basal texture, and the plasticity of the sheet increased (9.8% ± 1.9% and 14.5% ± 1.1% respectively) after annealing. The high proportion of the Mg2Sn phase hinders grain growth, and DSR treatment reduces the basic texture strength of the sheet.
5.4.3. Progress in forging technology
The multi-directional forging is a simple and economical method, and it has significant deformation. Therefore, it has great application potential in the industrial mass production of magnesium alloys.
Kun-ming Zhang et al. [270] designed a new closed forging extrusion (CFE) process for AZ31 magnesium alloy, as shown in Fig. 19. The results show that the CFE process can promote dynamic recrystallization (DRX), eliminate coarse non-DRX grain regions, and effectively refine grains. After 60-s closed forging and continuous extrusion, the ultimate tensile strength, plasticity, and anisotropy of the alloy are significantly improved (337 MPa, 27%, and 0.97, respectively).Furong Cao et al. [271] developed a new multi-directional forging and asymmetric rolling process to prepare Mg-9.55Li-2.92Al-0.027Y-0.026Mn alloy, as shown in Fig. 20. The results show that this forming process can bring about significant grain refinement, transforming the as-cast grain size of∼145 μm into the rolled grain size of 1.9 μm. Its good ductility (obtained to be 228% at 523 K at 1 × 10-2s-1) is suitable for high strain rate superplastic forming.
Chao Cui et al. [272] developed a new small strain multidirectional forging (MDF) method to adjust the microstructure of AZ31 magnesium alloy and improve its mechanical properties. With increasing MDF cumulative strain, the yield strength is affected by grain size and texture. The fluctuation of initial strength is mainly attributed to the change of texture. When the texture is stable, the increase of strength is due to grain refinement, and finally, a uniform fine-grain microstructure with bimodal basal texture is obtained. After 27 forgings, the yield strength along LFD, LTFD, and LSFD increased to 191 MPa, 215 MPa and 189 MPa, respectively. Songhe Lu et al. [273] designed a multi-directional impact forging (MDIF) method to successfully prepare Mg-4.96Gd-2.44Y-0.43Zr alloy with high strength and toughness. The results show that the alloy has a fine-grained microstructure after forging and aging. The average grain size is about 5.7 μm, and the tensile yield strength (TYS)(337 MPa ± 2.2 MPa), elongation (11.5% ± 1.3%) and static toughness (ST) (50.4 MJ/m3± 5.3 MJ/m3) are significantly improved.
5.4.4. Progress in friction stir processing technology
Friction stir processing (FSP) is also considered to be an effective technique for refining microstructure and improving performance. Friction stir welding (FSW) is a promising solid-state joining technology, especially suitable for joining dissimilar aluminum/magnesium alloys, with good welding quality and low residual stress and deformation.
Junjie Zhao et al. [274] changed the microstructure of the weld nugget zone(WNZ)of dissimilar Al/Mg alloy joints during FSW by ultrasonic vibration (UV). As shown in Fig. 21,they found that the applied ultraviolet light changed the main dynamic recrystallization (DRX) mechanism of the magnesium alloy side grains from continuous DRX (CDRX) to discontinuous DRX (DDRX). At the same time, along the thickness direction, the average grain size near the WNZ weld interface increases first and then decreases, and the maximum grain size appears in the middle of the weld. In particular,in UVeFSW more locations in WNZ have high-strain shear textures. Xiaoqing Jiang et al. [275] proposed a pulse currentassisted FSW to improve the mechanical properties of dissimilar Al/Mg alloys, as shown in Fig. 22. The results show that with the increase of pulse current, the tensile strength and elongation of the alloy increase to ∼216 MPa and ∼5.2%,respectively, and the fracture mode changes from brittle fracture to a compound fracture,which eliminates the defects such as cracks and tunnels in conventional Al-Mg fusion welding. Jianheng Cai et al. [276] proposed an immersion friction stir processing (SFSP) process for preparing high-strain rate superplastic ZK60 magnesium alloy. After SFSP, the as-cast microstructure is remarkably refined, and the average grain size reaches 1.84 μm. This is because with the fast cooling rate, severe plastic deformation occurs, and dynamic recrystallization occurs, but the grain growth will be significantly delayed.

Fig. 20. The schematic sketch of (a) multidirectional forging and (b) asymmetrical rolling. A, B, and C are pressing planes. F is the force [271].
Fig. 21. Schematic of the FSW and UVeFSW of dissimilar Al/Mg alloys[274].
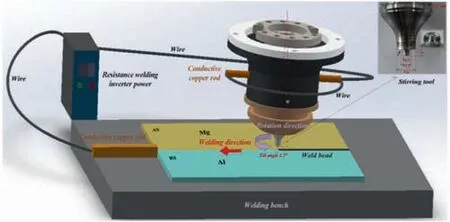
Fig. 22. Schematic of pulse current assisted FSW system [275].
5.4.5. Drawing technology
Fig. 23. The diagram of the micro-tube preparation technique [277].
National Engineering Research Center for Mg Alloys developed a novel method including multi-pass drawing and separation of a mandrel from a tube to obtain highly precise micro-tubes, which provides a reference for the development of biomedical magnesium alloys.
The specific diagram is shown in Fig. 23. In the first step,the Mg ingot needs to reduce its cross-section to achieve hollow profiles, and then the heated ingot passes through the die under pressure to obtain tubes in a single step [277]. Through the multi-pass drawing process, the tensile stress is applied to control the wall thickness of micro-tubes and the clamped mandrel can be used to keep the inner diameter from changing, leading to an improved dimensional accuracy as well as decreased cross-section area. The final step is the separation of the mandrel from the tube wall using a special die [278].The results of animal studies show that the self-developed stent exhibits excellent endothelialization, homogeneous endothelium, without hyperplasia and restenosis. Furthermore,the tensile strength of the stent exceeds that of foreign materials by 15.9% and the elongation by 21.4%.
5.5. Welding and joining technology of Mg alloys
Welding and joining of magnesium alloys are critical for their engineering applications. In the past year, the welding process optimization of magnesium alloys and the welding of dissimilar materials were developed [279–282].
5.5.1. Welding process
Eisha Khalid et al. [283] investigated the microstructure and tensile behavior of a Bobbin friction stir welded (BFSW)AZ31B alloy. The results show that a relatively homogeneous microstructure is obtained in BFSW welds compared to the conventional FSW. BFS welded specimens showed a maximum of 67% and 48% joint efficiency (a ratio of weld UTS to base material UTS) at room temperature and 200 °C, respectively. Qi Sun et al. [284] used pre-ultrasonic surface rolling on docking surfaces (PURS) to improve the mechanical properties of friction stir welded AZ31 joints. Compared with the FSW joint, the tensile results showed that the UTS(244 MPa±4.5 MPa)and%EL(10.3%±0.4%)of the PURS joint were improved by about 7% and 43.1%, respectively.
Chao Meng et al. [285] improved the mechanical properties of AZ31B magnesium alloy TIG welded joints by laser bionic treatment of joints. After laser bionic treatment, due to the rapid heating and cooling of laser processing, the crystal orientations of the laser treated zone (LZ) in the TIG welded joint were more random without obvious texture. The grain size in the LZ was refined. As the laser bionic stripe spacing was 4 mm, compared with the untreated welded joint sample, the tensile strength and elongation of the L-4 sample increased by 23% and 105%, while the yield strength slightly reduced by 1.8%. Huijing Zhang et al. [286] developed a navel AC/DC hybrid inert gas TIG (MIX-TIG) to improve the welding quality of AZ31B alloy TIG. At the same welding conditions, the average grain size andβphase number in the MIX-TIG were less than those of the AC-TIG. Compared with AC-TIG welded joints, the strain of MIX-TIG welded joints increased by ∼24%.
Jie Ning et al. [287] improved the thermal efficiency and stability of magnesium alloy laser welding process by combining power modulation and subatmospheric pressure environment. During laser welding in subatmospheric environment, the weld penetration can be greatly increased through power modulation. Besides, power modulation can inhibit the occurrence of bulges in local zones on the backwall of the keyhole during laser welding in subatmospheric environment,thus further curbing the significant growth of the weld widths of hump-shaped welding beads and local zones in the lower part of welded seams. Bin Jiang’s group [288] developed a new criterion to predict the solid-state welding quality of magnesium alloys during the porthole dies extrusion of hollow profiles. For Mg-Al-Zn-RE alloy under investigation, a sound weld seam with a strength higher than 316 MPa is achieved during the extrusion when the billet temperature is higher than 350 °C, and the extrusion speed is lower than 16.67 mm/s.
5.5.2. Welding of dissimilar materials
Jonova Thomas et al. [289] analyzed the three-dimensional microstructure of a friction stir welded AZ31/Zn-coated DP590 steel interface characterized via high-energy synchrotron X-rays. Diffraction assessment of the AZ31/Zncoated DP590 steel interface revealed the presence of several complex Mg-Zn intermetallic compounds and differences in the crystalline orientations of the Mg phase at the weld interface. Taotao Li et al. [290] studied the forming characteristics and control mechanisms of the AZ31B Mg alloy/Q235 steel welded joint by laser-TIG butt fusion welding. The results show that the key to controlling the formation of magnesium/steel butt fusion welding is to compensate the combustion loss of magnesium alloy and promote the interfacial reaction. The addition of the eutectic allows for Mg/steel singleside welding, and the tensile strength of the joint reaches nearly 165 MPa. Zhaoguo He et al. [291] explored a novel hybrid joining method of rivet plug oscillating laser welding (RPOLW) for joining DP590 dual-phase steel to AZ31B magnesium alloy. The tensile-shear load of the RPOLW joint reached 2.2 kN using the optimal parameters, which was 36%higher than that of the riveting joint.
Mustafa Acarer et al. [281] attempted to prepare AZ31-Al5005 laminated composites by explosive welding. The results illustrate that the UTS (178 MPa), YS (96 MPa) and EL(4%)of the composite were lower than those of the individual components owing to the cracks formed in the explosive welding process. Mingzhe Bian et al. [292] used the subsequent hot rolling process to improve the mechanical properties of explosively welded Mg-6Al-1Zn-1Ca (AZX611)/Al-0.6Mg-0.6Si-0.1Fe(A6005C)composite plates.The joint sheet rolled at 250 °C with a thickness reduction per pass of 28% shows a large fracture elongation of 15%,which is significantly higher than 1.2% of the explosively welded clad plate. Yingzong Liu et al. [293] controlled intermetallic compounds (IMCs)in AZ31 Mg and 6061 Al by ultrasonic-assisted Sn soldering to improve the mechanical properties. The results show that this prevents the formation of Mg-Al IMCs such as Al3Mg2and Al12Mg17. The average maximum shear strength of the water-cooled Mg/Sn-9 Zn/Al joint was ∼53 MPa, which was 70% higher than that of air-cooled Mg/Sn-9 Zn/Al and 95%higher than that of air-cooled Mg/pure-Sn/Al joints. Han Yan et al. [294] studied the evolution of interfacial IMCs and the mechanical performance of Sn-9 Zn solder joints of Mg/Al dissimilar metals by using Ga-coating protection. The Ga film replaced the original oxide film and formed a protective layer on the substrates. The joints exhibited the best shear strength up to 38 MPa because of the thinner discontinuous IMC layer and lower blocky Mg2Sn ratio among the fractured surface.
To sum up,the recovery and reuse of waste magnesium and magnesium alloys were developed in the past year.For plastic processing technology, the accumulative backward extrusion(ABE), slope extrusion (SE), asymmetric rolling (AR), ultrasonic surface rolling (USRP), drawing technology, etc. were developed. Both bio-Mg alloys with complex pore structures were successfully produced. Extrusion-based AM, followed by debinding and sintering,has been recently demonstrated as a powerful approach to fabricating such Mg scaffolds, which can avoid some crucial problems encountered when applying powder bed fusion AM techniques. In the aspect of joining and welding, the number of papers focusing on TIG welding and laser welding has increased. Welding of dissimilar materials mainly focuses on magnesium/aluminum, magnesium/steel, etc.
6. Corrosion and protection of Mg alloy
Magnesium has extremely low electrode potential(-2.363 V) and high chemical activity, which is usually considered to be easily corroded. In recent years, researchers mainly tried to understand the corrosion mechanisms of magnesium alloys in different environments and explore possible ways to improve the corrosion resistance of magnesium alloys through the actual corrosion behavior of magnesium alloys under different service conditions (including human environment) [295–297]. The corrosion resistance of magnesium alloys can be effectively enhanced by improving the purity of magnesium alloy, adding appropriate alloying elements, adopting special preparation process and appropriate surface treatment, which is crucial for the industrialization of magnesium alloys.
6.1. Corrosion behavior of Mg alloys
6.1.1. Corrosion mechanism of Mg alloys
Magnesium is one of the most active metals among all industrial alloys,and its corrosion behavior varies greatly with environmental changes.In the dry atmosphere,the magnesium surface can form an oxide film, which has a protective effect on the substrate. However, when the environment is humid or in the presence of Cl-,the corrosion resistance of magnesium is poor due to the loose and porous surface oxide film, which is prone to corrosion.
Many researchers usually use laboratory accelerated methods (EIS, Tafel, etc.) to simulate the environmental corrosion behavior of magnesium alloys in a corrosive media environment. Yuxiang Liu et al. [298] investigated the pitting corrosion behavior of AZ91 alloy in 3.5% NaCl solution by cyclic potentiodynamic polarization (CPDP). They found that the formation of a corrosion product layer during the 1st CPDP process is beneficial for improving corrosion performance.Huanwen Chen et al. [299] reported that the localized corrosion of AZ31B alloy in SO42--containing chloride solution was slowed down due to the competitive adsorption of SO42-.Evgeniy Merson et al. [300] found that the pre-immersion of ZK60 magnesium alloy in NaCl-based corrosion solution increases the stress corrosion cracking tendency. This is mainly due to the ’repair’ effect caused by the hydrogen produced by the initial corrosion and the storage of the corrosion solution sealed in the corrosion products after exposure to air.
However, the laboratory environment is usually a singlefactor or two-factor study, which cannot truly reflect the corrosion process of the alloy under actual operating conditions(pressure, solar irradiation, high temperature, high humidity,etc.). Quantong Jiang et al. [301] studied the sea-water corrosion behavior of EW75 Mg alloy used in the research vessel KEXUE. Due to the high temperature, high humidity, high salinity, and intense ultraviolet radiation in the marine atmospheric environment, and the alternating state of dry and wet on the alloy surface, severe corrosion occurred during the exposure of EW75 magnesium alloy. In addition, Ying Wang et al. [302] also investigated the dynamic marine atmospheric corrosion behavior of AZ31 magnesium alloy. The corrosion was initiated by pitting corrosion and gradually evolved into general corrosion. This study provides valuable data for the application of magnesium alloy in shipboard aircraft and other equipment and provides a reference for indoor simulation experiments.
Magnesium alloys have attracted great interest in the biomedical field in recent years. A.S. Gnedenkov et al.[303] studied the detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions (MEM,NaCl solutions) and found the Mg2Ca phase creates a localized micro-galvanic cell withα-matrix which facilitates the dissolution of Mg2Ca compounds at the grain boundaries(and withinα-Mg grains) during the corrosion process. This provides faster degradation, higher susceptibility to pitting formation, and increased alloy corrosion. N. Pulido-González et al. [304] compared the biocorrosion behaviors of ZX11 and ZX30 alloys in Hank’s solution. The ZX11 alloy generally shows better biocorrosion behavior than the ZX30 due to its lower content in the Ca2Mg6Zn3phase, thus reducing galvanic corrosion.
6.1.2. Influencing factors of Mg alloy corrosion
The factors affecting the corrosion of magnesium alloys are very complex, including alloying elements, microstructure, corrosion medium, ion concentration, pH value of the solution, etc. In 2022, a series of studies were carried out on the factors, and certain progress was made.
As we know, alloying elements play a vital role in corrosion behavior. Michiaki Yamasaki et al. [305] found that Al reduced the hydration of the magnesium alloy surface oxide film, which promoted the formation of the surface protective film of extruded Mg97Zn1Y2alloy. Umer Masood Chaudry’s group [306] found that by adding Ca into AZ31 alloy, the segregation of Ca and Al formed (Mg, Al)2Ca intermetallic compound, which reduced the sensitivity of the alloy to micro-galvanic corrosion by decreasing the concentration of Al and changes the morphology of theβ-Mg17Al12phase.Minjie Liang’s group [307] found that the formation of theβ-Mg17Al12phase will be inhibited by adding rare-earth Nd elements into the as-cast AZ80 alloy.β-Mg17Al12phase changed from a coarse reticular structure to fine and discontinuous distribution, improving the corrosion resistance.
It has been widely investigated that the microstructure greatly influences the corrosion behavior of magnesium alloy. Peihua Du et al. [308] found that the HTHEed Mg-Zn-Y-Nd alloy microtubules had a firm texture, and the basal plane was parallel to the longitudinal section, which provides better in-vitro corrosion resistance. Yuxiang Liu’s group[309] found theβ-Mg2Ca phase dissolves and forms a uniform structure during laser treatment; meanwhile, the MgO phase was formed on the alloy surface. This is beneficial for reducing the corrosion rate of the laser-treated alloy at the beginning of immersion corrosion. Kai Ma et al. [310] found that the adequately heat-treated Mg-Gd-Cu alloy contained a large number of solute separation stacking faults (SFs), which shows a high corrosion rate at the initial stage of corrosion,but the average corrosion rate is lower than that of as-cast alloy due to the corrosion film quickly formed on the surface.Xuewei Fang et al. [311] found that the AZ31 magnesium alloy thin wall prepared by gas tungsten arc-based wire arc additive manufacturing (WAAM-GTA) technology had high dislocation density, uniform and fine twin equiaxed grains,which makes its corrosion resistance better than hot-rolled AZ31 plate. Fatemeh Iranshahi’s group [312] carried out solution heat treatment on ZKX50 alloy. They found that the refinement of alloy grains, dissolution and spheroidization of Ca2Mg6Zn3particles reduce the micro-couple corrosion and improve the corrosion resistance in the solution heat treatment state. N. Pulido-Gonz á lez et al. [304] found that the corrosion resistance of as-cast ZX11 alloy was enhanced due to the reduced number of coarse second phases and galvanic corrosion; as for ZX30 alloy, the undissolved second phase at the grain boundary was redistributed and more connective,leading to the enhancement of galvanic corrosion.
Magnesium alloys exhibit different corrosion characteristics in various corrosion media. A.S. Gnedenkov’s group[303] found that Mg-0.8Ca alloy formed a magnesium carbonate substituted hydroxyapatite film on the sample surface during the degradation process in MEM, which reduced the electrochemical activity and delayed the corrosion rate. Baojing Feng et al. [313] found that the surface facial mask of WE43 alloy in NaCl solution has better protection than that in Na2SO4solution. This is because the film composed of rareearth oxides and hydroxyl radicals covers the second phase,but it is vulnerable to attack by SO4-and loses its protection.
The ion concentration in the electrolyte is also an essential factor affecting the corrosion performance of magnesium alloys. Baojie Wang et al. [314] found that localized pitting was very sensitive to Cl-concentration, the hydrogen evolution rate of dual-phase Mg-8Li alloy in 3.5 wt.% NaCl solution was three times higher than that in 0.9 wt.% NaCl solution when the immersion time exceeded 8 h. Branimir N. Grgur’s group [315] studied the initial corrosion behavior of AZ63 magnesium alloy in 1, 3, 5, and 7 wt.% NaCl solution and found that the corrosion rate increases with increasing chloride concentration, and the corrosion potential decreases linearly. Xing Gao et al. [316] found that when ionic liquid corrosion inhibitors are added to the corrosive medium below 200 ppm, the inhibitors can effectively adsorb on the surface of Mg alloys, preventing the corrosion medium from contacting with substrate. However, when the inhibitor concentration exceeds 200 ppm, the concentration of negative ions and cations in the solution may increase, leading to an increase in the conductivity of the solution and enhanced corrosion. Yumiao Jiang et al. [317] found that the increase of inhibitor concentration was positively correlated with the inhibition ability because the inhibitor molecules adsorbed on the interface between Mg alloy and NaCl solution, blocking the active site of corrosion to a certain extent. With the increase of inhibitor concentration, the adsorption behavior became stronger.
The acid and alkali environment of the solution also has a certain impact on the corrosion performance of magnesium alloys. Tao Zhu’s group [318] found that the increase in pH value provoked an increase in the corrosion resistance of AZ80 magnesium alloy. In the acidic environment, the Mg(OH)2protective film is destroyed by a large number ofH+and Cl-, resulting in the precarious existence of massive Mg(OH)2film, thus reducing the corrosion resistance of Mg alloys. On the contrary, in neutral and alkaline environments,the stability of massive Mg(OH)2films improves the corrosion resistance of Mg alloys. Abdelmoheiman Zakaria Benbouzid et al. [319] studied the electrochemical impedance diagrams of Mg electrodes in a sodium sulfate solution at three different pHs (1.8, 2.9 and 7.7). It is found that the cathodic reaction is only considered in the medium with a low pH value. On the other hand, when the pH value is neutral to alkaline, the anodic branch dominates the corrosion mechanism.
6.2. Corrosion resistance improvement of Mg alloys
Improving the corrosion resistance of magnesium and magnesium alloys can usually be carried out from the following three aspects: Firstly, improve the overall corrosion resistance of magnesium alloy products, such as improving the purity of magnesium alloy, adding unique alloying elements, using rapid solidification treatment and other preparation methods,reducing the content of harmful elements in magnesium alloy or improving the solid solution limit of impurities, optimizing the microstructure of magnesium alloy and its corrosion products, thereby improving the corrosion resistance of magnesium alloy itself. Secondly, adding corrosion inhibitors, through adsorption or consumption of ions in the solution a physical protective layer is formed on the surface to prevent the substrate from contacting with aggressive ions. Thirdly, the surface treatment of magnesium alloy is performed by chemical conversion, anodic oxidation, surface infiltration layer, organic coating, metal coating, and surface modification. The corrosion-resistant coating is obtained on the surface of magnesium alloy products to prevent the corrosive medium in the environment from entering the magnesium alloy substrate.
6.2.1. Micro-alloying for Mg alloys
Improving the purity of magnesium alloy and adding special alloying elements are effective methods to improve the corrosion performance of magnesium alloy. In 2022, a large number of studies have been carried out to improve the corrosion resistance of magnesium alloys by alloying, such as Zn [320–322], Ca [323–326], Ni [327], Ti [328], Y [329–331],Gd[329,332],La[328,333],Nd[333,334],Sr[335]and other alloying elements. The corrosion rate is summarized in Table 14.
The strong micro-galvanic interaction between Fe and Mg can significantly reduce the corrosion resistance of Mg.Strictly controlling the Fe impurity content in Mg alloys is essential for enhancing corrosion resistance. The effect of alloying elements (Al, Y, Mn, and Nd) on the solubility of Fe in the Mg melt (Mg-rich liquid phase) was studied by Shiyu Jiang et al. [337]. It was found that a small amount of Al,Y, Mn, or Nd in the melt can significantly decrease the solubility of Fe in the Mg melt. In addition, Mengxuan Li et al.[338] investigated several Fe/Mg models to explore how Fe accelerates Mg corrosion by first-principles calculations based on the Quantum Espresso package. Fe and Mg form a double layer with a negative Fe layer and a positive Mg layer, whichincreases the work function of the system relative to pure Mg. This finding provides insight into the corrosion process of Mg at an atomic level that might inform future measures to prevent corrosion.
Table 14 Corrosion rates of Mg alloys by adding different elements reported in 2022.
Adding an appropriate amount of Zn is beneficial to improve the mechanical strength and corrosion resistance of magnesium alloy. The corrosion rate of Mg-11Li-1 Zn was∼0.5 mg·cm-2·d-1,about 3 times lower than L11 alloy[321].Zheng Jia et al. [322] improved the corrosion resistance of homogenized Mg-3Sn-1Ca-1Cu alloy in 3.5% NaCl solution by adding 1% Zn, and the corrosion rate decreased to 80 mm·a-1from 132 mm·a-1due to the significant refinement of the grains, the decreases of Mg2Cu phases and the formation of MgZnCu phase.
Ca is one of the common alloying elements to improve the corrosion resistance of magnesium alloys. Umer Masood Chaudry et al. [323] reduced the corrosion rate of AZ31 sheet from 18.2 mm·a-1to 9.4 mm·a-1by adding 0.5 wt.% Ca.The corrosion properties of Mg-0.3Sc alloys with different Ca contents were studied by Cheng Zhang et al. [324]. Due to the combined effect of Ca and Sc, the grain size of the alloy was significantly refined, and more Mg2Ca phases were formed in the matrix.When 2 wt.%Ca is added,the corrosion rate is the lowest (1.01 mm·a-1). Adding trace Ca and Ce is also beneficial to improve the corrosion resistance of Mg-Zn alloy [325].
Adding an appropriate amount of rare-earth (RE) elements into magnesium alloys is an effective way to improve the comprehensive properties of magnesium alloys. When the Y content was 0.3 wt.%, Mg-2Zn-0.1Mn-0.3Ca alloy showed the best corrosion resistance in the simulated body fluid(SBF)[330].The increased Y addition transformed solute-rich stacking faults (SFs) into 18R-LPSO and 14H-LPSO structures,suppressing the interior corrosion of theα-Mg matrix. The as-cast Mg-4Gd-xY-1Zn-0.5Ca-1Zr alloy with 3 wt.% Y has the most uniform corrosion morphology and the lowest corrosion rate [331]. The addition of trace Gd can refine the microstructure, reduce the content of AlLi softening phase and form the Al2Gd strengthening phase, and improve the corrosion performance of homogenized Mg-8Li-3Al-2Zn-0.2Zr alloy. When the Gd content is 1.0 wt.%, the alloy shows the best corrosion rate of 0.86 mm·a-1[332]. After the addition of rare-earth Nd, a new rod-like Al3Nd phase and block-like Al2Nd phase are formed in the as-cast alloy, which makes theβ-Mg17Al12phase change from coarse network structure to fine discontinuous distribution. After adding 0.5% Nd, the corrosion resistance of AZ80 alloy is the highest[334].Penbe Kurt et al. [328] reported that compared with Mg-4Sn-2Al alloy (46.60 mm·a-1), the corrosion rate of Mg-4Sn-2Al-0.15Ti alloy and Mg-4Sn-2Al-2La alloy decreased significantly after adding La and Ti, which were 11.68 mm·a-1and 29.69 mm·a-1, respectively.
6.2.2. Preparation and forming process of Mg alloys
The corrosion resistance of magnesium alloy can be effectively improved by heat treatments [304,334,339], different preparation methods [340–343], and deformation processing technologies [344–348] to optimize and regulate the microstructure.In 2022,the research on improving the corrosion resistance of magnesium alloys through special preparation methods and forming processes was summarized in Table 15.
Heat treatment (including solution treatment, quenching,aging, etc.) can improve the corrosion resistance of magnesium alloys by adjusting the microstructure. For example,Hang Xu et al. [339] found that after T5 and T6 heat treatment, the corrosion rate of Mg-11.46Gd-4.08Y-2.09Zn-0.56Zr alloy decreased by 17.6% and 20.4%, respectively. N. Pulido-González et al. [304] studied the effect of heat treatment on the bio-corrosion behavior of Mg-Zn-Ca alloy and found that the corrosion rate of the over-aged Mg-Zn-Ca alloy was the lowest. However, Minjie Liang et al. [334] found that the corrosion resistance of AZ80-0.5Nd alloy was improved after aging treatment, mainly due to the uniform and fine precipitation ofβ-Mg17Al12after dissolution in the matrix during aging treatment.
Recently, some studies found that some new preparation processes can significantly improve the corrosion resistance of magnesium alloys. For example, the corrosion current density(Icorr)of the new Mg-13Li-X alloy fabricated by rapid solidification process(RSP)was significantly reduced to 5.83×10-7A·cm-2after immersion in HBSS solution for 2 h [340].V. Beura et al. [341] adjusted the microstructure of AZ31 magnesium alloy by shear-assisted processing and extrusion(ShAPE), which improved the corrosion resistance and mechanical properties compared with the deformed AZ31 alloy.The Mg-13 wt.% Li alloy prepared by ultra-high pressure(UHP) technology had excellent corrosion resistance because the Li2CO3film formed on the surface hinders the penetration of corrosive chloride ions, and the average corrosion rate is about 1.81 mm·a-1[343].
Table 15 Corrosion rates of Mg alloys with different preparation and forming process reported in 2022.
Taking appropriate processing methods to adjust the microstructure is one common method to improve the corrosion resistance of magnesium alloys. Extrusion, rolling and forging are the most common deformation processing methods.Guonan Liu et al. [344] and Bo Deng et al. [346] studied the corrosion performance of extruded, forged, and rolled WE43 Mg alloy in 0.6 M NaCl and Hank’s solution, respectively.R. Reyes-Rivero et al. [345] compared the corrosion resistance of extruded cast and powder metallurgical Mg-1Zn alloy in chloride-containing solutions and found the corrosion rate of PM Mg-1Zn alloy was significantly higher than that of cast Mg-1Zn alloy.Compared with the traditional deformation processing methods, many studies in 2022 have adopted new processing methods such as large plastic deformation,friction stir welding, and multi-axis deformation to improve the corrosion resistance of magnesium alloys. Abdulrahman I.Alateyah et al. [348] fabricated the biodegradable ZK30 magnesium alloy by equal channel angular processing (ECAP)and found that the corrosion rates of the four-passes sample in simulated body fluid and NaCl solution were significantly reduced by 99% and 45.25%, respectively, compared with the as-annealed alloy. Denis A. Aksenov et al. [347] prepared Mg-Al-Zn alloy by equal channel angular pressing and subsequent ultrasonic irradiation. It was found that the corrosion resistance of the alloy in HCl almost doubled after ultrasonic treatment.
6.2.3. High-efficiency corrosion inhibitors of Mg alloys
Corrosion inhibitors can effectively reduce the corrosion rate of magnesium alloys and even delay the corrosion process of metals at relatively low concentrations,which is more easygoing and promising than other corrosion protection technologies. Common corrosion inhibitors can be divided into inorganic (molybdate [349], phosphate [350], etc.) and organic(pyrazole ionic liquid corrosion inhibitors [351,352], sodium dodecyl sulfate (SDS) [353], polyethylene glycol trimethyl nonyl ether (PGTE) [353], cationic surfactant benzalkonium chloride (BC) [353], etc.) compounds. In 2022, the methods of improving the corrosion resistance of magnesium alloys by corrosion inhibitors and the corrosion inhibition efficiency are summarized in Table 16.
Inorganic corrosion inhibitors are generally used in neutral water media and have been fully applied in many fields.Maria A. Osipenko et al. [349] investigated the corrosion inhibition of AZ31 magnesium alloy in NaCl solution containing different amounts of sodium molybdate inhibitors and discussed the corrosion inhibition mechanism. It was found that the Na2MoO4inhibitor provided a reliable inhibition at a high concentration (150 mM), mainly due to the formation of a protective surface layer of mixed Mo (VI, V, IV) compounds.
However, the efficiency of inorganic corrosion inhibitors is usually low, which is greatly affected by the pH value of the corrosive medium, and the selection is generally limited. In recent years, various organic corrosion inhibitors have been developed to expand the further applications of magnesium alloys. Yumiao Jiang et al. [351] and Xing Gao et al. [352] compared corrosion inhibition of Pyrazole ionic liquid corrosion inhibitors on magnesium alloy and found[DMIm][NTf2] showed the best corrosion inhibition ability,up to 93.91%. Yan Li et al. [353] found that SDS reduced the anodic dissolution rate of AM50 magnesium alloy due to its physical adsorption in the outer porous corrosion layer.Yanan Cui et al. [354] studied the effects of SDS, PGTE, and BC on the corrosion resistance of AZ91D magnesium alloy.It was found that the inhibition efficiency of the corrosion inhibitor composite addition was much higher than that of the single branch, and the inhibition efficiency of SDS/PGTE was up to 91.47%.In 2022,many studies focused on developing environmentally friendly inhibitors from natural sources.Zeqi Liu et al. [355] synthesized a new type of green corrosion inhibitor (pheophorbide), and the inhibition efficiency was up to 92.3% in 0.5 mg·L-1NaCl solution. In addition,Haonan Li et al. [356] screened three kinds of corrosion inhibitors(GUE,PDE and TOE)from seven types of traditionalChinese medicine extracts. When the concentration of PDE reached 2.0 g·L-1, the inhibition efficiency was up to 87.6%.
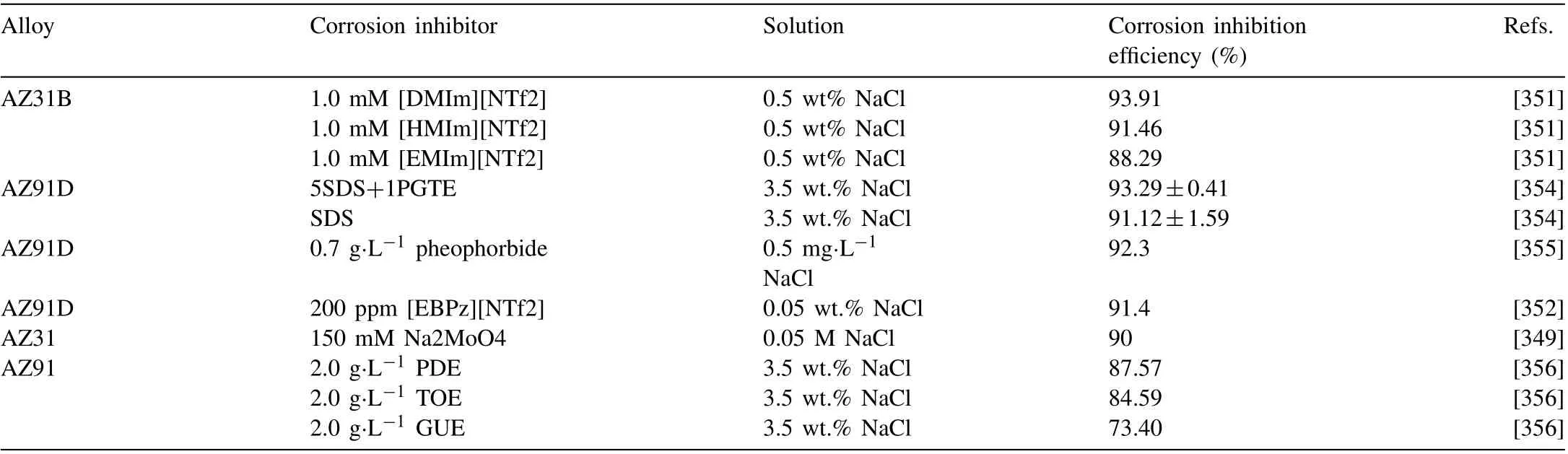
Table 16 Corrosion inhibition efficiency of Mg alloys with different corrosion inhibitors in 2022.
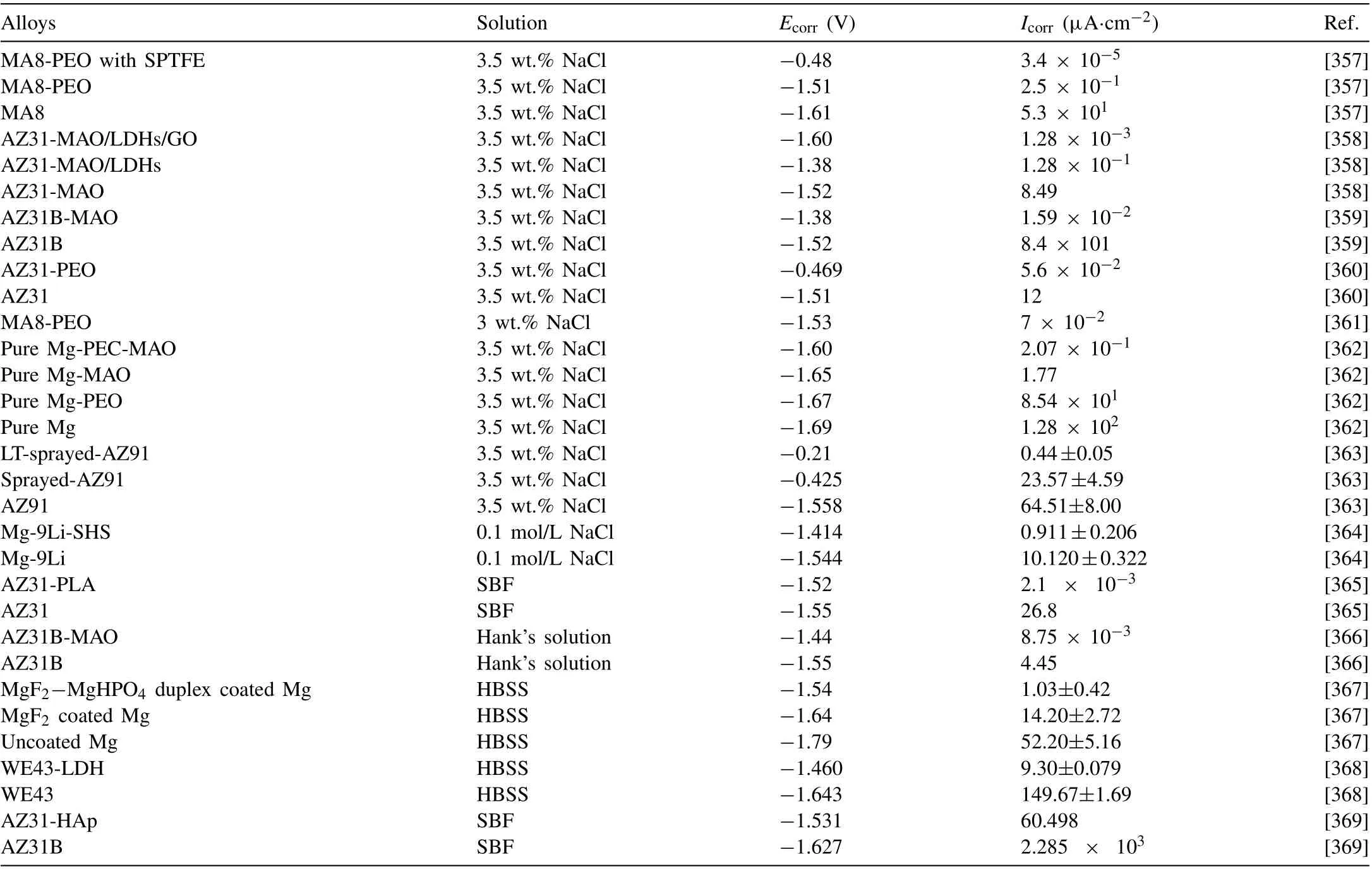
Table 17 Corrosion rate of magnesium alloys in different surface treatment conditions reported in 2022.
6.2.4. Surface treatment for Mg alloys
Surface treatment is one of the most commonly used and reliable strategies to improve the service life of magnesium alloys in corrosion conditions. The corrosion rates of magnesium alloys in different surface treatment conditions reported in 2022 are summarized in Table 17.
The single-layer coating on the surface of magnesium alloy has become popular because of its superior application prospect and economy. Anca Constantina Parau et al.[369] fabricated hydroxyapatite film on the surface of AZ31B alloy by magnetron sputtering and found that the film deposited at 200 °C showed the best corrosion behavior, the corrosion current density was only 2.6% of the substrate.Lei Liu et al. [370] prepared layered double hydroxides with the uniform structure on Mg-Gd-Y-Zn-Zr alloy, which effectively avoided the micro-current effect between the second phase and theα-Mg matrix, and the corrosion current density became as low as 2.3 × 10-7A·cm-2. Manuela Elena Voicu et al. [365] prepared a biodegradable magnesium alloy modified by polylactic acid (PLA) nanofibers by electrospinning technology, and the hydrophilicity of the alloy changed to hydrophobicity (59° vs. 98°). The corrosion rate on the uncoated Mg alloy was 2.03 mm·a-1, while on Mg alloy-PLA, it was 0.36 mm·a-1. Plasma electrolytic oxidation(PEO) is a promising surface treatment method, S. Fatimah et al. [360] used an electrolyte below zero (268 K) to change the plasma characteristics of the protective film on the surface of AZ31 magnesium alloy. Weikang Xu et al. [371] innovatively added double magnetic into the PEO process, and the film’s porosity decreased from ∼17.5% to ∼0.6%. The corrosion current density decreased by an order of magnitude compared with the non-magnetic group. At the same time,some scholars have improved the corrosion resistance of PEO films by optimizing the substrate morphology [372], process sequence [373], electrolyte nanoparticle additives [361,366],etc.
With the development of high-end equipment, the application environment of materials is becoming more and more severe, which puts forward higher requirements for the surface protection of materials.As a result,surface films/coatings are developing towards composite, micro-nano, and functionalization.
To overcome the defects of more cracks and insufficient corrosion resistance of fluoride conversion coating, M. Sathyaraj et al. [367] prepared magnesium phosphate coating based on fluoride coating to prepare magnesium fluoridemagnesium phosphate double coating to prevent excessive dissolution of magnesium. Martin Buchtík et al. [363] used a laser to remelt the HVOF sprayed iron-based layer deposited on AZ91 magnesium alloy to obtain a crystallineamorphous composite coating structure. The porosity decreased from 0.7% to 0.0%, the corrosion potential increased from -425 mV to -210 mV, and the corrosion current density decreased from 23.57 μA·cm-2to 0.44 μA·cm-2. Another composite coating was prepared on pure magnesium by plasma electrolytic carburization (PEC) and micro-arc oxidation (MAO) [362]. The corrosion current was ranked as follows: the PEC+MAO hybrid coatings (0.207 μA·cm-2)< MAO coatings (1.77 μA·cm-2)< PEC surface modification layers (85.4 μA·cm-2)< pure magnesium substrate (128 μA·cm-2).
Among the composite treatment methods [357,359, 374],layered double hydroxides (LDHs) as a nanocontainer have recently been considered as a new and effective method to reduce corrosion due to their ion exchange properties and easy modification [368]. Yanning Chen et al. [358] successfully prepared a MgAl-LDHs/graphene oxide (GO) composite coating based on AZ31 magnesium alloy micro-arc oxidation film. The synergistic effect of nano-scale GO and LDHs(1.28×10-9A·cm-2)can significantly improve the corrosion resistance of MAO coating (8.49 × 10-6A·cm-2). Yahya Jafari Tarzanagh et al. [374] used a LDH coating to load an 8-hydroxyquinoline (8-HQ) corrosion inhibitor, and a composite sol-gel layer as a surface layer, which actively protected AM60B magnesium alloy from corrosion. At the same time, Jesslyn K. E. Tan et al. [375] used citric acid (CA)to modify the LDH-8HQ composite coating on the surface of magnesium alloy WE43. The anodic and cathodic dissolution reactions were inhibited and the corrosion resistance was further improved.
The functionalization of coatings is increasingly studied.Guowei Wang et al. [364] constructed a superhydrophobic coating (contact angle of 154°) on Mg-9Li alloy by hydrothermal method, which simultaneously realized the decontamination and corrosion resistance of the layer. Qiang Li et al. [376] designed a micro/nanostructured composite coating (La-LDH powder/polydimethylsiloxane (PDMS)/La-LDH film) with double functions of self-healing and active anti-corrosion. The layer can self-heal after physical damage, which can realize maintenance-free in engineering applications. Najme Shahverdi et al. [377] fabricated nanohydroxyapatite/chitosan nanocomposite coating on Mg-2Zn scaffold by pulse electrodeposition (PED). The corrosion rate of the coating was 0.58 mm·a-1,much lower than that of Mg-2Zn and 2.09 mm·a-1. At the same time, the cell adhesion,cytotoxicity and alkaline phosphatase (ALP) assay of MG63 cells showed that it also had excellent in-vitro biocompatibility.
In conclusion, pressure, sunlight, temperature, and humidity significantly affect the corrosion behavior of magnesium alloys. In addition, the pH value of the corrosion medium,microstructure, impurities, and second phase of the alloy significantly influence the corrosion behavior of magnesium alloy. The corrosion resistance of magnesium alloys can be significantly improved by adding corrosion inhibitors, enhancing the purity, adding alloying elements, using unique preparation processes and processing methods, and surface treatment.
7. Other progresses
7.1. Standards
In 2022, ISO 4155:2022 is published successfully, which is proposed by China, with the detailed information shown in Table 18. It focuses on the content of nickel in magnesium and magnesium alloys, which is mainly determined by inductively coupled plasma optical emission spectrometric method.In addition,ISO 4155:2022 international standard was equally settled as British standards,which is BS ISO 4155:2022,indicating that the standard quality has been highly recognized by developed countries such as the UK. Actively promoting the revision or compilation of various standards in the magnesium industry, and further improving and perfecting the standardization system of the magnesium industry, are of great significance for the high-quality development of the magnesium industry.
7.2. Patents
The granted Mg-related patents in 2022 were searched in SooPAT with “Mg or magnesium alloy” as a keyword. After manually eliminating the irrelevant data, 758 granted patentsin total were found, which represents an increase compared with the number of the authorized invention patents in 2022.
Table 19 Distribution of granted patents on magnesium and magnesium alloys in different countries and regions in 2022.
According to the number of Mg-related granted patents published in 2022, the top 10 countries and regions of the granted patens are listed in Table 19. China granted the most patents on Mg and Mg alloys, followed by USA, Japan, Europe and South Korea. In addition, many papers on magnesium and magnesium alloys have been published in these countries, indicating that they value the academic and industrial development of magnesium and magnesium alloys.
7.3. International awards
In 2022, both International Mg Society (IMS) and International Magnesium Association (IMA) issued awards on magnesium and magnesium alloys. Both awards are of great importance to the R&D and applications of magnesium and magnesium alloys.
IMS issued the 2022 International Mg Science and Technology Award (Annual Award). Eight types of awards were conferred to 26 winners who have made significant contributions to the magnesium R&D and applications. These awards covered many aspects including magnesium smelting,Mg-based structural materials, Mg-based functional materials and so on. The award effectively promoted the discussion and communication between scientists,young researchers,and students, stimulated the interaction and collaboration between universities, research institutes and enterprises, boosted the development and application of magnesium and magnesium alloy science and technology, and provided a long-term platform for international communication and collaboration.
IMA issued six types of awards, which are Automotive Cast Product/Process, Commercial Cast Product/Process, Process, Wrought Product, Environmental Responsibility, Future Technology.The award effectively boosted the application and industrial development of magnesium and magnesium alloys.
8. Summary and outlook
The research and development of magnesium alloys have received more and more attention. Over 4600 papers on magnesium and magnesium alloys were published and indexed in the database of “Web of Science Core Collection” last year.According to bibliometric analysis, the microstructures and mechanical properties of magnesium alloys are still the main research focus, and the corrosion and protection of magnesium alloys continue to receive widespread attention. Emerging research hotspots involve mainly functional magnesium materials, such as magnesium ion batteries, hydrogen storage magnesium materials, and bio-magnesium alloys.
In the structural Mg alloys, some new progress in cast and wrought Mg alloys are made in 2022. For cast magnesium alloys, the Mg-7.8Gd-2.7Y-2.0Ag-0.4Zr alloy fabricated by gravity casting exhibits an ultimate tensile strength of ∼411 MPa and an elongation to failure of ∼4.9%. For wrought magnesium alloys, the ultimate tensile strength and elongation of Mg-15Gd alloy via extrusion, warm-rolling and aging have exceeded 518 MPa and 4.5%, respectively. The development of superlight wrought Mg alloys is fruitful. Mg-7Li-2Al-1.5Sn alloy prepared by conventional extrusion exhibits an excellent tensile strength of 324 MPa and a relatively good plasticity of 11.9%. Controlling defects is of great significance for improving the performance of Mg alloys. The solid-liquid-gas multiphase-field lattice-Boltzmann model has been proposed. It has made significant progress in solving the formation of the gas porosity and is suitable for addressing the issues involving the solid-liquid-gas multiphase and multi-physical characteristics.
Functional magnesium materials are becoming an emerging focus and have made some new progress in 2022. In the aspect of bio-magnesium alloys,the developed Mg-30Sc alloy exhibits an acceptable in-vivo degradation rate (0.06 mm/y),without cytotoxicity on the MC3T3 cell model, and excellent mechanical properties. The surface treatment as an effective method to improve the corrosion resistance and biocompatibility of biomedical magnesium alloys needs to be further developed to achieve the controllable degradation of Mg alloys.In the aspect of Mg batteries, a new CuS0.96Te0.04cathode material with a reversible capacity of up to 446 mAh·g-1and a life-span of 1500 cycles has been developed. In the case of hydrogen storage materials, some new catalysts have been used to reduce the activation energy of hydrogen release and accelerate the rate of hydrogen desorption under isothermal conditions,such as YH2,NiCp2,Ni/BN,Ni/V2O3,and so on. Additionally, some new preparation methods which can directly generate nano-powders and improve the kinetic properties of hydrogen storage alloys at the same time have been developed.
The preparation and processing technologies of magnesium alloys were further improved in 2022. For cast technology, a Mg-0.5Zn-0.5Ca alloy fabricated by a new twinroll casting process (TRC) showed excellent refinement with grain sizes less than 150 μm and exhibited a UTS and EL of 221.9 MPa and 9.3%, respectively. For plastic processing technology, the accumulative backward extrusion (ABE),slope extrusion (SE), asymmetric rolling (AR), ultrasonic surface rolling (USRP), etc., were developed. For additive manufacturing technology, extrusion-based AM followed by debinding and sintering is a powerful approach to fabricating Mg scaffolds. In addition, the recovery and reuse of waste magnesium and magnesium alloys were developed in the past year. Inert treatment of waste magnesium alloy dust forms a composite conversion film on the surface of the dust particles, blocking the contact between external water molecules and Mg2+ions, avoiding hazards.
In the aspect of corrosion and protection, the corrosion resistance of magnesium alloys can be significantly improved by adding corrosion inhibitors, enhancing the purity of the alloy, adding alloying elements, using appropriate preparation processes and processing methods, and surface treatments. Surface treatment is a simple and efficient method,which has received extensive attention from researchers. Mg-Mn-Ce alloy with PEO/superdispersed polytetrafluoroethylene composite coating exhibits an extremely low corrosion rate(3.4 × 10-5μA·cm-2), which is reduced by more than 6 orders of magnitude in comparison with the uncoated magnesium alloy. The surface films/coatings are developing towards composite, micro-nano, and functionalization. In the future,high corrosion-resistant magnesium alloys and effective surface treatment of magnesium alloys should be further developed to meet the application requirements on most occasions.
Although the research and development of magnesium alloys have been widely carried out, there are some challenges that still need to be overcome. For structural Mg alloys, the comprehensive properties of magnesium alloys need to be further improved. For functional magnesium materials, alloying design and surface modification of bio-magnesium alloys are the key to enhancing corrosion resistance, but in-vivo biological properties,including healing rates,inflammatory reactions,and side effects, should be considered systematically. Magnesium batteries still face challenges such as the incompatibility of electrolytes and the scarcity of the high-performance cathode and anode materials, which require further research. The large-scale use of magnesium-based hydrogen storage materials is hindered because of the high thermodynamic stability and the slow reaction kinetics. Further research needs to be conducted in relation to alloying, nanostructuring, adding catalysts, changing preparation methods, etc. to overcome these problems. For the preparation technologies, the microstructural evolution and heat treatment of vacuum die casting, low cost and high safety of large and complex additive manufacturing Mg alloy parts, and welding of Mg alloy castings with complex morphology deserve to be further investigated. The corrosion resistance of magnesium alloys should be improved to meet the demand of wider applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Yan Yang, Xiaodong Peng, Daolun Chen, Fusheng Pan are editorial board members/editor-in-chief for Journal of Magnesium and Alloys and were not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Acknowledgments
The authors would like to thank the experts worldwide(including Prof. Guangsheng Huang and Prof. Qian Li, Dr.Jia She, Dr. Ang Zhang, Dr. Shuangshuang Tan, etc.) who have contributed to this review by providing important information and commenting on the manuscript preparation. We also express our gratitude to some students (including Hao Wang, Xiaofei Cui, Gang Zhou, Haohao Mi, Lizou Shen,Yangyang Luo, Weihao Liu, Chen He, etc.) for providing us with a lot of assistance. This work was financially supported by the National Key Research and Development Program of China (No. 2021YFB3701100), the National Natural Science Foundation of China (Nos. 52171104 and U20A20234), the Chongqing Research Program of Basic Research and Frontier Technology, China (Nos. cstc2021ycjh-bgzxm0086 and 2019jcyj-msxmX0306), the fundamental Research funds for Central Universities, China (Nos. SKLMT-ZZKT-2022R04,2021CDJJMRH-001, and SKLMT-ZZKT-2022M12).
杂志排行
Journal of Magnesium and Alloys的其它文章
- NaH doped TiO2 as a high-performance catalyst for Mg/MgH2 cycling stability and room temperature absorption
- Microstructure, mechanical properties and fracture behaviors of large-scale sand-cast Mg-3Y-2Gd-1Nd-0.4Zr alloy
- Enhanced initial biodegradation resistance of the biomedical Mg-Cu alloy by surface nanomodification
- Elucidating the evolution of long-period stacking ordered phase and its effect on deformation behavior in the as-cast Mg-6Gd-1Zn-0.6Zr alloy
- Effect of long-period stacking ordered structure on very high cycle fatigue properties of Mg-Gd-Y-Zn-Zr alloys
- The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition
