PCOS患者血清血管生成素样蛋白2、4水平与卵巢间质血流动力学的关系
2023-11-08赵芳园邹红时思毛徐芳左冬冬
赵芳园 邹红 时思毛 徐芳 左冬冬
摘要:目的 探討多囊卵巢综合征(PCOS)患者血清血管生成素样蛋白2、4(Angptl2、Angptl4)与卵巢间质血流动力学的关系。方法 选取123例PCOS患者(PCOS组)及41例健康志愿者(对照组),比较2组血清Angptl2、Angptl4水平及卵巢间质收缩期最大血流速度(PSV)、搏动指数(PI)、阻力指数(RI);分析Angptl2、Angptl4与PSV、PI、RI及PCOS的关系及两者对PCOS的预测价值。结果 PCOS组左右侧卵巢间质PI、RI均低于对照组,PSV高于对照组(P<0.05)。Pearson分析显示,PCOS患者血清Angptl2、Angptl4与左右侧卵巢间质PSV呈正相关,与PI、RI呈负相关(P<0.01)。Logistic回归结果显示,高HOMA-IR(OR=1.921,95%CI:1.017~4.154)、高BMI(OR=1.459,95%CI:1.085~3.220)、高Angptl2(OR=2.625,95%CI:1.330~6.324)、高Angptl4(OR=3.543,95%CI:1.915~8.147)是影响PCOS发生的危险因素(P<0.05)。受试者工作特征(ROC)曲线显示,血清Angptl2、Angptl4单独应用时,预测PCOS的AUC(95%CI)为0.747(0.661~0.821)、0.769(0.685~0.841),低于两者联合应用的0.879(0.793~0.921,P<0.05)。结论 血清Angptl2、Angptl4在PCOS患者中异常升高,与卵巢间质血流动力学相关,且两者与PCOS发生密切相关,可作为评估PCOS的参考指标。
关键词:多囊卵巢综合征;卵巢间质;血流动力学;血管生成素样蛋白2;血管生成素样蛋白4
中图分类号:R711.75文献标志码:ADOI:10.11958/20222119
Changes of serum angiopoietin-like protein 2 and 4 levels and their relationship with ovarian interstitial hemodynamics in patients with PCOS
ZHAO Fangyuan ZOU Hong SHI Simao XU Fang ZUO Dongdong
1 Department of Ultrasound Medicine, 2 Department of Gynaecology, 3 Preventive Medicine Centre, the First Affiliated
Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin 150040, China
Corresponding Author E-mail: 87312569@qq.com
Abstract: Objective To investigate changes of serum Angptl2 and Angptl4 levels in patients with polycystic ovary syndrome (PCOS) and their relationship with ovarian interstitial hemodynamics. Methods A total of 123 PCOS patients (the PCOS group) and 41 healthy volunteers (the control group) were selected. The serum Angptl2 and Angptl4 levels, the peak systolic velocity (PSV), pulsation index (PI) and resistance index (RI) of ovarian interstitial were compared between the two groups. The relationship between Angptl2, Angptl4, PSV, PI, RI and PCOS and their predictive value to PCOS were analyzed. Results The PI and RI values of left and right ovarian interstitial were lower in the PCOS group than those in the control group, and PSV values were higher than those in the control group (P<0.05). Pearson analysis showed that serum Angptl2 and Angptl4 were positively correlated with PSV of left and right ovarian interstitial, and negatively correlated with PI and RI of left and right ovarian interstitial in patients with PCOS (P<0.01). Logistic regression results showed that high HOMA-IR (OR=1.921, 95%CI: 1.017-4.154), high BMI (OR=1.459, 95%CI: 1.085-3.220), high Angptl2 (OR=2.625, 95%CI: 1.330-6.324), high Angptl4 (OR=3.543, 95%CI: 1.915-8.147) levels were related factors affecting the occurrence of PCOS (P<0.05). Receiver operating characteristic curve (ROC) showed that the AUC (95%CI) of predicted PCOS were 0.747 (0.661-0.821) and 0.769 (0.685-0.841) when serum Angptl2 and Angptl4 were used alone. It was lower than that of 0.879 (0.793-0.921, P<0.05) for the combined application. Conclusion The abnormal levels of serum Angptl2 and Angptl4 in PCOS patients are related to the hemodynamics of ovarian interstitial, and they are closely related to the occurrence of PCOS, which can be used as reference indexes for the evaluation of PCOS.
Key words: polycystic ovary syndrome; ovarian interstitial; hemodynamics; angiopoietin-like protein 2; angiopoietin-like protein 4
多囊卵巢综合征(polycystic ovary syndrome,PCOS)是内分泌系统常见病,在我国育龄期女性中发病率为7%~9%,且逐年升高,呈年轻化趋势[1]。目前关于PCOS的诊断主要依据临床症状、血清激素及超声检查。近年来研究显示,彩色多普勒超声血流显像技术通过评估卵巢及子宫血流改变情况,有助于指导PCOS的诊断及治疗[2-3]。有研究指出,PCOS患者存在卵巢血管增生及血流动力学异常改变,认为血管增生失常可能在PCOS发生及发展中起重要作用[4]。血管生成素样蛋白(angiopoietin-like protein,Angptl)是一类与血管生成素(angiopoietin,Ang)结构相似的分泌型糖蛋白,但功能上却有明显差别,在糖脂代谢异常、炎症反应及血管生成等过程中发挥重要调控作用[5]。Angptl2和Angptl4是Angptl家族的重要成员。Park等[6]研究发现,超重及肥胖者血清Angptl2水平升高,且与动脉僵硬度呈正相关,在接受12周的饮食干预后,血清Angptl2水平明显降低。另有研究发现,在接受体外受精-胚胎移植治疗的PCOS患者卵巢颗粒细胞中Angptl4高表达[7]。因此,Angptl2和Angptl4可能与脂代谢密切相关,但其与PCOS患者卵巢间质血流的关系尚未可知。本研究通过分析PCOS患者血清Angptl2和Angptl4的表达变化及与卵巢间质血流动力学参数的关系,为临床PCOS早期防治提供参考。
1 对象与方法
1.1 研究对象 选取2020年5月—2022年5月黑龙江中医药大学附属第一医院收治的123例PCOS患者(PCOS组),年龄23~37岁,平均(31.30±3.44)岁。纳入标准:(1)PCOS符合2003年鹿特丹共识[8](3条中符合任意2条),即长期无排卵或稀发排卵;伴高雄激素血症并排除其他病因;超声提示卵泡≥12个、卵巢体积>10 mL。(2)近1个月内未接受非甾体抗炎药或影响胰岛素水平的药物者。排除标准:(1)近期严重感染性疾病者。(2)伴其他妇科疾病,如卵巢良恶性肿瘤、子宫内膜异位等。(3)伴其他内分泌系统疾病,如甲状腺疾病、代谢综合征等。(4)合并恶性肿瘤者。(5)伴可引起排卵障碍的其他病因者。(6)存在心脑血管疾病、原发性高血压等血管相关疾病者。(7)有精神病史者。另选取同期体检中心41例健康志愿者(对照组),均为女性,年龄22~36岁,平均(31.12±3.16)岁。所有研究对象均签订知情同意书并获得医院伦理委员会批准(批号HZYLLKY202201601)。
1.2 研究方法
1.2.1 阴道超声检查 月经结束3~5 d后,采用彩色多普勒超声诊断仪(GE Voluson E10型,探头频率5.0~8.0 MHz),先二维模式下整体扫查卵巢形态,再切换血流显像模式,记录双侧卵巢间质内动脉的收缩期最大血流速度(peak systolic velocity,PSV)、搏动指数(pulsation index,PI)、阻力指数(resistance index,RI),测量3次取均值,均由同一名经验丰富的超声科医师操作。
1.2.2 臨床资料收集及实验室指标检测 记录年龄、体质量指数(BMI),PCOS组及对照组均在月经第3—5天上午采集空腹肘静脉血5 mL,离心(3 000 r/min,半径11.5 cm)10 min,取上清液并分成2份。一部分检测血清激素:卵泡刺激素(follicle stimulating hormone,FSH)、黄体生成素(luteinizing hormone,LH)、雌二醇(estradiol,E2)、睾酮(testosterone,T)、泌乳素(prolactin,PRL)。空腹血糖(FPG)和血清激素均采用全自动生化仪(日本东芝,型号FR2000)及其配套试剂盒检测,用电化学发光法检测空腹胰岛素(FINS),胰岛素抵抗指数(HOMA-IR)=FPG×FINS/22.5;另一部分采用酶联免疫吸附试验(ELISA)检测血清Angptl2和Angptl4水平,Angptl2检测试剂盒购自无锡东林科技发展有限公司(货号DL-ANGPTL2-Hu),Angptl4检测试剂盒购自美国Thermo Fisher公司(货号EHANGPTL4),按照试剂盒说明书于490 nm波长处测量吸光度值,根据标准曲线计算Angptl2和Angptl4浓度。
1.3 统计学方法 采用SPSS 26.0进行数据分析,计量资料以均数±标准差(x±s)表示,组间比较采用t检验;2样本间的相关性用Pearson法分析;采用Logistic回归分析PCOS影响因素,绘制受试者工作特征(ROC)曲线进行预测价值分析。以双侧P<0.05为差异有统计学意义。
2 结果
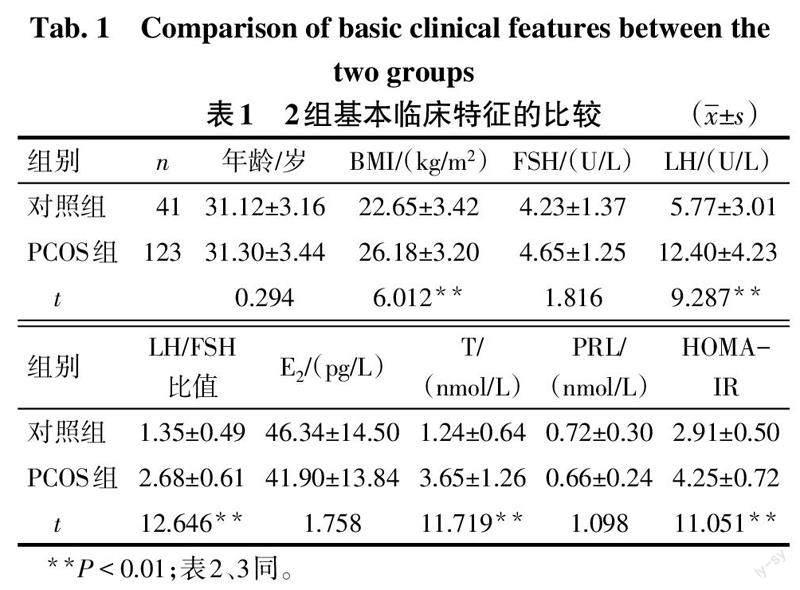
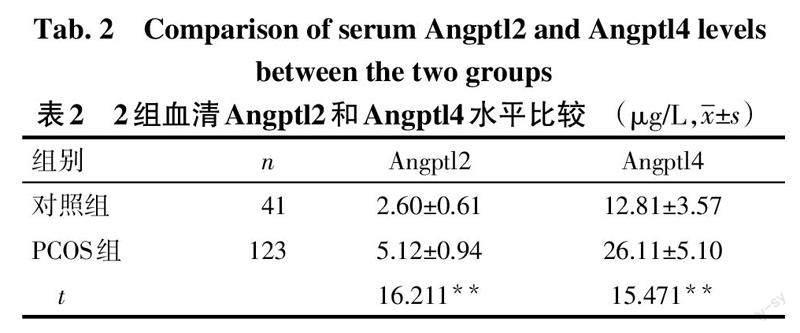
2.1 2组基线资料及血清Angptl2、Angptl4水平比较 PCOS组BMI、LH、LH/FSH比值、T、HOMA-IR、Angptl2、Angptl4水平高于对照组(P<0.01),2组年龄、FSH、E2、PRL比较差异无统计学意义,见表1、2。
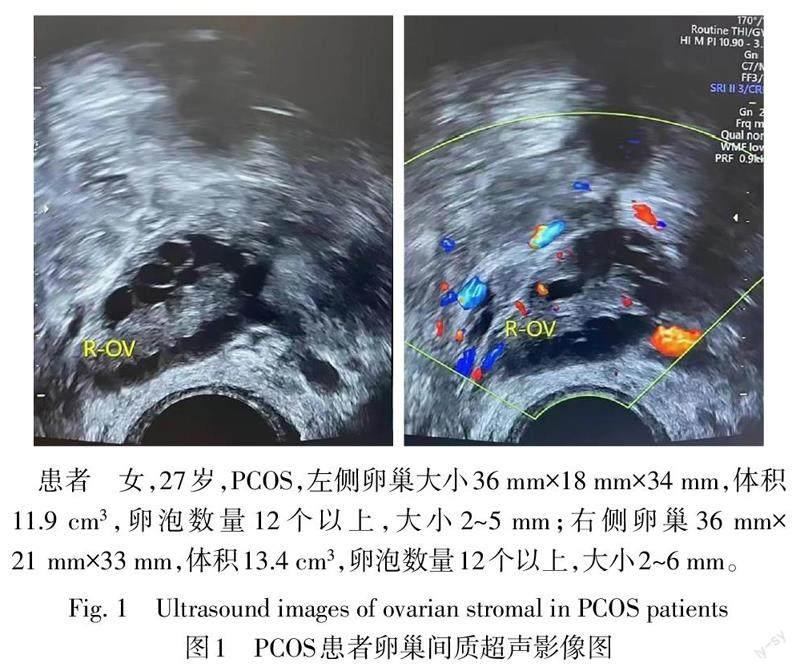
2.2 2组卵巢间质血流动力学参数比较 卵巢超声检查提示PCOS患者卵巢和卵泡体积较大,卵泡数量较多(图1)。与对照组比较,PCOS组左右侧卵巢间质PI、RI值降低,PSV值升高(P<0.01),见表3。
2.3 PCOS患者血清Angptl2和Angptl4水平与卵巢间质血流动力学参数的关系 PCOS患者血清Angptl2和Angptl4与左右侧卵巢间质PSV呈正相关(P<0.01);血清Angptl2和Angptl4与左右侧卵巢间质PI、RI呈负相关(P<0.01)。见表4。
2.4 PCOS的影响因素分析 以年龄、BMI、血清LH/FSH、LH、T、HOMA-IR、Angptl2、Angptl4为自变量,以PCOS为因变量(有=1,无=0),进行Logistic回归分析,结果显示,升高的HOMA-IR、BMI、Angptl2和Angptl4均是影响PCOS发生的危险因素(P<0.05),见表5。
2.5 血清Angptl2、Angptl4水平对PCOS的预测价值 以PCOS组为阳性、对照组为阴性,建立ROC诊断模型,联合应用按照LogP模式进行拟合诊断,ROC分析结果显示,血清Angptl2和Angptl4单独应用时AUC均低于Angptl2、Angptl4联合应用,差异有统计学意义(Z分别为4.258、2.135,P<0.05)。见表6、图2。
3 讨论
PCOS是不孕症的主要病因之一,给育龄期女性身心健康均造成严重影响[9]。关于PCOS发病机制尚无统一定论,多认为与肥胖、内分泌紊乱、胰岛素抵抗(insulin resistance,IR)、炎症反应等有关。此外,血管生成失调与PCOS的关系也有诸多研究证实[10-11]。研究显示,PCOS卵巢血供呈现周期性改变,但当血供异常增加时会导致血管增生,引发卵巢囊肿、肿瘤等疾病,因卵巢血流灌注对卵泡发育成熟及排卵有重要影响[12]。监测卵巢间质血供异常改变在临床中有一定难度,寻找与之密切相关的血清标志物有重要临床意义。宋小青等[13]研究发现,PCOS患者血清AngⅡ高表达,且与IR有关,提示血管功能异常参与PCOS发生及发展。Angptl2、Angptl4与Ang结构类似,但功能更丰富,不仅能调节血管内皮细胞功能、参与血管内皮修复,还与糖脂代谢、炎症反应等有关[14-15]。因此,探讨血清Angptl2、Angptl4水平改变与卵巢间质血流动力学改变的关系有助于监测PCOS发生及病情发展。
本研究发现,PCOS组左右侧卵巢间质PI、RI值均低于对照组,PSV值及Angptl2、Angptl4表达高于对照组,说明PCOS患者血清Angptl2、Angptl4水平呈升高状态,卵巢间质血流阻力降低。Angptl2、Angptl4是调节代谢与血管稳态的多功能糖蛋白[16]。Zhou等[17]研究发现,外泌体miR-211可靶向抑制Angptl2表达,从而抑制肥胖母猪脐静脉内皮细胞增殖及血管生成。Li等[18]发现,PCOS患者Angptl2、Angptl4升高可促进卵巢组织血管生成,而机体为应对过度的血管生成可能启动了代偿机制,从而引起卵巢局部环境紊乱,最终导致卵巢组织病理损害。PCOS患者卵巢间质血流波峰圆钝,呈现“高速低阻”现象,提示PCOS卵巢血流异常丰富,这与陈奕男等[19]研究结果相似。本研究结果显示,PCOS患者血清Angptl2、Angptl4与左右侧卵巢间质PSV呈正相关,与左右侧卵巢间质PI、RI呈负相关,提示PCOS患者血清Angptl2、Angptl4水平与卵巢间质血流速度正相关,与血流阻力负相关。分析其可能机制为,Angptl2、Angptl4能够直接诱导卵巢间质血管生成。此外,卵巢血供增加伴随机体激素水平改变,为新生血管提供更适应生长的环境,进一步加速异常血管增生,形成恶性循环[20-21]。
本研究结果显示,高HOMA-IR、BMI、Angptl2和Angptl4均是影响PCOS发生的危险因素。Angptl2在PCOS大鼠模型卵巢组织中高表達,给予二甲双胍干预后Angptl2及IR降低,推测Angptl2可能通过PI3K信号通路影响内分泌环境而参与疾病发展[22]。Spitler等[23]研究发现,Angptl4基因敲除小鼠在长期高脂喂养期间葡萄糖耐量及三酰甘油摄取量均无明显改变,证实Angptl4参与脂代谢及IR的调节。Schinzari等[24]指出,与非肥胖患者相比,不同肥胖亚型(代谢正常、代谢异常、2型糖尿病)患者循环血中Angptl4均升高,血管反应性降低,提示Angptl4与内皮功能障碍有关。由此可推测,Angptl2、Angptl4作为代谢调节因子,可能通过影响IR、卵巢间质血管内皮功能导致PCOS发生。ROC曲线分析发现,血清Angptl2、Angptl4单独应用时,均低于Angptl2、Angptl4联合应用的AUC,提示血清Angptl2、Angptl4水平与PCOS发生密切相关,可作为PCOS发生的参考指标。
综上,血清Angptl2、Angptl4在PCOS患者中升高,与卵巢间质血流速度正相关,与血流阻力负相关,且血清Angptl2、Angptl4水平可作为评估PCOS发生的参考指标。本次开展的是单中心小样本研究,2项血清指标进行预测的最佳截断值可能存在一定偏倚,在后续研究中应该开展多中心大样本试验,提高血清Angptl2、Angptl4对PCOS预测截断值的准确性,为临床提供可靠依据。
参考文献
[1] 乔杰,李蓉,李莉,等. 多囊卵巢综合征流行病学研究[J]. 中国实用妇科与产科杂志,2013,29(11):849-852. QIAO J,LI R,LI L,et al. Epidemiology of polycystic ovary syndrome[J]. Chinese Journal of Practical Gynecology and Obstetrics,2013,29(11):849-852.
[2] WEI L,WU F,ZHANG J,et al. Evaluation of endocrine and metabolic changes in polycystic ovary syndrome by ultrasonic imaging features under an intelligent algorithm[J]. Comput Math Methods Med,2022,2022:1411943. doi:10.1155/2022/1411943.
[3] 魏鏡讚,赵彦艳. 多囊卵巢综合征的卵巢血管生成[J]. 中国实用妇科与产科杂志,2020,36(6):567-570. WEI J Z,ZHAO Y Y. Ovarian angiogenesis in polycystic ovarian syndrome[J]. Chinese Journal of Practical Gynecology and Obstetrics,2020,36(6):567-570. doi:10.19538/j.fk2020060121.
[4] DAMBALA K,PASCHOU S A,MICHOPOULOS A,et al. Biomarkers of endothelial dysfunction in women with polycystic ovary syndrome[J]. Angiology,2019,70(9):797-801. doi:10.1177/0003319719840091.
[5] LIU Y Z,ZHANG C,JIANG J F,et al. Angiopoietin-like proteins in atherosclerosis[J]. Clin Chim Acta,2021,521:19-24. doi:10.1016/j.cca.2021.06.024.
[6] PARK J,CHOI Y,MIZUSHIMA R,et al. Dietary modification reduces serum angiopoietin-like protein 2 levels and arterial stiffness in overweight and obese men[J]. J Exerc Nutrition Biochem,2019,23(3):39-44. doi:10.20463/jenb.2019.0021.
[7] JIANG Q,PAN Y,LI P,et al. ANGPTL4 expression in ovarian granulosa cells is associated with polycystic ovary syndrome[J]. Front Endocrinol(Lausanne),2021,12:799833. doi:10.3389/fendo.2021.799833.
[8] GEISTH?VEL F. A comment on the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine consensus of the polycystic ovarian syndrome[J]. Reprod Biomed Online,2003,7(6):602-605. doi:10.1016/s1472-6483(10)62081-0.
[9] WITCHEL S F,TEEDE H J,PE?A A S. Curtailing PCOS[J]. Pediatr Res,2020,87(2):353-361. doi:10.1038/s41390-019-0615-1.
[10] CHEN W,PANG Y. Metabolic syndrome and PCOS:pathogenesis and the role of metabolites[J]. Metabolites,2021,11(12):869. doi:10.3390/metabo11120869.
[11] CELIK ?,YILMAZ E,CELIK N,et al. Salusins,newly identified regulators of hemodynamics and mitogenesis,increase in polycystic ovarian syndrome[J]. Gynecol Endocrinol,2013,29(1):83-86. doi:10.3109/09513590.2012.706667.
[12] DWIVEDI A,GANESH V,SHUKLA R C,et al. Colour Doppler evaluation of uterine and ovarian blood flow in patients of polycystic ovarian disease and post-treatment changes[J]. Clin Radiol,2020,75(10):772-779. doi:10.1016/j.crad.2020.05.023.
[13] 宋小青,蔡遷,王革玲,等. 多囊卵巢综合征患者血清FGF-21、MCP-1、AngⅡ表达与胰岛素抵抗的关系[J]. 中国医师杂志,2022,24(5):790-792. SONG X Q,CAI Q,WANG G L,et al. Relationship between expression of FGF-21,MCP-1,AngⅡ and insulin resistance in patients with polycystic ovary syndrome[J]. Journal of Chinese Physician,2022,24(5):790-792. doi:10.3760/cma.j.cn431274-20210416-00441.
[14] YANG L,LI T,ZHA L. Foxc2 alleviates ox-ldl-induced lipid accumulation,inflammation,and apoptosis of macrophage via regulating the expression of angptl2[J]. Inflammation,2020,43(4):1397-1410. doi:10.1007/s10753-020-01217-w.
[15] HE Y,YANG W,GAN L,et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol,2021,44(5):355-365. doi:10.1016/j.gastrohep.2020.09.014.
[16] FERN?NDEZ-HERNANDO C,SU?REZ Y. ANGPTL4:a multifunctional protein involved in metabolism and vascular homeostasis[J]. Curr Opin Hematol,2020,27(3):206-213. doi:10.1097/MOH.0000000000000580.
[17] ZHOU Y,XIA M,CUI C,et al. Circulating exosomal mir-221 from maternal obesity inhibits angiogenesis via targeting angptl2[J]. Int J Mol Sci,2021,22(19):10343. doi:10.3390/ijms221910343.
[18] LI M,HU J,YAO L,et al. Decreased ANGPTL4 impairs endometrial angiogenesis during peri-implantation period in patients with recurrent implantation failure[J]. J Cell Mol Med,2020,24(18):10730-10743. doi:10.1111/jcmm.15696.
[19] 陈奕男,秦将均. 超声监测PCOS患者卵巢間质血流动力学变化及其与血清ES、VEGF的相关性分析[J]. 中国超声医学杂志,2021,37(4):453-456. CHEN Y N,QIN J J. Ultrasound monitoring of ovarian stromal hemodynamic changes and its correlation with serum Es and VEGF in patients with PCOS[J]. Chinese Journal of Ultrasound in Medicine,2021,37(4):453-456.
[20] WEI J,ZHAO Y. MiR-185-5p Protects against angiogenesis in polycystic ovary syndrome by targeting VEGFA[J]. Front Pharmacol,2020,11:1030. doi:10.3389/fphar.2020.01030.
[21] WEI Y,LU S,HU Y,et al. MicroRNA-135a regulates VEGFC expression and promotes luteinized granulosa cell apoptosis in polycystic ovary syndrome[J]. Reprod Sci,2020,27(7):1436-1442. doi:10.1007/s43032-020-00155-0.
[22] WANG D,GUO Y,CHAI S,et al. Expression of angiopoietin-like protein 2 in ovarian tissue of rat polycystic ovarian syndrome model and its correlation study[J]. Reprod Biol Endocrinol,2020,18(1):94. doi:10.1186/s12958-020-00651-7.
[23] SPITLER K M,SHETTY S K,CUSHING E M,et al. Chronic high-fat feeding and prolonged fasting in liver-specific ANGPTL4 knockout mice[J]. Am J Physiol Endocrinol Metab,2021,321(4):E464-E478. doi:10.1152/ajpendo.00144.2021.
[24] SCHINZARI F,VIZIOLI G,CAMPIA U,et al. Variable changes of circulating ANGPTL3 and ANGPTL4 in different obese phenotypes:relationship with vasodilator dysfunction[J]. Biomedicines,2021,9(8):1037. doi:10.3390/biomedicines9081037.
(2022-12-27收稿 2023-03-14修回)
(本文编辑 胡小宁)
